Abstract
In this review, we synthesize the current knowledge of the biology, ecology, and impact of Sinanodonta freshwater mussels (Bivalvia, Unionidae), native to East Asia, that have successfully invaded Europe, Central America, North Africa, and several Asian regions. The main introduction pathways of Sinanodonta were reconstructed based on DNA sequence data and distribution records. We show that invasive lineages of Sinanodonta belong to three species, namely, S. woodiana s. str. (“temperate invasive” lineage), S. pacifica (“tropical invasive” lineage), and S. lauta. Their generalist fish-dispersed larvae, short life span, high fecundity, use by humans for multiple purposes, and ability to establish populations in anthropogenically disturbed conditions were identified as crucial traits driving their invasions. Information on the consequences is scarcer, but Sinanodonta can impact native species through larval parasitism, host fish/food competition, and parasite transmission. In addition, ecosystem effects through their filtration—biodeposition—excretion activity and the occurrence of massive die-offs were detected. Ecosystem services and disservices have not yet been quantified, even at local scales, and management methods in the invasive range are understudied. A better understanding of Sinanodonta ecology, impacts, and management options is urgently needed to make informed decisions and set realistic and impactful restoration goals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and current research effort
Invasive freshwater bivalves are a global problem with significant and growing adverse effects on the economy, environment, and biodiversity (Early et al., 2016; Haubrock et al., 2022). Dominant reproductive and ecological traits in this group include a simple life cycle involving planktonic larval stage, environmental tolerance, and short generation time, which create preconditions for a high invasive potential and negative impacts on a global scale (Sousa et al., 2014). However, the invasive lineages of the freshwater mussel genus Sinanodonta Modell, 1945, native to East Asia (Lopes-Lima et al., 2020), are an ecological exception among other invasive bivalve species. Belonging to the Unionidae, Sinanodonta species have a complex life cycle, which includes an obligatory parasitic larval stage involving a fish host (Dudgeon & Morton, 1983). Most members of Unionidae are sensitive to environmental changes, rendering this family one of the most threatened animal groups (Haag & Williams, 2013; Lopes-Lima et al., 2018; Zieritz et al., 2018a; Benson et al., 2021). Accordingly, exhaustive research focused on problematic invasive bivalves from the families Dreissenidae and Cyrenidae (for example Ward & Ricciardi, 2007; Sousa et al., 2009; Zieritz et al., 2022) is only partially applicable to Sinanodonta.
Results from a systematic review and classification of available literature (Supplementary material S1) show that research related to Sinanodonta mussels conducted to date (n = 266 research articles) has focused predominantly on the research categories of “Ecology”, “Ecotoxicology”, and “Biogeography”, while categories “Reproduction biology”, “Conservation”, and “Systematics” were least represented in the dataset. The majority of the articles that were related to invasion biology (27.4% of the whole dataset) were focused on “Distribution”, followed by “Impact” and “Ecology in invasive range”. Surprisingly, only two studies focused on “Prevention”, and no articles focused on the “Control” of these invasive bivalves. This extensive collection of data and new genetic samples offer an opportunity for a more thorough understanding of the group's global invasion.
Here we present a new phylogeny with new data and synthesize information on the (1) taxonomy, systematics, and evolution, (2) native and non-native distribution, (3) major pathways and vectors of introduction, (4) ecology, (5) impacts and (6) management options of all invasive lineages (sensu lato) of the genus Sinanodonta, namely the two globally invasive lineages of S. woodiana (Lea, 1834) [often referred to as Anodonta woodiana (Lea, 1834)] (Bolotov et al., 2016) and S. lauta (Martens, 1877), which has been introduced to central Asia (Bespalaya et al., 2018) and Borneo (Zieritz et al., 2020), respectively. Newly discovered phylogenetic relationships within the genus Sinanodonta and advancements in understanding ecological requirements and effects provide a fresh perspective on the primary knowledge gaps, management opportunities and the way forward.
Taxonomy, systematics, and evolution
Most species currently recognized within Sinanodonta Modell, 1945 were originally placed in Anodonta Lamarck, 1799 until the middle of the last century (Haas, 1969). In recognition of several morphological distinctions, the genus Sinanodonta was created to include East Asian species previously assigned to the genus Anodonta. The validity of Sinanodonta was only widely accepted and confirmed with the development of molecular tools at the end of the last century, but other combinations, notably Anodonta woodiana, have been widely used until now.
Sinanodonta species, like most anodontines, are particularly difficult to identify based on morphological characters, which has led to extensive misidentifications of species within this group (Guarneri et al., 2014; Lopes-Lima et al., 2020). Based on the combined evidence of morphology and DNA sequence data, most commonly mitochondrial (mtDNA) data, several species have been assigned to Sinanodonta (Bolotov et al., 2016; Bespalaya et al., 2018; Burzyński & Soroka, 2018; Kondakov et al., 2018; Wu et al., 2018; Huang et al., 2019; Lopes-Lima et al., 2020; Sano et al., 2022). More recently, a revision of the evolutionary relationships of Sinanodonta species in Japan using genomic data showed the presence of mtDNA introgression in some species, supporting further splitting of the genus (Sano et al., 2022). Therefore, critical uncertainties remain regarding the species composition of this genus.
Phylogenetic reconstruction and species delineation
In this review, we constructed mtDNA phylogenetic trees using all 403 publicly available sequences from the most widely available gene fragment, i.e., the cytochrome c oxidase subunit I (COI) and included 150 new sequences (see Supplementary material S2 for specimens used; for materials and methods see Supplementary material S3). We then estimated the number of species-level molecular operational taxonomic units (MOTUs) using three distinct methods, i.e., the distance-based (1) BIN system implemented in BOLD (Ratnasingham & Hebert, 2013) and (2) Assemble Species by Automatic Partitioning (ASAP) (Puillandre et al., 2021), and the parsimony-based (3) haplotype network reconstructions in TCS 1.21 (Clement et al., 2000), with a 95% statistical parsimony connection limit.
Both (ML and BI) COI phylogenies for the genus Sinanodonta showed similar topologies, recovering eleven clades that were defined as MOTUs by a consensus of all species delineation methods, three of which are known to occur outside of their native range (Figs. 1, 2; Table 1).
Bayesian Inference (BI) and Maximum Likelihood (ML) phylogenetic tree inferred from the cytochrome c oxidase subunit I (COI) gene fragment and species delineation of Sinanodonta species. Support values above the branches are percent posterior probabilities/ultrafast bootstraps. Vertical bars correspond to molecular operational taxonomic units by various species delimitation methods: red—TCS (95%); green—ASAP; blue—BINS of BOLD; and black—consensus. Support values > 95% for both phylogenetic analyses are represented by an asterisk; support values within each recognized MOTU were erased for clarity. Species introduced outside the native range are in bold
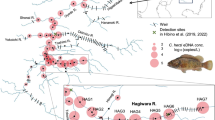
Global distribution of invasive lineages of the genus Sinanodonta in countries with sparse occurrence (points) and widespread occurrence (fill color). Arrows indicate estimated directions of spread, and purple and green colors indicate probable source areas of S. woodiana s. str. and S. lauta, respectively) (Data source: samples listed in Supplementary material S2; literature review; Global Biodiversity Information Facility, https://www.gbif.org/, last accessed: June 12, 2023). Colored boxes: Median-joining networks of the CO1 sequences of invasive Sinanodonta lineages (see Supplementary material S2 for samples included). Each haplotype is represented by a circle, the size of which is proportional to its overall frequency in the dataset (see legend showing 1–10 samples). Crossing lines on the branches indicate the number of mutational changes between the haplotypes. Haplotype colors correspond to the sampling regions annotated in the legends of haplotype networks. The occurrence of introgression in S. lauta based on Sano et al. (2022) is indicated
These COI phylogenies show a first split within the genus Sinanodonta of a clade comprising two species from the Yangtze River Basin in China, S. lucida (Heude, 1877) and S. angula (Tchang, Li & Liu, 1965), and a second split of a clade including the Far East Asian species S. tumens (Haas, 1910), S. calypigos (Kobelt, 1879), S. schrenkii (Lea, 1870) and a fourth species, as yet unnamed (here referred to as Sinanodonta sp. A (Japan)), which has only recently been recognized based on nuclear data since it has mitochondrial DNA introgression from S. calipygos (Sano et al., 2022). The remaining clade represents the S. woodiana species complex, which was consistently split into six MOTUs by all three methods applied. Of these, three MOTUs are already recognized as valid species, i.e., S. jourdyi (Morlet, 1886), S. elliptica (Heude, 1878), and S. lauta (which includes an additional unnamed species, here named Sinanodonta sp. B (Japan), based on nuclear data alone as in Sano et al. (2022)). The other three lineages are here named Sinanodonta woodiana s. str. (also called “temperate invasive”), S. pacifica (Heude, 1878) (also called “tropical invasive”), and Sinanodonta sp. (Yangtze). Two of these lineages (i.e., S. woodiana s. str. and S. pacifica) were introduced outside of their native range and are the main subject of this review.
Species validation of the lineage Sinanodonta sp. (Yangtze) needs further morphological, biogeographical, and molecular evidence, due to the low level of COI divergence with S. lauta and S. pacifica (uncorrected p-values of interspecific distances in Supplementary material S4). For S. woodiana s. str. and S. pacifica, the high level of mitochondrial divergence of these lineages supports their validation as distinct species.
This section highlights that mitochondrial DNA is very useful for species identification and consequently for clarifying invasion pathways, but genome-wide data are needed to define species boundaries more accurately and better estimate evolutionary relationships within this genus. A detailed study, including molecular and morphological data, of specimens from regions of China outside the Yangtze basin is essential to fully understand the taxonomy of the species within the Sinanodonta woodiana complex and to clarify the major invasion pathways of its lineages.
Taxonomy of S. woodiana
A large variety of shell forms attributed to S. woodiana (Lea, 1834) have been described as separate species, resulting in 59 synonyms recognized by Haas (1969), and another 14 taxa being added based on the work of the Russian Comparatory method (Graf, 2007). Sinanodonta woodiana has been treated as widespread across Asia (Brandt, 1974; He & Zhuang, 2013; Zieritz et al., 2018a). However, as shown above, S. woodiana represents a cryptic species complex, from which three of the clades have been introduced into non-native regions.
Here, we largely follow the traditional concept of S. woodiana s. str. and consider it to be the species that was widely introduced into Europe and designated as the “temperate invasive” lineage (Bolotov et al., 2016). The nominal species S. woodiana has a vague type locality in China (Lea, 1837). One of the two syntypes was labeled "China", while the second syntype was said to have been sent from Canton (now Guangzhou) to Philadelphia. However, the collection site of the second syntype is also unclear. A growing body of phylogeographic research indicates that native populations of S. woodiana s. str. are found in the Yangtze basin (Kondakov et al., 2018; Konečný et al., 2018).
Based on available DNA sequence data, representatives of the “tropical invasive” lineage of S. woodiana were recorded from the southern part of the Yangtze basin (Jiangxi) and Taiwan. Here, we tentatively link the “tropical invasive” lineage to the nominal species Sinanodonta pacifica (Heude, 1878) (species status revived from synonymy). The type locality (TL) of this species is situated in Anhui Province (La rivière de Ning-kouo-fou, Ngan-houé [China: a river at Ningguo, approx. 30.6267°N, 118.9861°E, Anhui Province]), which borders Jiangxi. Although the exhaustive synonymy of the “tropical invasive" lineage will be published elsewhere, our first reviser’s action on the precedence of simultaneous synonyms based on morphological similarity and close proximity of the type localities is as follows: Sinanodonta pacifica over Anodon friniana Heude, 1878 syn. nov. (TL: Lac de Ho-kieou-hien, Ngan-houé); A. fusca Heude, 1878 syn. nov. (TL: Le lac Pai, au nord de la sous-préfecture, Ngan-houé); A. joreti Heude, 1878 syn. nov. (TL: Les fosses a rizieres des vallees du Kien-te sud, Ngan-houé); and A. striata Heude, 1878 syn. nov. (TL: Lac de Tong-lieou, Ngan-houé). There is no available taxonomic name for a population of this species from Taiwan.
Native range, introduction events, and current distribution
Sinanodonta woodiana (Lea, 1834) s. str. [= “temperate invasive” lineage]
There is some uncertainty about the native range of S. woodiana s. str., but the species is generally assumed to be native to the Yangtze River basin and possibly other river basins in southern China (Kondakov et al., 2018; Konečný et al., 2018). Earlier records from the Amur River basin in the Russian Far East were not confirmed using a DNA-based approach (Bolotov et al., 2020). A native origin of this invasive species in at least the Yangtze Basin is supported by the haplotype network analysis, revealing by far the highest genetic diversity within this river basin, while no COI sequence data are currently available for other river basins in China and North Korea (Fig. 2).
The species has been introduced and widely dispersed across Europe, where it now ranges from Spain in the west to Romania, Ukraine and Russia in the east, and from Italy and Greece in the south to southern Sweden in the north (Fig. 2). The introduced range in Asia includes Kazakhstan, Uzbekistan, Western and Eastern Siberia in Russia, South Korea and Myanmar, where this species is known from the Irrawaddy and Salween basins (Kondakov et al., 2020a, b; Bolotov et al., 2022). Interestingly, the vast majority of these non-native populations—with the sole exception of populations from Uzbekistan and South Korea—appear to belong to a single haplotype (Fig. 2).
In Europe, the first records of S. woodiana s. str. came from fish hatcheries in 1959–1965 from Hungary and Romania in connection with imports of East Asian fish species from the Yangtze and Amur basins (summarized in Watters, 1997). Between 1979–1982, S. woodiana s. str. started to appear in the water systems in Romania (Sárkány-Kiss et al., 2000), Hungary (Benkő-Kiss et al., 2013), and France (Prié, 2023), followed by records in other European countries (summarized in Watters, 1997; Konečný et al., 2018; Kondakov et al., 2020a). Considering the results of the haplotype network analysis, only the introductions from the Yangtze basin are likely to be the primary source of S. woodiana s. str. populations established in Europe. An alternative scenario of the origin of current S. woodiana s. str. populations in Europe, i.e., via imports of fish to Hungarian hatcheries in the 1960s that most likely involved populations from the Amur River basin (Watters, 1997), is not supported due to the lack of shared COI haplotypes between European and Amur basin populations (Kondakov et al., 2018; Konečný et al., 2018; this study). Using microsatellite marker data, Konečný et al. (2018) further supported the scenario of a single introduction event (or single native range source population) of S. woodiana s. str. to Europe, and early establishment of two distant populations, i.e., in the Romanian/Hungarian region and the southeastern France.
Documented introduction events of S. woodiana s. str. to temperate Asia appear to have also been via host fish. For example, the species was introduced to Uzbekistan with silver carp Hypophthalmichthys molitrix (Vallenciennes, 1844) and grass carp Ctenopharyngodon idella (Valenciennes, 1844) from China between 1960 and 1965 (Izzatullaev & Boymurodov, 2016). This pathway is also in accordance with the results of the haplotype analysis, as populations in central and western Russia and Kazakhstan share a haplotype with the Yangtze River basin, which is in turn closely related to the haplotype present only in Uzbekistan (Fig. 2).
Sinanodonta has also recently been recorded in Africa (Morocco, Algeria), where several established populations have been found independently (Mabrouki & Fouzi Taybi, 2022; Bensaâd-Bendjedid et al., 2023). Due to the proximity of the localities to the European continent and the presumed introduction route via transport of fish hosts from Hungary (Bensaâd-Bendjedid et al., 2023), it can be hypothesized that it is also S. woodiana s. str., but verification by genetic methods is necessary.
Sinanodonta pacifica (Heude, 1878) [= “tropical invasive” lineage]
The native distribution of S. pacifica is still unresolved, mainly due to a lack of adequate DNA sequence data from the species’ presumed native region in eastern China and Taiwan (Fig. 2). The COI sequence data of this lineage from China (southern part of the Yangtze basin) are restricted to seven specimens from two sites in Jiangxi Province and a single haplotype, which is genetically distant from all other haplotypes and not shared with any of the invasive populations (Fig. 2). Data from Taiwan are also restricted to a single haplotype, which is, however, shared by non-native populations in Japan, Peninsular Malaysia, Singapore, Java, the Philippines and Costa Rica (Fig. 2). Considerably more DNA sequence data will be required from the Yangtze basin and other Chinese and Taiwanese river basins to resolve the native range of this lineage.
The species has spread widely across parts of Southeast and East Asia, where it is now known from Myanmar, Indonesia, Malaysia, Singapore, the Philippines, and Japan, as well as the USA and Costa Rica (Fig. 2). In Indonesia, the species is commonly referred to as “Kijing Taiwan” (= Taiwan mussel), referring to its introduction to the country, specifically in 1970 to a pond in Java, via fish from Taiwan (Djajasasmita, 1982). The species has now been recorded from all major Indonesian islands, including Java, Sumatra, Lombok, and Sulawesi. In parts of Malaysia, especially Peninsular Malaysia (Zieritz et al., 2016) and Sabah, Borneo (Zieritz et al., 2018b), S. pacifica is now arguably the most common unionid species. In the Philippines, the species has been recorded from the islands of Leyte, Mindanao, and Mindoro (Demayo et al., 2012; Fornillos et al., 2021). In 2022, S. pacifica was first recorded from Myanmar at two sites on the Shan Plateau (Irrawaddy basin) close to the Chinese border (Bolotov et al., 2022). The species is also known from Honshu Island, Japan, although it is currently unclear whether it is native here or represents an invasion (Lopes-Lima et al., 2020).
Sinanodonta pacifica was also introduced to North and Central America, specifically to Costa Rica by 1994, probably via fish from Taiwan (Watters, 1997; Bauer et al., 2021); the New Jersey Conservation Foundation's fishponds in the USA, presumably via the introduction of bighead carp, common carp and/or grass carp (Bogan et al., 2011); and the Dominican Republic since the 1980s (Gomez et al., 1986) (but note that no sequences are available from Dominican Republic specimens; thus, assignment to this lineage is preliminary). However, records in North and Central America are sparse, indicating that the species currently maintains a localized and restricted distribution on this continent.
Finally, the record in Iraq is also considered to belong to the “tropical invasive” lineage (Abdul Razak & Zwair, 2021; Bogan et al., 2021), but further data are needed to clarify its origin there, similarly to African localities.
Sinanodonta lauta (Martens, 1877)
Sinanodonta lauta is native to Japan (Kyushu, Honshu, and Hokkaido islands), the Korean Peninsula, and the Prymorye region in eastern Russia (Lopes-Lima et al., 2020). As expected, considerable genetic diversity is present in this species across each of these regions, with several haplotypes being unique to specific islands (e.g., Honshu, Hokkaido) and countries (e.g., South Korea, Russia) (Fig. 2).
The species was introduced to central Asia, where it was first recorded in 2018 in a thermally polluted river channel of the Yenissei River (Bespalaya et al., 2018), and the same haplotype is now additionally known from a site in the Ob River basin, the Ili River basin, a tributary of Balkhash Lake, Kazakhstan (Kondakov et al., 2020a, b), and various reservoirs and tributaries of the Volga River (all new sites; Fig. 2, Supplementary material S2). In 2018, S. lauta was recorded for the first time from Borneo, i.e., in a pond in Lawas District, Limbang Division, Malaysia, sharing the same haplotype as a native population from Honshu Island, Japan (Fig. 2). These specimens had been bought from a market in Lawas and were intentionally introduced to the pond by the owner (Zieritz et al., 2020).
More recently, S. lauta has also been recorded in southwestern Iran in a fish farm and the nearby Dez River (Alwanzadegan et al., 2023). Analysis of CO1 haplotypes (not yet included in our analysis) and data from farmers in this case also suggests that it is probably the result of imports of East Asian fish.
Life cycle
Available information on the life history of invasive Sinanodonta is based primarily on S. woodiana s. str. in the European region, so future research will be necessary to assess variations across species, lineages and climatic regions. Sinanodonta woodiana s. str. is mainly gonochoristic, but studies have found up to 2.3% hermaphroditic individuals in some populations (Dudgeon & Morton, 1983; Hliwa et al., 2015; Labecka & Domagala, 2018). In Europe, the species was observed to reach sexual maturity already in the second growing season (in the summer of the following year after detachment from the fish), when the shell length is at least 3 cm (Dudgeon & Morton, 1983; Labecka & Domagala, 2018; Douda et al., 2021; Fig. 3). The maximal life span is approximately 10 or 12 years (Dudgeon & Morton, 1983; Afanasyev et al., 2001).
The seasonal cycle of gametogenesis in invasive Sinanodonta has been studied primarily in S. woodiana s. str. in thermally polluted waters. Labecka & Domagala (2018) observed that partial spawning of S. woodiana s. str. occurs each month, and females have multiple ovulations during the whole year. Similar results, with a season of higher spawning activity in spring, were also found in the heated Konin lakes (Hliwa et al., 2015). This continuous reproduction could give Sinanodonta a considerable advantage in competition with native species, although reproductive rates in these cold-blooded animals are likely to be lower in water bodies that do not experience artificial heating. That said, S. woodiana s. str. has considerable cold tolerance, and was observed to have higher recruitment success and faster reproduction than native unionid species in a pond in Poland with a natural temperature regime (Urbańska et al., 2021).
There are two pathways of spermatogenesis in Sinanodonta males, and Labecka & Domagala (2019) described typical and atypical spermatozoa. The typical pathway is located close to the lumen in the seminiferous epithelium. The atypical pathway occurs less frequently, and spermatozoa are released from multinucleated cysts and have shorter heads than typical ones. The production of spermatozoa is continuous throughout the year (Hliwa et al., 2015; Labecka & Domagala, 2019). During brooding, males release sperm into the water, which are subsequently inhaled by females, where eggs can be fertilized in the suprabranchial cavity (Dudgeon & Morton, 1983). After fertilization, eggs are transferred into marsupia (the outer gill arches) in which eggs are kept until maturation into parasitic larvae called glochidia.
Sinanodonta woodiana s. str. is producing glochidia multiple times per year, and one individual female can produce several hundred thousand glochidia every year (Douda et al., 2012; Labecka & Domagala, 2018). The main period of glochidia release in European waters with a natural temperature regime is during the summer months (Sárkány-Kiss et al., 2000; Douda et al., 2012). In warmer waters, glochidia production can be year-round (Labecka & Domagala, 2018), which is more consistent with the situation in the native East Asian range (Dudgeon & Morton, 1984).
Glochidia of Sinanodonta species are relatively large (390–400 µm), obligatory parasites of fish and attach primarily to fins or gills, but can also be found encapsulated in the mouth, operculum, or nostrils (Dudgeon & Morton, 1984; Douda et al., 2012; Šlapanský et al., 2016; Labecka & Domagala, 2018). After attachment to the host, glochidia are covered by epithelial cells and remain inside the capsule for several days to weeks. The mean parasitic period can be as short as 6 days and is prolonged with decreasing temperature (Dudgeon & Morton 1984; Wen et al., 2006; Douda et al., 2012, 2017b). Metamorphosis into juvenile mussels starts with the rupture of the capsule when the juvenile individual falls off the fish host and starts to feed by pedal feeding (using the foot) and filtration of water within the sediment.
Ecology
Habitat requirements
The invasive Sinanodonta species have relatively high ecological plasticity and tolerance to different environmental conditions (Fig. 4) and can thus be found in lentic (standing water) habitats (lakes, oxbows, ponds) as well as in lotic (slow-flowing) habitats, such as streams, canals, and large rivers (Kraszewski, 2007; Spyra et al., 2012; Konečný et al., 2018; Zieritz et al., 2021; Bolotov et al., 2022; Dobler et al., 2022; Sahidin et al., 2021, 2022), similar to the native range (Liu et al., 2022). In Europe, Sinanodonta occurs in large rivers draining to all major seas, such as the Danube (Black Sea), Oder (Baltic Sea), Elbe (North Sea), Rhône (Mediterranean Sea), and Loire (Atlantic Sea) (e.g., Paunovic et al., 2006; Szlauer-Łukaszewska et al., 2017; Prié, 2023). It also occurs in European natural lakes, such as Lake Balaton (Hungary), Lake Maggiore, and Garda (Italy); dam reservoirs, oxbows; and artificial fish farming reservoirs, such as in the Třeboň Pond System (Czech Republic), Bavaria (Germany), and the Wojnowice Pond System (Poland) (Cappelletti et al., 2009; Benkő-Kiss et al., 2013; Kamburska et al., 2013; Konečný et al., 2018; Urbańska & Andrzejewski, 2019; Dobler et al., 2022). The occurrence in other parts of the invasive range is even more diverse as document examples from fishponds (Myanmar, Java), abandoned mining pools (Malaysia), rice paddy channels (Malaysia), rivers (Iraq, West Java, Malaysia), and (reservoir) lakes (Myanmar, West Java) (Zieritz et al., 2021; Bolotov et al., 2022; Sahidin et al., 2022). The unifying element of the physical habitat of Sinanodonta species seems to be the presence of fine substrate (silt, fine sand, and organic material), and in river systems, their occurrence is often concentrated in areas of slow-moving sections where sedimentation occurs (Popa & Murairu, 2009; Lajtner & Crnčan, 2011; Szlauer-Łukaszewska et al., 2017). However, note that in the analysis of Poznańska-Kakareko et al. (2021), the substrate requirements of S. woodiana s. str. were found to be less constrained than those of native unionid species, as the species was frequently recorded in coarse gravel substrate, which other species avoided. The available studies further agree that S. woodiana s. str. avoids zones with dense aquatic vegetation (Kraszewski & Zdanowski, 2007; Urbańska et al., 2019a), although more detailed physical habitat studies are needed, especially for S. pacifica and S. lauta.
Example localities of Sinanodonta species in non-native ranges: a S. pacifica—rice paddy channel, Perlis, Peninsular Malaysia (credit: A. Zieritz); b S. pacifica aquaculture lake, Sabah, Borneo (credit: A. Zieritz); c S. lauta—warm channel to the artificially heated Zainsk Reservoir, Russia (credit: I. V. Vikhrev); d S. woodiana s. str.—oxbow lake, Morava River, Czech Republic (credit: K. Douda)
Temperature range
Thermal optima and thresholds of the invasive Sinanodonta are not well described, although they are considered key factors influencing the invasion process. In addition, temperature tolerance may vary among species and over time within individual lineages. For example, during its initial invasion stage in Europe in the 1970s, S. woodiana s. str. occurred mostly in warmer areas and artificially warmed waters (as recipients of water from cooling systems), whilst in the 1990s, the species started to spread into waters with natural temperature regimes (Kraszewski, 2007). It has therefore been suggested that over time, the European populations of S. woodiana s. str. have adapted to colder waters (Konečný et al., 2018; Urbańska et al., 2019a). In fact, possible ongoing expansion of physiological thermal limits is indicated by relatively recent records in some subalpine lakes (Lake Garda, Italy) (Cappelletti et al., 2009) and Scandinavia (von Proschwitz, 2008). Similar to observation in Europe, the invasion of Sinanodonta species in Russia was first detected in artificially heated habitats, including S. woodiana s. str. and S. lauta in the Yenisei River, Eastern Siberia (Bespalaya et al., 2018), and the Ob River basin, where the species’ range now extends to 54–56 degrees north latitude (Kondakov et al., 2020a).
The extent to which global climate change is contributing to Sinanodonta’s spread to formerly colder regions is unknown, but changing climate and heat pollution may certainly be an advantage for Sinanodonta in many habitats. Recently, Sinanodonta larvae have been confirmed to have significantly higher thermal tolerance than native Unio crassus Philipsson, 1788 in Europe, which indicates that the species can have a competitive temperature-driven advantage at multiple life stages (Bielen et al., 2016; Benedict & Geist, 2021).
Water quality requirements and tolerance to stressors
Sinanodonta prefers nutrient-rich waters with high levels of suspended organic food particles (Kraszewski & Zdanowski, 2007; Zieritz et al., 2018a), which possibly limits the spatial extent of its invasion and restricts its occurrence along a river or trophic gradient. Commonly, Sinanodonta species appear to be linked to eutrophicated sites. In a study in northern Borneo, the occurrence of S. pacifica was positively associated with higher concentrations of chlorophyll-a (range 1.2–135.9 µg/L), total dissolved solids (25.7–139.8 mg/L), and NH4-N (0.8–1020 µg/L) compared to that of native unionid species (Zieritz et al., 2018b). The high level of ecological plasticity of S. pacifica is also confirmed by its occurrence along a wide altitudinal gradient in Java, with chlorophyll-a concentrations ranging from 3.4 µg/L (in the highlands) to 10.6 µg/L (in the lowlands) (Sahidin et al., 2022).
S. woodiana s. str. in Europe exhibits a similarly high tolerance to eutrophication. For example, the species was documented in fishponds with up to 160–290 mg/L total dissolved solids, 0.92–2.29 mg NH4/L, 10.6–17.3 mg NO3/L, and 0.07–0.6 mg PO4/L (Spyra et al., 2012). The species is also frequently found at localities with cyanobacterial blooms (Andrzejewski et al., 2013). Survival and successful reproduction in such eutrophicated water bodies with fluctuating water quality appears to be facilitated, amongst other characteristics, by the strategy of rapid growth and early maturation exhibited by Sinanodonta.
In addition to the tolerance to eutrophication, invasive Sinanodonta species can exist in a wide variety of impacted environmental conditions, as indicated by a range of studies focused on a variety of contaminants (Corsi et al., 2007; Bielen et al., 2016; Giari et al., 2017; Jing et al., 2019). For example, S. woodiana s. str. exhibited a much higher tolerance to physiological stress posed by exposure to temperature extremes and zinc pollution compared to the native species Anodonta anatina (Linnaeus, 1758), as measured by biological response proxies, including the multixenobiotic resistance mechanism, respiration estimates, and enzymatic biomarkers (Bielen et al., 2016). Earlier research conducted by Corsi et al. (2007) proposed that increased activity of certain enzymes, specifically cholinesterase, may be linked to this greater tolerance to environmental stress in Sinanodonta. Considering the widespread presence of anthropogenic impacts in fresh waters, tolerance toward stressors is one of the aspects that can drive the invasion success of Sinanodonta.
Host fish relationships
Sinanodonta woodiana s. str. is a broad host generalist and was confirmed to efficiently use all fish species from European waters tested thus far during experimental infestations (Douda et al., 2012; Huber & Geist, 2019), and its glochidia were also observed on a variety of fish species after natural infestation (Šlapanský et al., 2016; Douda et al., 2017a). It can be assumed that their host range is similarly generalistic in other areas of the invasion range, although experimental host data are lacking there. In the native range, S. woodiana s. str. development seems to be more dependent on immunologically compatible hosts, and a substantial portion of glochidia do not complete development because of the immune reaction of the host (Douda et al., 2017a; Chen et al., 2022). Sympatric species of bitterlings (fish species which live in close contact with mussels and lay their eggs into the mantle cavity of live mussels) are typically resistant to glochidia of local mussel species, including Sinanodonta, and can thus help reveal the mechanisms of evolution of resistance to invasive mussels (Reichard et al., 2006; 2012; Douda et al., 2017a). Bitterling immune response to S. woodiana s. str. was documented to be population-specific (Reichard et al., 2015) and hypothesized to be related to the structure of the major histocompatibility complex genes (MHC class II, involved in the immune response of vertebrates to extracellular pathogens). However, there is only a slight relationship between the major allelic lineages (and the functional diversity) of the MHC complex and the gradient of sites with varying stages of S. woodiana invasion in Central Europe (Lorencová et al., 2015; Talarico et al., 2022), which is also associated with the high temporal variation in host response. Understanding the mechanisms influencing the success of glochidia of invasive Sinanodonta and its evolution will thus necessitate additional research.
Even less information is available on the host requirements of invasive Sinanodonta outside Europe. Data from introductions suggest that at least S. pacifica has a wide fish host range as well, including tilapia and several carp species (e.g., Djajasasmita, 1982; Watters, 1997; Hamidah, 2006; Chen et al., 2022). Nevertheless, it is still not clear which fish hosts Sinanodonta species can use in South and Southeast Asia, Africa, or Central America, and whether these include imported fish from other areas, co-invasive fish species, and/or local fish fauna.
More data on the natural prevalence and intensity of infestation on fish by Sinanodonta glochidia can indicate potential hosts, and follow-up host compatibility tests can determine metamorphosis success rates. Such testing can currently be performed even without robust local laboratory infrastructure by monitoring metamorphosis success in simple and low-cost floating pontoon devices (Douda et al., 2020), which can also minimize the risks associated with transporting invasive species. Knowledge of host use in different locations in the invasive range can be essential for our understanding of the dispersal pathway and host-parasite dynamics.
Symbionts, parasites, and predators
In addition to fish hosts, other closely interacting species, such as symbionts, parasites, and predators, may strongly affect Sinanodonta invasion dynamics. Symbionts and parasites, for example, can influence the fitness of invading species (Brian & Aldridge, 2019; Taskinen et al., 2021; Creed et al., 2022), with the size of the effect depending on a number of other conditions (e.g., environmental conditions, species similarity, duration of invasion) and potentially changing over time.
Research focused on S. woodiana s. str. has shown that symbiotic and parasitic relationships with native taxa (e.g., ciliates, trematodes, annelids, insects, acarids) are already occurring in the species’ invaded range (Yuryshynets & Krasutska, 2009; Cichy et al., 2016; Yermoshyna & Pavliuchenko, 2021; Supplementary material S5). However, evidence to date suggests that compared with native unionid species, parasite/symbiont species richness and prevalence tends to be lower in S. woodiana s. str. compared to native Unionidae. An example for this is provided by endoparasitic bucephalid trematodes (Digenea: Bucephalidae), which cause partial or complete castration, changes in shell shape, and increased mortality in their unionid hosts (Taskinen et al., 1997; Zieritz & Aldridge, 2011; Muller et al., 2015). In a study by Cichy et al. (2016), Rhipidocotyle fennica Gibson, Taskinen & Valtonen, 1992 was the only bucephalid parasite in Polish populations of S. woodiana s. str., where it occurred with a lower prevalence (maximum 3.4%) than in native species from the same ecosystem. Similarly, in thermally polluted waters, infection prevalence across three native unionid species [Anodonta anatina, Unio tumidus Philipsson, 1788, and U. pictorum (Linnaeus, 1758)] was 13–20% and thus much higher than in S. woodiana s. str., with 3% prevalence (Taskinen et al., 2021). That said, compared to other, non-unionid invasive freshwater bivalve species, such as Corbicula fluminea and Dreissena polymorpha, species richness and prevalence of endosymbiont infestation appear to be higher in S. woodiana s. str., potentially due to the closer evolutionary proximity of Sinanodonta to native unionid species and original host organisms (Taskinen et al., 2021).
Another relationship that can reduce the viability of invasive Sinanodonta populations is predation. A typical species for which S. woodiana s. str. is reported to be attractive prey is the otter Lutra lutra (Linnaeus, 1758) (Tajer, 2020). Other species that have been reported to feed on S. woodiana s. str. also include the oystercatcher Haematopus ostralegus Linnaeus, 1758 during autumn migration, the white-tailed eagle Haliaeetus albicilla (Linnaeus, 1758), the wild boar Sus scrofa Linnaeus, 1758, and the fox Vulpes vulpes (Linnaeus, 1758) (Urbańska et al., 2013). However, these behaviors are local and related to the occurrence of S. woodiana s. str. in fishponds that are regularly drained (Urbańska et al., 2013). In the delta of the Ili River in Kazakhstan, the introduced muskrat Ondatra zibethicus (Linnaeus, 1766) was observed to actively feed on Sinanodonta species (Shiryaev, 1976). Dobler & Geist (2022) showed in laboratory experiments that the native European noble crayfish Astacus astacus (Linnaeus, 1758) and the invasive signal crayfish Pacifastacus leniusculus (Dana, 1852) may also prey on S. woodiana s. str. and suggest that the impact of predation will likely increase with the ongoing spread of P. leniusculus replacing A. astacus populations in Europe.
In summary, there is increasing evidence of the suitability of Sinanodonta for various freshwater symbionts, parasites, and predators in the invaded range. Inevitably, some of these interactions may impair Sinanodonta populations, but the extent is to be determined.
Community ecology
Invasive Sinanodonta can be considered fast-growing and fast-reproducing r-strategists. Their habitat requirements and ranges often overlap with native mussel species. In Europe, S. woodiana s. str. has been found to live in sympatry with most native unionid species, including Unio pictorum, U. crassus, U. tumidus, U. elongatulus Pfeiffer, 1825, U. mancus Lamarck, 1819, Pseudanodonta complanata (Rossmässler, 1835), Anodonta anatina, Anodonta cygnea (Linnaeus, 1758), Anodonta exulcerata Porro, 1838, and Potomida littoralis (Cuvier, 1798) (Froufe et al., 2017; Szlauer-Łukaszewska et al., 2017; Urbańska et al., 2019a; Kondakov et al., 2020a; Taskinen et al., 2021). Many of these species are endangered (IUCN Red list database) and protected, and the invasive spread of Sinanodonta may potentially represent an additional threat to these species. However, to date, no robust long-term data sets are available to allow for a quantification of how the population dynamics of native unionids are affected by and after the arrival of Sinanodonta. Short-term data are available on their effect on relative species abundances of native bivalve communities, but these are contradictory. Thus, at some sites in Europe, S. woodiana s. str. appears to have almost completely displaced native species (Fabbri & Landi, 1999; Niero, 2003; Benkő-Kiss et al., 2013) or has become the dominant species (e.g., Kyjovka River—Czech Republic—Douda et al., 2012; Pond Bolek—Poland—Urbańska et al., 2019a, 2021; Lower Volga—Russia—Kondakov et al., 2020a). At other sites, the species remains at low relative abundances for many years (Lužnice River, Czech Republic, Douda et al., 2021) and/or co-occurs with other species (Szlauer-Łukaszewska et al., 2017). In addition, considerable temporal fluctuations in population sizes can be observed, as Sinanodonta is prone to mass mortalities similar to other bivalve species (Bódis et al., 2014a). Interestingly, novel eDNA methods indicate that S. woodiana s. str. had the highest number of significant negative co-occurrences with other bivalve species (especially sphaeriids) in French freshwaters, which the authors hypothesize may reflect the negative impacts of this invasive species on native bivalve communities (Prié et al., 2023).
Major pathways and main vectors of introduction
Natural dispersal
The main mode of natural dispersal of Sinanodonta and other Unionidae is via fish hosts during the parasitic larval stage (Watters, 1997). During this period, glochidia can be transported up to tens of kilometers regardless of the direction of water flow depending on the migratory movement of the fish. This mode of dispersal is therefore very efficient within individual catchments and in migratory connected river systems (including connections by artificial canals—see Prié, 2023). In contrast, the actual locomotor activity of adults using their muscular feet can be considered negligible in terms of invasive spread. In general, for adult unionid mussels, the speed of relocation movement and their actual relocation distance are not high (tens of meters at most) (Kappes & Haase, 2012).
Other modes of natural dispersal, such as endo- or ecto-zoochory, in addition to the glochidia encapsulated on fish, have not yet been described in the literature but cannot be ruled out. However, given the fragile shells and low persistence outside water, modes such as juvenile transmission in the digestive tract of fish or on the fur/feathers of mammals/birds can likely be considered even less common in comparison with some other freshwater molluscs (Coughlan et al., 2017).
Human-driven dispersal
Humans spread Sinanodonta both intentionally and unintentionally at different life stages and various distances. It is believed that the primary mode of Sinanodonta introduction to novel regions relates to the transfer of fish species parasitized accidentally by glochidia (Watters 1997; Kondakov et al., 2018; Konečný et al., 2018; Zieritz et al., 2018a). Other pathways of unintentional spread by humans are apparently not as common in Sinanodonta as in some other invasive freshwater bivalves. Unlike Dreissena, they do not have the ability to attach to mobile technical infrastructure by byssal threads, nor do they have mobile-free planktonic larvae, such as Dreissena and Corbicula (allowing, e.g., transfers by ballast water) (Karatayev & Burlakova, 2022; Modesto et al., 2023).
Importantly, Sinanodonta is often introduced by humans intentionally. In the native range, Sinanodonta has significant economic value as a natural source of pearl production, an important protein source for animals and humans, and other uses (Table 2). For the majority of such purposes, it is important that mussels can be taken from local sources (Putro et al., 2010), and their ability to survive or even reproduce in anthropogenic and heavily human-influenced habitats, including small artificial earthen fishponds, makes available habitats widespread and the introductions for this purpose easy. For example, in Tuscany (Italy), S. woodiana s. str. was reported to have been intentionally introduced to produce artificial pearls (Berni et al., 2004). Deliberate introduction for food but also for ornamental purposes may be major vectors in Indonesia and Malaysia (Djajasasmita, 1982; Zieritz et al., 2018a) and common in Europe. For example, Sinanodonta is one of the freshwater mussel genera sold at garden centers and pet shops across the globe, including Germany (Huber & Geist, 2019), Singapore (Ng et al., 2016), the Czech Republic (Patoka et al., 2017), and even countries where the species has not yet been detected in the wild (e.g., Portugal; Lopes-Lima, personal observation).
Ecological impacts in the non-native range
The global spread of Sinanodonta is associated with a range of confirmed or potential negative impacts (Table 2). Here, we briefly summarize the key ecological impact mechanisms.
Larval parasitism
The parasitic stage of Sinanodonta generates unique impact mechanisms that have only recently been described. High population densities with massive production of glochidia (Wächtler et al., 2001; Zieritz et al., 2016; Labecka & Czarnoleski, 2021) may make these mechanisms particularly relevant. Sinanodonta woodiana s. str. glochidia infestation has been shown to alter biochemical blood plasma parameters in host fish, indicating that infestation can disturb the homeostasis of the host similarly to other gill parasites (Yin et al., 2014; Douda et al., 2017b). Importantly, these changes were reflected by an increase in energy consumption in fish parasitized by glochidia (Slavík et al., 2017), altered respiration rates (Methling et al., 2019), and increased stress hormone cortisol (Reichard et al., 2023), ultimately leading to a decreased condition factor and growth of the parasitized host fish (Douda et al., 2017b).
Arguably of greater conservation concern may be that Sinanodonta glochidia can alter the relationships between (endangered) native mussel species and their fish hosts, specifically by inducing host cross-resistance to glochidia. Competition among sympatric unionid species over their hosts has been a known phenomenon for some time (e.g., Bauer et al., 1991; Dodd et al., 2005). Fish hosts can develop-specific adaptive immunity reactions to glochidia after infestation, which can result in cross-resistance to subsequent infestations by other species, ultimately reducing success rate of glochidia metamorphosis on the hosts (O’Connell & Neves, 1999; Dodd et al., 2005). Sinanodonta appears to induce a particularly strong cross-resistance response. Thus, in an experimental study by Donrovich et al. (2017), metamorphosis success rate of native A. anatina glochidia declined by 42.1% after a single S. woodiana s. str. priming infestation. This mechanism can be particularly dangerous for an ecologically similar species, such as A. cygnea, with a very similar habitat preferences and shared fish hosts (Wojton et al., 2012; Huber & Geist, 2017; Geist et al., 2023), as well as for all the other sympatric species.
Water filtration, biodeposition, excretion
Water filtration and subsequent deposition or transformation of the cleared matter is considered the key mechanism of how non-native freshwater bivalves affect invaded ecosystems and species, as shown in the extensive literature on the well-studied invasive freshwater bivalves within the genera Dreissena, Corbicula, and Limnoperna (Boltovskoy et al., 2022b; Karatayev & Burlakova, 2022; Modesto et al., 2023). To what extent these impacts also apply to invasive Sinanodonta is not well documented, and available data on Sinanodonta clearance, deposition and excretion rates in its invaded range are restricted to a few isolated studies in Europe and Malaysia. Adult individuals of S. woodiana s. str. in Czechia were observed in laboratory to filter 26.9 to 85.7 L of water per day (Douda & Čadková, 2018). Considering that at some localities, the absolute biomass of this species can reach up to 27 kg/m2 (Kraszewski & Zdanowski, 2007), in situ filtration rates can be up to several thousands of liters per square meter per day. In artificial tropical freshwater habitats in Malaysia, S. pacifica activity led to a steep increase in biodeposition of suspended material and altered N:P ratios in the water (Zieritz et al., 2019, 2021). In northern Italy, S. woodiana s. str. was observed to alter physico-chemical conditions of the sediment, including benthic respiration and nutrient recycling rates (Benelli et al., 2017). More studies are needed for a more complete understanding of the clearance, biodeposition and excretion rates of Sinanodonta populations in different habitats and across its invaded range. Recently published biomass prediction equations can thereby aid to rapidly determine biologically active Sinanodonta biomass (Coughlan et al., 2021: Appendix S1).
In addition to ecosystem-wide effects, competition for food is a key mechanism of how invasive freshwater bivalves negatively impact native species (Sousa et al., 2014). Sinanodonta woodiana s. str. has been shown to reduce seston loads to levels comparable to those by the native U. tumidus, indicating potential for food competition with native species (Douda & Čadková, 2018). More data are, however, needed, particularly when considering the complexity of biofiltration interactions, such as the involvement of selective feeding identified in A. cygnea (Lopes-Lima et al., 2014) and Sinanodonta (Wu et al., 2005). As the filtration activity of freshwater mussels is not only species-specific but also dependent on environmental factors, such as ambient temperature, and abundance and species composition of the bivalve community (Vaughn, 2010).
The filtering interaction between mussel species is not limited to food competition. Another important factor of filtering activity is the possibility that Sinanodonta can also filter out sperm or even larvae (depending on the size of the species) of native species (Sousa, 2014; Urbańska et al., 2019a), but there are no data on these potential effects in Sinanodonta currently available.
Massive die-offs
Massive die-offs (large-scale sudden mortality) of freshwater bivalves can be caused, for example, by poisoning (Fleming et al., 1995; Ćmiel et al., 2019) and extreme weather events, such as temperature extremes or long-term drought (Sousa et al., 2012; White et al., 2015), although triggers are often not or insufficiently understood (McDowell & Sousa 2019). Whilst massive die-offs are common in non-native Sinanodonta s. str. populations (Bódis et al., 2014a), information about the consequences these have on ecosystems and biodiversity is scarce. However, data from other invasive freshwater bivalve species highlight the risk of a range of negative impacts. These include toxic levels of ammonia nitrogen (Cherry et al., 2005), oxygen deficiencies, and the spread of pathogens, with their potential spread to other species and ecosystem components (summarized in McDowell & Sousa, 2019). Massive freshwater bivalve die-offs can also act as a resource pulse for both the aquatic and adjacent terrestrial ecosystem (Sousa et al., 2012; Bódis et al., 2014a).
Physical habitat modifications
Sinanodonta can also play a role in benthic habitat modification. Living individuals have been shown to affect sediment stability and bioturbation (Monaco et al., 2016), but gradually accumulating dead shells are likely to have similar impacts. Bódis et al. (2014c) showed that larger individuals of unionids such as S. woodiana s. str. attracted a denser and more diverse macroinvertebrate community than smaller shelled species (but see Ilarri et al., 2015). Additionally, the posterior part of their shell exposed to the water-column provides a suitable substrate for D. polymorpha, but due to the large size and deep burial behavior of Sinanodonta, D. polymorpha abundance in relation to the total shell area is relatively low (Bódis et al., 2014b; Urbańska et al., 2019b). It can be expected that with the growing abundance of Sinanodonta in invaded areas, other types of interactions will emerge.
Hybridization with native unionids
A rather overlooked and understudied risk of Sinanodonta invasions is the potential interbreeding with native Sinanodonta species and possibly other genera. As first documented in the work of Sano et al. (2022) and shown in Fig. 1, there have been several cases of hybridization between S. lauta, S. calipygos, and S. pacifica in historical or recent times, resulting in mtDNA introgression. This may pose significant risks and negative impacts in terms of erosion of genetic diversity, potential ecological impacts, and further increasing the unpredictability of future Sinanodonta invasions and spread.
Disease and parasite reservoir and transfer
Sinanodonta is known to host a range of parasites and symbionts in its invasive range, potentially including pathogens that present a risk to native bivalves. For example, the presence of inflammatory capsules and infiltrates linked to bacterial infection has been observed in Italian S. woodiana s. str. population (Carella et al., 2016). Whilst the origin of these pathogens is unknown, they may have been introduced from the species’ native range, which could lead to a ‘spillover’ event (i.e., introduction of parasites and/or pathogens from native range that subsequently exploit native species). Additionally, ‘spillback’ events, whereby parasites of native species exploit invading S. woodiana s. str., have already been confirmed and can potentially lead to increased opportunities of native species infections (Taskinen et al., 2021).
Economic impact and ecosystem services
Cost of Sinanodonta invasions
Data on the negative economic impacts of Sinanodonta invasions are surprisingly sparse and mostly based on indirect quantification of management costs (regulation, prevention) (InvaCost database, Diagne et al., 2020; Table 2). Economic quantification of direct impacts, such as the costs associated with biofouling of technical infrastructure by dreissenid bivalves (Diagne et al., 2020), is currently lacking for invasive Sinanodonta. Direct impacts of invasive Sinanodonta that should be quantified include the economic losses associated with reduced fish vitality due to intense glochidial parasitism, the increased costs for the conservation of endangered freshwater bivalve species in natural reserves negatively impacted by Sinanodonta, and the costs of reduced recreational functions of fresh water sites caused by Sinanodonta introductions (Benkő-Kiss, 2012; Bódis et al., 2014a). For example, repeated mass die-off events of Sinanodonta s. str. populations in touristic parts of Lake Balaton, Hungary, resulted in intensive visual and sensory pollution that lasted for weeks, thus severely disrupting the recreation season (Benkő-Kiss, 2012). Quantifying the economic losses associated with Sinanodonta invasions should be a research priority considering its importance for management decisions (prevention, regulation) and implementation of legislation.
Ecosystem services
In comparison to data on disservices and ecological risks, more data are available on ecosystem services of Sinanodonta (Table 2), which, are often based on experience from their native ranges. That said, these and other potential ecosystem services may become increasingly more relevant in the non-native ranges of Sinanodonta species when considering their continued global spread, combined with their characteristically rapid growth rates and large body and population sizes. In fact, collection and post-processing of individuals or shells to exploit potential ecosystem services may help their management in invaded ranges.
In some Asian countries, including in invaded ranges, Sinanodonta is eaten and provides a source of proteins, micronutrients, and amino acids (Yan et al., 2002; Chen et al., 2012; Saiful & Lumenta, 2014; Wijayanti et al., 2017; Zieritz et al., 2018a). The mussels are often harvested from the wild and directly consumed or otherwise used, but also commonly cultured (Rahayu & Rachman, 2015). More recently, projects have been developed in its invasive range to produce alternative food, such as protein bars enriched with Sinanodonta shell flour (Fatmi & Rustiani, 2018) and surimi-like material from Sinanodonta tissue (Stangierski et al., 2021). Liposome-encapsulated aqueous extracts, and calcium nano- and micro-particles have been extracted from Sinanodonta and studied for their potential use in medicine and cosmetics (e.g. Liu et al., 2008; Aminingsih et al., 2018). Sinanodonta also shows potential in nacre and pearl culture (FAO 1983; Sahidin et al., 2022). Sinanodonta meat, either in fresh or dried form, has been successfully used as a feed in the culture of Nile tilapia Oreochromis niloticus (Linnaeus, 1758), Indonesian shortfin eel Anguilla bicolor McClelland, 1844, carp Cyprinus carpio Linnaeus, 1758 (Koroh & Lumenta, 2014; Mangkapa et al., 2017; Saiful et al., 2019), and crustaceans (Dan et al., 2006). Significant amounts of taurine (150.73 mg/100 g of dry mass), an essential micronutrient, were found in Sinanodonta soft tissues, so attempts have been made to develop pets’ food based on Sinanodonta meat (Konieczny et al., 2022).
Due to their stress tolerance, Sinanodonta can potentially be used to regulate undesirable organisms in water (such as cyanobacteria, algae, bacteria, and diseases) or other harmful human wastes in aquatic habitats where native bivalve species cannot survive in the long term. Several studies document the potential of Sinanodonta to reduce certain undesired aspects of eutrophication (Jing et al., 2011; Xiaojun et al., 2011; Liu et al., 2014; Chen et al., 2015). However, these effects can be strongly context-dependent (Yu et al., 2020). In disrupted natural habitats where Sinanodonta replaced native unionids (e.g., due to water pollution), it could theoretically restore some of the lost functionalities, but caution is in order here because the few studies on ecosystem functions suggest that Sinanodonta has different effects (phytoplankton removal, excretion, biodeposition) than native unionids (Zieritz et al., 2019, 2021). The potential positive effects of Sinanodonta should not be neglected in future studies (Emery-Butcher et al., 2020), and more in-depth knowledge is needed (see, for example, discussion in Sotka & Byers, 2019; Boltovskoy et al., 2022a).
In addition to provisioning and regulating ecosystem services discussed above, invasive Sinanodonta can be used as bioindicators of the aquatic environment (cultural ecosystem services; Zieritz et al., 2022). In the native range, a method has been developed to use a standardized Sinanodonta population for biomonitoring (Chen et al., 2014, 2019), as well as assessment of the contamination of organotins, organochlorines, and heavy metals (Yang et al., 2008; Bian et al., 2009; Liu et al., 2010), and these methods are also being developed and tested in the invasive range (Giari et al., 2017; Marić et al., 2020).
Despite the aforementioend potential ecosystem services provided by invasive Sinanodonta, we emphasize that a short-term economic profit should never be prioritized over potential long-term and irreversible risks of species invasions. The precautionary principle should be applied in all cases to prevent the spread of invasive Sinanodonta species into novel areas, and more data are needed to faciliatate a sustainable use of Sinanodonta species without increasing negative impacts in areas where they are already established.
Management options
Management methods of invasive Sinanodonta are remarkably unaddressed, as illustrated by the systematic literature search where not a single scientific article was found that focused primarily on any method of eradication, control, or prevention of Sinanodonta (Supplementary material S1). Here, we discuss current knowledge and practices of Sinanodonta invasion management reported in grey literature, categorized into prevention, early detection and monitoring, and eradication/regulation methods.
Prevention of unintentional spread
Unintentional spread with host fish transfers
To prevent the further spread of Sinanodonta, the key unintentional introduction pathway must be addressed, which is the long-distance transfer of parasitized host fish. Central in this regard is adherence to biosecurity principles for fish and fish brood stock that are transported to new locations. Currently used fish quarantine practices against the spread of fish parasites (e.g., Tonguthai, 1997; Hadfield & Clayton, 2011; Buchmann, 2022) should be highly efficient if applied to Sinanodonta. In practice, this involves a sufficiently long period (depending on temperature: 7 days to several weeks) in quarantine before transferring fish from sites with possible Sinanodonta presence and suitable quarantine tanks. It appears that the quarantine period for fish transport cannot be effectively accelerated. More detailed data on this are lacking, but pilot testing of the possibility of using chemotherapeutic agents (Formalin, NaCl) on encapsulated S. woodiana s. str. has shown no effect on glochidia metamorphosis success (Douda et al., 2016).
In contrast, spread of Sinanodonta through natural migration of infested fish hosts within a river network is difficult to prevent, as migration permeability is generally a key characteristic of an ecologically functioning river network, and artificial migration barriers are often undesirable for other reasons.
Escapees from ornamental use and pet trade
Direct trade of adult Sinanodonta for ornamental aquaria and garden ponds is another key introduction pathway in many areas, which can be mitigated through available solutions commonly applied in ornamental aquaculture trade (Padilla & Williams, 2004). Firstly, strict compliance with good practice principles for the sale of non-native species, including consistent reporting about non-nativity and all potential risks during the sale, is urgently needed but currently not widely observed. For example, in Germany and the Czech Republic, S. woodiana s. str. is sometimes sold under the incorrect generic name "Anodonta" and without information about non-nativity (Bahr & Wiese, 2018). In addition, all precaution and biosecurity principles must be applied at the level of water transfer or fish stock that may be contaminated by glochidia, as Sinanodonta can produce glochidia and infest fish if kept in good conditions (such as large garden ponds).
Commercial use and regulating services
Prevention of Sinanodonta escapes from large-scale aquaculture farms appears to be almost non-feasible in the long term. The nature of the localities suitable for Sinanodonta farming does not allow them to be kept in completely closed systems. Any such use therefore carries this risk, and such activities should not be carried out in areas where escape into the wild is undesirable.
The legislative context of prevention and management
Preventing unintentional introductions of species is set within the framework of general protection against the spread of invasive species and biosecurity principles globally. However, within individual regions, this is relatively neglected and the human-mediated spread of Sinanodonta does not appear to be adequately controlled. There is no explicit codification of the trade ban in legislation in most countries, with only a few exceptions, such as Poland, where S. woodiana was added to the list of invasive species in 2012; thus, selling this species is illegal in this country (Urbańska et al., 2019a). In the European Union as a whole, which has a complex and developing strategy for invasive species, no representative of Sinanodonta is included in the list of priority invasive species (which would forbid their breeding and selling) according to EU Regulation 1143/2014 on Invasive Alien Species. Currently (November 2023), the only invasive freshwater bivalve listed is Limnoperna fortunei (Dunker, 1857).
First, it is necessary to consider whether the sale and breeding of this species should be completely banned in the given region. The argument for a ban on the sale and culture of the species is based on the documented or anticipated risks, and the argument against it can be based on the already wide distribution, the risk of replacement in trade by another species, and potential positive provisioning or regulating ecosystem services in degraded and already invaded areas (see discussion about Dreissena and Corbicula in Burlakova et al., 2022).
Even more complex is the question of legislation in terms of deliberate spread in areas with higher levels of Sinanodonta use by humans. Examples include Indonesia and other areas of the non-native range where S. pacifica is used or planned to be used for provisioning or regulating ecosystem services. More knowledge of impacts and risks is needed so that this can be weighed against potential benefits. Otherwise, a similar ban may be controversial and ineffective. In the non-native areas where Sinanodonta is not yet present and negative impacts cannot be ruled out, we strongly encourage a precautionary approach and support for legislative solutions preventing the spread and cultivation of invasive Sinanodonta species.
Methods of early detection and monitoring
The traditional, standard method for surveying freshwater mussel populations, including Sinanodonta, is sampling by tactile or visual search on the bottom of a water body, which is most commonly restricted to wadable areas but can also include deeper areas via scuba diving (Strayer & Smith, 2003). Surveying is relatively time- and labor-consuming, requires expertise, and is most efficient during periods of reduced water levels, particularly at low population densities. Due to their semi-buried life habits in usually deep, muddy sediment, early detection of invasive Sinanodonta is therefore uncommon, and populations are most often first detected as adults in the course of general freshwater mollusk surveys. Alternatively, when water levels are lowered (e.g., due to construction work, fish extraction from reservoirs, drought), and shells or live specimens are found, even non-specialists often note the occurrence. In either case, detection is only achieved when populations are already established at the site.
One of the newly available methods for the early detection of invasive bivalve species is environmental DNA (eDNA). Sinanodonta, as well as several other invasive freshwater bivalve species, have been detected using this approach in, for example, Spain (Clusa et al., 2017), France (Prié et al., 2021, 2023), and Italy (Egeter et al., 2022). eDNA can facilitate early detection of a species even when the population density is very low, which allows a well-timed swift management response. The method is demanding in terms of laboratory infrastructures, but its potential use for Sinanodonta may be even more beneficial than for other invasive bivalve species when considering its cryptic microhabitat preferences.
Other emerging methods of early detection rely on the use of citizen science through the involvement of data from volunteers and web-based nature monitoring projects to detect species at new localities (Crall et al., 2011). In the case of freshwater mussels, there can be problems with incorrect determination because of the lack of obvious morphological characteristics and the cryptic nature of most of the Sinanodonta microhabitats. However, at most of the sites with more abundant occurrences, it is possible to find shells that attract public attention even at the shore in lower water-level conditions. Thus, even passive crowdsourcing methods such as automated searches of online photos and social media for Sinanodonta photos can be used. For example, some new locations of S. woodiana s. str. in the Czech Republic have been detected by searching web forums based on photographs only (Douda, unpublished).
Eradication and containment methods, and biomass processing
Neither eradication (complete removal from a locality) nor regulation (reduction in abundance) interventions are commonly carried out on Sinanodonta populations in most of its invasive range. The absence of a direct and strong economic damage (in comparison to, for example, Dreissena fouling of water pipes) probably leads to a low perceived cost-effectiveness of interventions. While eradication is almost unfeasible in riverine and lake habitats, it can be accomplished by manipulating the water-level in aquaculture facilities and drainable fishponds in combination with chemical treatment. A likely successful example of Sinanodonta eradication is the case of occurrence in New Jersey, USA, where the species was found in 2010 restricted to a small fishpond area (Bogan et al., 2011). Eradication action consisted of lowering the water-level in all fishponds with detected occurrences and killing all fish with Rotenone. Further actions are not described in detail in the available report, but included draining of ponds and leaving mussels to freeze over winter (Bogan et al., 2011). It is believed that the mussels were successfully eradicated based on a repeated treatment by the copper-based molluskocide EarthTec QZ, as indicated by a subsequent survey from 2019 (Benson, 2023), but there is no available study describing the procedure used in detail.
Another option for Sinanodonta eradication/regulation is the mechanical removal of individuals, which is very time consuming and demanding (Urbańska et al., 2019a). Manual removal of individuals seems feasible only in small sites, and a single surviving hermaphrodite adult buried in fine sediment may produce hundreds of thousands of larvae in the next season. Thus, manual collections are currently used to regulate Sinanodonta in situations where unionid communities are managed. That is, in the case of rescue transfers (native species are moved to an alternate site while Sinanodonta is removed). The method currently used in Europe for handling removed Sinanodonta biomass is processing in a rendering plant, but methods are also being tested to make more efficient use of the biomass (Table 2).
It is also important to consider the risks associated with specific methods of eradication or regulation of Sinanodonta in individual cases. The main risk is the potential impact on non-target organisms, including endangered freshwater mussels. Due to the similar morphology, ecology and habitat requirements of sympatric species, native mussels can be at risk even during the manual collection of specimens (e.g., if species are incorrectly identified), especially if carried out by non-specialists. Even greater is the risk posed by eradication methods based on chemical or water-level manipulation, as these methods are not selective at all for mussels. For example, long-term management aimed at eliminating Sinanodonta from fishponds could lead to the undesirable elimination of all freshwater mussels, despite the fact that the fishponds can be an essential anthropogenic habitat type for endangered freshwater mussel conservation (Sousa et al., 2021). Therefore, before any intervention plan against Sinanodonta is carried out, an expert assessment of the effectiveness, necessity of the intervention, and clear strategy for exclusion of possible risks to non-target organisms is needed.
Main knowledge gaps and the way forward
Despite a rapid increase in our understanding of Sinanodonta invasions in recent years, key gaps remain in almost all aspects of our knowledge of the taxonomy, biology, ecology, and management of invasions. To highlight the key data gaps and lack of consensus in management strategies and methods, we briefly summarize here some of the aspects we consider critical in the coming years:
-
(1)
Critical lack of quantification of the impacts of Sinanodonta invasion on biodiversity, particularly the threats to endangered unionid bivalves.
-
(2)
Lack of knowledge of the environmental requirements of different Sinanodonta species, leading to generalizations across the genus Sinanodonta that may not be appropriate and may lead to dysfunctional management approaches. For example, we do not know the tolerance ranges and thresholds for several crucial water quality parameters (similar to many endangered unionid species), which would allow us to assess the effects of water quality on the species composition of the Sinanodonta-invaded freshwater mussel communities.
-
(3)
Insufficient knowledge of the impact of Sinanodonta filtration, biodeposition and excretion activity on the ecosystem of invaded reservoirs and watercourses.
-
(4)
Complete absence of testing the effectiveness of eradication and regulation methods and ways of their safe implementation.
-
(5)
Insufficient knowledge of the native range of S. pacifica, which makes it difficult to assess the actual non-native status of the species in different parts of its current range and to use this information for management.
-
(6)
Complete absence or anecdotal nature of data on host fish in different parts of the invasive range.
-
(7)
Potential benefits, but also the risks of widespread use of provisioning, regulating, and biomonitoring services provided by invasive Sinanodonta.
The implications of these knowledge gaps can be far-reaching and hinder the application of prevention, eradication, and regulation in the invasive range (but also potentially prevent the efficient use of Sinanodonta ecosystem services). Worse, they are likely one of the main reasons for the lack of consensus on a legislative approach to management strategies across the invasive range.
We would like to emphasize here that current methodological advances make it possible to address most of the knowledge gaps listed above, but a further increase in attention and funding is needed to advance our understanding and inform management decisions and conservation actions. We hope that this review will help to outline avenues for future research, ultimately leading to a better understanding and effective management of Sinanodonta invasions globally.
Data availability
The datasets generated during this review are available from the authors on reasonable request.
References
Abdul Razak, A. & H. Zwair, 2021. Molecular study of new record species Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) in middle of Iraq. Turkish Journal of Physiotherapy and Rehabilitation 32: 22202–22217.
Afanasyev, S. A., B. Zdanowski & A. Kraszewski, 2001. Growth and population structure of the mussel Anodonta woodiana (Lea, 1834) (Bivalvia, Unionidae) in the heated Konin lakes system. Archives of Polish Fisheries 9: 123–131.
Alwanzadegan, K., H. Kashiri, I. N. Bolotov, E. Ercan, A. Shirangi & E. Ghaderi, 2023. Discovery and DNA analysis of the invasive freshwater mussel Sinanodonta lauta (Unionidae) in south Iran. International Journal of Aquatic Biology 11: 86–97. https://doi.org/10.22034/ijab.v11i2.1796.
Aminingsih, T., S. Y. S. Rahayu & Y. Yulianita, 2018. Formulation of instant granule containing nano calcium from the freshwater mussels (Anodonta woodiana) for autism children. Indonesian Journal of Pharmaceutical Science and Technology 1: 49–56. https://doi.org/10.24198/ijpst.v1i1.16125.
Andrzejewski, W., M. Urbańska, J. Mazurkiewicz, H. Gierszal & J. Golski, 2013. The current invasion status of Anodonta woodiana (Lea, 1934) in Poland—study of habitat parameters. Oceanological and Hydrobiological Studies 42: 173–180.
Arfiati, D., C. D. G. Putra, A. H. Tullah, S. W. A. Permanasari & A. W. Puspitasari, 2019. The dynamics of total organic matter (tom) on sangkuriang catfish (Clarias gariepinus) farming at upt ptpbp2kp and the effectiveness of freshwater bivalve (Anodonta woodiana) in reducing the total organic matter with varying density. IOP Conference Series: Earth and Environmental Science 236: 012022. https://doi.org/10.1088/1755-1315/236/1/012022.
Bahr, A. L. & V. Wiese, 2018. Freilandvorkommen von Sinanodonta woodiana (Lea 1834) in Ostholstein und Trockenfallen eines Teiches mit umfangreichen Verlusten von Großmuscheln (Bivalvia: Unionidae). Schr Malakozool 30: 39–44 ((in German)).
Bauer, G., S. Hochwald & W. Silkenat, 1991. Spatial distribution of freshwater mussels: the role of host fish and metabolic rate. Freshwater Biology 26: 377–386. https://doi.org/10.1111/j.1365-2427.1991.tb01405.x.
Bauer, W. G., D. T. Stewart, R. Q. Céspedes, S. A. Valverde & R. H. Easy, 2021. DNA barcoding evidence of the tropical invasive lineage of Sinanodonta woodiana in Costa Rica. Neotropical Naturalist 5: 351–359.
Benedict, A. & J. Geist, 2021. Effects of water temperature on glochidium viability of Unio crassus and Sinanodonta woodiana: implications for conservation, management and captive breeding. Journal of Molluscan Studies 87: eyab011. https://doi.org/10.1093/mollus/eyab011.
Benelli, S., M. Bartoli, E. Racchetti, P. C. Moraes, M. Zilius, I. Lubiene & E. A. Fano, 2017. Rare but large bivalves alter benthic respiration and nutrient recycling in riverine sediments. Aquatic Ecology. https://doi.org/10.1007/s10452-016-9590-3.
Benkő-Kiss, Á., 2012. The invasive Chinese pond mussel (Sinanodonta woodiana, LEA 1834 as a danger for waterside tourism. Lucrari Stiintifice Seria Management Agricol 14: 5–12.
Benkő-Kiss, Á., Á. Ferincz, N. Kováts & G. Paulovits, 2013. Spread and distribution of Sinanodonta woodiana in Lake Balaton. Knowledge and Management of Aquatic Ecosystems 408: 09. https://doi.org/10.1051/kmae/2013043.
Bensaâd-Bendjedid, L., S. Telailia, F. Alliouche, H. Touati & I. Ladjama, 2023. First record of the occurrence of the Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) in African freshwaters: Oubeira Lake, Algeria. Turkish Journal of Zoology 47: 94–102. https://doi.org/10.55730/1300-0179.3119.
Benson, A. J., 2023. Sinanodonta woodiana (Lea, 1834): U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL, [available on internet at https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=2824, Revision Date: 5/26/2020, Access Date: 3/21/2023.
Benson, J. A., B. A. Stewart, P. G. Close & A. J. Lymbery, 2021. Freshwater mussels in Mediterranean-climate regions: Species richness, conservation status, threats, and conservation actions needed. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 708–728.
Berni, P., S. Bitossi, M. Salvato, M. Orlandi, J. Salviati, M. Silvestri, P. Megale, P. Orlandi, & R. Billiard, 2004. Valorizzazione del territorio attraverso produzioni alternative di perle di acqua dolce di elevata qualita`. Con tecniche di policoltura eco-sostenibile. International Workshop ‘Tinca e acquacoltura nelle acque interne, Ceresole d’Alba, Italy, 179–185.
Bespalaya, Y. V., I. N. Bolotov, O. V. Aksenova, M. Y. Gofarov, A. V. Kondakov, I. V. Vikhrev & M. V. Vinarski, 2018. DNA barcoding reveals invasion of two cryptic Sinanodonta mussel species (Bivalvia: Unionidae) into the largest Siberian river. Limnologica 69: 94–102. https://doi.org/10.1016/j.limno.2017.11.009.
Bian, X., H. Liu, J. Gan, R. Li & J. Yang, 2009. HCH and DDT residues in bivalves Anodonta woodiana from the Taihu Lake, China. Archives of Environmental Contamination and Toxicology 56: 67–76. https://doi.org/10.1007/s00244-008-9173-y.
Bielen, A., I. Bošnjak, K. Sepčić, M. Jaklič, M. Cvitanić, J. Lušić, J. Lajtner, T. Simčič & S. Hudina, 2016. Differences in tolerance to anthropogenic stress between invasive and native bivalves. Science of the Total Environment 543: 449–459. https://doi.org/10.1016/j.scitotenv.2015.11.049.
Bódis, E., B. Tóth & R. Sousa, 2014a. Massive mortality of invasive bivalves as a potential resource subsidy for the adjacent terrestrial food web. Hydrobiologia 735: 253–262. https://doi.org/10.1007/s10750-013-1445-5.
Bódis, E., B. Tóth & R. Sousa, 2014b. Impact of Dreissena fouling on the physiological condition of native and invasive bivalves: interspecific and temporal variations. Biological Invasions 16: 1373–1386. https://doi.org/10.1007/s10530-013-0575-z.
Bódis, E., B. Tóth, J. Szekeres, P. Borza & R. Sousa, 2014c. Empty native and invasive bivalve shells as benthic habitat modifiers in a large river. Limnologica 49: 1–9.
Bogan, A. E., J. Bowers-Altman & M. E. Raley, 2011. The first confirmed record of the Chinese Pond Mussel (Sinanodonta woodiana) (Bivalvia: Unionidae) in the United States. Nautilus 125: 41–43.
Bogan, A. E., A. A. Al-Fanharawi & M. Lopes-Lima, 2021. First record of Sinanodonta woodiana and report for freshwater bivalves from Iraq (Mollusca: Bivalvia: Unionidae). Ecologica Montenegrina 46: 52–60. https://doi.org/10.37828/em.2021.46.2.
Bolotov, I. N., Y. V. Bespalaya, M. Y. Gofarov, A. V. Kondakov, E. S. Konopleva & I. V. Vikhrev, 2016. Spreading of the Chinese pond mussel, Sinanodonta woodiana, across Wallacea: One or more lineages invade tropical islands and Europe. Biochemical Systematics and Ecology 67: 58–64. https://doi.org/10.1016/j.bse.2016.05.018.
Bolotov, I. N., A. V. Kondakov, E. S. Konopleva, I. V. Vikhrev, O. V. Aksenova, A. S. Aksenov, Y. V. Bespalaya, A. V. Borovskoy, P. P. Danilov, G. A. Dvoryankin, M. Y. Gofarov, M. B. Kabakov, O. K. Klishko, Y. S. Kolosova, A. A. Lyubas, A. P. Novoselov, D. M. Palatov, G. N. Savvinov, N. M. Solomonov, V. M. Spitsyn, S. E. Sokolova, A. A. Tomilova, E. Froufe, A. E. Bogan, M. Lopes-Lima, A. A. Makhrov & M. V. Vinarski, 2020. Integrative taxonomy, biogeography and conservation of freshwater mussels (Unionidae) in Russia. Scientific Reports 10: 3072. https://doi.org/10.1038/s41598-020-59867-7.
Bolotov, I. N., E. S. Konopleva, N. Chan, Z. Lunn, T. Win, M. Y. Gofarov, A. V. Kondakov, A. A. Tomilova & I. V. Vikhrev, 2022. A riverine biodiversity hotspot in northern Myanmar supports three new and narrowly endemic freshwater mussel species. Aquatic Conservation: Marine and Freshwater Ecosystems 32: 1490–1508. https://doi.org/10.1002/aqc.3850.
Boltovskoy, D., R. Guiasu, L. Burlakova, A. Karatayev, M. A. Schlaepfer & N. Correa, 2022a. Misleading estimates of economic impacts of biological invasions: including the costs but not the benefits. Ambio 51: 1786–1799. https://doi.org/10.1007/s13280-022-01707-1.
Boltovskoy, D., E. Paolucci, H. J. MacIsaac, A. B. Zhan, Z. Q. Xia & N. Correa, 2022b. What we know and don’t know about the invasive golden mussel Limnoperna fortunei. Hydrobiologia. https://doi.org/10.1007/s10750-022-04988-5.
Brandt, R. A. M., 1974. The non-marine aquatic Mollusca of Thailand. Archiv Für Molluskenkunde 105: 1–423.
Brian, J. I. & D. C. Aldridge, 2019. Endosybionts: An overlooked threat in the conservation of freshwater mussels? Biological Conservation 237: 155–165.
Buchmann, K., 2022. Control of parasitic diseases in aquaculture. Parasitology 149: 1985–1997. https://doi.org/10.1017/S0031182022001093.
Burlakova, L. E., A. Y. Karatayev, D. Boltovskoy & N. M. Correa, 2022. Ecosystem services provided by the exotic bivalves Dreissena polymorpha, D. rostriformis bugensis, and Limnoperna fortunei. Hydrobiologia. https://doi.org/10.1007/s10750-022-04935-4.
Burzyński, A. & M. Soroka, 2018. Complete paternally inherited mitogenomes of two freshwater mussels Unio pictorum and Sinanodonta woodiana (Bivalvia: Unionidae). PeerJ 6: e5573. https://doi.org/10.7717/peerj.5573.
Cappelletti, C., S. Cianfanelli, M. E. Beltrami & F. Ciutti, 2009. Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae): a new non-indigenous species in Lake Garda (Italy). Aquatic Invasions 4: 685–688. https://doi.org/10.3391/ai.2009.4.4.15.
Carella, F., G. Villari & N, Maio & G. De Vico, 2016. Disease and disorders of freshwater unionid mussels: a brief overview of recent studies. Frontiers in Physiology 7: 489. https://doi.org/10.3389/fphys.2016.00489.
Chen, X., J. Yang, H. Liu, Y. Su, L. Sun & Y. Oshima, 2012. Element concentrations in a unionid mussel (Anodonta woodiana) at different life stages. Journal of the Faculty of Agriculture, Kyushu University 57: 139–144. https://doi.org/10.5109/22061.
Chen, X., Y. Su, H. Liu & J. Yang, 2014. Biomonitoring of heavy metal pollution in Wulihu Bay of Taihu Lake by transplanting “standardized” Anodonta woodiana. China Environmental Science 34: 225–231 ((in Chinese with English abstract)).
Chen, X., H. Liu, Y. Su, X. Ge & J. Yang, 2015. Purification capacity of Anodonta woodiana on fishpond water. Chinese Journal of Environmental Engineering 9: 1757–1762.
Chen, X., Y. Su, H. Liu & J. Yang, 2019. Active biomonitoring of metals with cultured Anodonta woodiana: a case study in the Taihu Lake, China. Bulletin of Environmental Contamination and Toxicology 102: 198–203. https://doi.org/10.1007/s00128-018-2482-6.
Chen, X., G. Duan, M. Yan, H. Liu, T. Jiang & J. Yang, 2022. Host fish suitability for freshwater bivalve Anodonta woodiana breeding programs. Fishes 7: 329. https://doi.org/10.3390/fishes7060329.
Cherry, D. S., J. L. Scheller, N. L. Cooper & J. R. Bidwell, 2005. Potential effects of Asian clam (Corbicula fluminea) die-offs on native freshwater mussels (Unionidae) I: water-column ammonia levels and ammonia toxicity. Journal of the North American Benthological Society 24: 369–380. https://doi.org/10.1899/04-073.1.
Cichy, A., M. Urbańska, A. Marszewska, W. Andrzejewski & E. Żbikowska, 2016. The invasive Chinese pond mussel Sinanodonta woodiana (Lea, 1834) as a host for native symbionts in European waters. Journal of Limnology 75: 288–296. https://doi.org/10.4081/jlimnol.2016.1334.
Clement, M., D. Posada & K. A. Crandall, 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1659. https://doi.org/10.1046/j.1365-294x.2000.01020.x.
Clusa, L., L. Miralles, A. Basanta, C. Escot & E. Garcı, 2017. eDNA for detection of five highly invasive molluscs. A case study in urban rivers from the Iberian Peninsula. PloS ONE 12(11): e0188126. https://doi.org/10.1371/journal.pone.0188126.
Ćmiel, A. M., K. Zając, A. M. Lipińska & T. Zając, 2019. Is Pseudanodonta complanata the most vulnerable of widespread European species of unionids? An intense stress test leading to a massive die-off. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 2185–2192. https://doi.org/10.1002/aqc.3216.
Corsi, I., A. M. Pastore, A. Lodde, E. Palmerini, L. Castagnolo & S. Focardi, 2007. Potential role of cholinesterases in the invasive capacity of the freshwater bivalve, Anodonta woodiana (Bivalvia: Unionacea): A comparative study with the indigenous species of the genus, Anodonta sp. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 145: 413–419. https://doi.org/10.1016/j.cbpc.2007.01.011.
Coughlan, N. E., A. L. Stevens, T. C. Kelly, J. T. A. Dick & M. A. K. Jansen, 2017. Zoochorous dispersal of freshwater bivalves: an overlooked vector in biological invasions? Knowledge & Management of Aquatic Ecosystems 418: 42. https://doi.org/10.1051/kmae/2017037.
Coughlan, N. E., E. M. Cunningham, R. N. Cuthbert, P. W. S. Joyce, P. Anastácio, F. Banha, N. Bonel, S. J. Bradbeer, E. Briski, V. L. Butitta, Z. Čadková, J. T. A. Dick, K. Douda, L. E. Eagling, N. Ferreira-Rodríguez, L. A. Hünicken, M. L. Johansson, L. Kregting, A. M. Labecka, D. Li, F. Liquin, J. Marescaux, T. J. Morris, P. Nowakowska, M. Ożgo, E. M. Paolucci, M. A. Peribáñez, N. Riccardi, E. R. C. Smith, M. J. Spear, G. T. Steffen, J. S. Tiemann, M. Urbańska, K. Van Doninck, M. Vastrade, G. Y. W. Vong, B. Wawrzyniak-Wydrowska, Z. Xia, C. Zeng, A. Zhan & F. Sylvester, 2021. Biometric conversion factors as a unifying platform for comparative assessment of invasive freshwater bivalves. Journal of Applied Ecology 58: 1945–1956. https://doi.org/10.1111/1365-2664.13941.
Crall, A. W., G. J. Newman, T. J. Stohlgren, K. A. Holfelder, J. Graham & D. M. Waller, 2011. Assessing citizen science data quality: an invasive species case study. Conservation Letters 4: 433–442. https://doi.org/10.1111/j.1755-263X.2011.00196.x.
Creed, R. P. & B. L. Brown, & J. Skelton, 2022. The potential impacts of invasions on native symbionts. Ecology 103: e3726. https://doi.org/10.1002/ecy.3726.
Dan, H., H. Wen, G. Xu & S. Weng, 2006. Growth and free amino acids in muscle and liver of Chinese mitten - hand crab Etiocheir sinensis fed clam Anodonta woodiana pacifica. Journal of Dalian Ocean University 21: 83–86 (in Chinese with English abstract). https://doi.org/10.3969/j.issn.1000-9957.2006.01.017
Demayo, C. G., K. M. C. Cabacaba & M. A. J. Torres, 2012. Shell shapes of the Chinese pond mussel Sinanodonta woodiana (Lea, 1834) from Lawis stream in Iligan City and Lake Lanao in Mindanao, Philippines. Advances in Environmental Biology 6: 1468–1473.
Diagne, C., B. Leroy, R. E. Gozlan, A. C. Vaissière, C. Assailly, L. Nuninger, D. Roiz, F. Jourdain, I. Jarić & F. Courchamp, 2020. InvaCost, a public database of the economic costs of biological invasions worldwide. Scientific Data 7: 277. https://doi.org/10.1038/s41597-020-00586-z.
Djajasasmita, M., 1982. The occurrence of Anodonta woodiana Lea, 1837 in Indonesia (Pelecypoda: Unionidae). Veliger 25: 175–175.
Dobler, A. H. & J. Geist, 2022. Impacts of native and invasive crayfish on three native and one invasive freshwater mussel species. Freshwater Biology 67: 389–403. https://doi.org/10.1111/fwb.13849.
Dobler, A. H., P. Hoos & J. Geist, 2022. Distribution and potential impacts of non-native Chinese pond mussels Sinanodonta woodiana (Lea, 1834) in Bavaria, Germany. Biological Invasions 24: 1689–1706. https://doi.org/10.1007/s10530-022-02737-2.
Dodd, B. J., M. C. Barnhart, C. L. Rogers-Lowery, T. B. Fobian & R. V. Dimock Jr., 2005. Cross-resistance of largemouth bass to glochidia of unionid mussels. Journal of Parasitology 91: 1064–1072. https://doi.org/10.1645/GE-511R.1.
Donrovich, S. W., K. Douda, V. Plechingerová, K. Rylková, P. Horký, O. Slavík, H.-Z. Liu, M. Reichard, M. Lopes-Lima & R. Sousa, 2017. Invasive Chinese pond mussel Sinanodonta woodiana threatens native mussel reproduction by inducing cross-resistance of host fish. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 1325–1333. https://doi.org/10.1002/aqc.2759.
Douda, K. & Z. Čadková, 2018. Water clearance efficiency indicates potential filter-feeding interactions between invasive Sinanodonta woodiana and native freshwater mussels. Biological Invasions 20: 1093–1098. https://doi.org/10.1007/s10530-017-1615-x.
Douda, K., M. Vrtílek, O. Slavík & M. Reichard, 2012. The role of host specificity in explaining the invasion success of the freshwater mussel Anodonta woodiana in Europe. Biological Invasions 14: 127–137. https://doi.org/10.1007/s10530-011-9989-7.
Douda, K., L. Kalous, P. Horký, O. Slavík, J. Velíšek & J. Kolářová, 2016. Methods for the elimination and prevention of spread of the invasive species Chinese pond mussel (Sinanodonta woodiana) in freshwater ecosystems and aquaculture facilities in the Czech Republic, Czech University of Life Sciences Prague, Prague: ((in Czech)).
Douda, K., H.-Z. Liu, D. Yu, R. Rouchet, F. Liu, Q.-Y. Tang, C. Methling, C. Smith & M. Reichard, 2017a. The role of local adaptation in shaping fish-mussel coevolution. Freshwater Biology 62: 1858–1868. https://doi.org/10.1111/fwb.13026.
Douda, K., J. Velíšek, J. Kolářová, K. Rylková, O. Slavík, P. Horký & I. Langrová, 2017b. Direct impact of invasive bivalve (Sinanodonta woodiana) parasitism on freshwater fish physiology: evidence and implications. Biological Invasions 19: 989–999. https://doi.org/10.1007/s10530-016-1319-7.
Douda, K., F. Escobar-Calderón, B. Vodáková, P. Horký, O. Slavík & R. Sousa, 2020. In situ and low-cost monitoring of particles falling from freshwater animals: from microplastics to parasites. Conservation Physiology 8: coaa08. https://doi.org/10.1093/conphys/coaa088.
Douda, K., W. R. Haag, F. Escobar-Calderón, B. Vodáková, M. Reichard, X. Chen, M. McGregor, J. Yang & M. Lopes-Lima, 2021. Effects of in vitro metamorphosis on survival, growth, and reproductive success of freshwater mussels. Biological Conservation 254: 108964. https://doi.org/10.1016/j.biocon.2021.108964.
Dudgeon, D. & B. Morton, 1983. The population dynamics and sexual strategy of Anodonta woodiana (Bivalvia: Unionacea) in Plover Cove Reservoir, Hong Kong. Journal of Zoology 201: 161–183.
Dudgeon, D. & B. Morton, 1984. Site selection and attachment duration of Anodonta woodiana (Bivalvia: Unionacea) glochidia on fish hosts. Journal of Zoology, London 204: 355–362.
Early, R., B. A. Bradley, J. S. Dukes, J. J. Lawler, J. D. Olden, D. M. Blumenthal, P. Gonzalez, E. D. Grosholz, I. Ibañez, L. P. Miller, C. J. B. Sorte & A. J. Tatem, 2016. Global threats from invasive alien species in the twenty-first century and national response capacities. Nature Communications 7: 12485. https://doi.org/10.1038/ncomms12485.
Egeter, B., J. Veríssimo, M. Lopes-Lima, C. Chaves, J. Pinto, N. Riccardi, P. Beja & N. A. Fonseca, 2022. Speeding up the detection of invasive bivalve species using environmental DNA: a nanopore and illumina sequencing comparison. Molecular Ecology Resources 22: 2232–2247. https://doi.org/10.1111/1755-0998.13610.
Emery-Butcher, H. E., S. J. Beatty & B. J. Robson, 2020. The impacts of invasive ecosystem engineers in freshwaters: a review. Freshwater Biology 65: 999–1015. https://doi.org/10.1111/fwb.13479.
Fabbri, R. & L. Landi, 1999. Nuove segnalazioni di molluschi, crostacei e pesci esotici in Emilia-Romagna e prima segnalazioni di Corbicula fluminea (O.F. Müller, 1774) in Italia (Mollusca Bivalvia, Crustacea Decapoda, Osteichthyes Cypriniformes). Quaderno Di Studi e Notizie Di Storia Naturale Della Romagna 12: 9–20 (in Italian with English abstract).
FAO, 1983 Freshwater aquaculture development in China. Report of the FAO/UNDP study tour organized for French-speaking African countries. 22 April–20 May 1980. FAO Fisheries Technical Paper 215: 125
Fatmi, M. & E. Rustiani, 2018. Development of snack bar (Snack Bar) in rich in calcium with fortification of shell flour Kijing (Anadonta woodiana). Journal of Science Innovare 1: 27–30.
Fleming, W. J., T. P. Augspurger & J. A. Alderman, 1995. Freshwater mussel die-off attributed to anticholinesterase poisoning. Environmental Toxicology and Chemistry: an International Journal 14: 877–879.
Fornillos, R. J. C., G. C. L. Que, R. V. D. Mendoza, I. K. C. Fontanilla & P. S. Ong, 2021. Molecular identification of the Chinese pond mussel Sinanodonta woodiana (Lea, 1834) from Mindoro and Leyte Islands, Philippines. Science Diliman 33: 77–96.
Froufe, E., M. Lopes-Lima, N. Riccardi, S. Zaccara, I. Vanetti, J. Lajtner, A. Teixeira, S. Varandas, V. Prié, A. Zieritz, R. Sousa & A. E. Bogan, 2017. Lifting the curtain on the freshwater mussel diversity from the Italian Peninsula and Croatian Adriatic coast. Biodiversity and Conservation 26: 3255–3274. https://doi.org/10.1007/s10531-017-1403-z.
Geist, J., A. Benedict, A. H. Dobler, R. Hoess & P. Hoos, 2023. Functional interactions of non-native aquatic fauna with European freshwater bivalves: implications for management. Hydrobiologia. https://doi.org/10.1007/s10750-022-05121-2.
Giari, L., F. Vincenzi, E. A. Fano, I. Graldi, F. Gelli & G. Castaldelli, 2017. Sensitivity to selected contaminants in a biological early warning system using Anodonta woodiana (Mollusca). Water SA 43: 200–208.
Graf, D. L., 2007. Palearctic freshwater mussel (Mollusca: Bivalvia: Unionoida) diversity and the comparatory method as a species concept. Proceedings of the Academy of Natural Sciences of Philadelphia 156: 71–88.
Guarneri, I., O. P. Popa, L. Gola, L. Kamburska, R. Lauceri, M. Lopes-Lima, L. O. Popa & N. Riccardi, 2014. A morphometric and genetic comparison of Sinanodonta woodiana (Lea, 1834) populations: does shape really matter? Aquatic Invasions 9: 183–194. https://doi.org/10.3391/ai.2014.9.2.07.
Gomez, J. D., M. Vargas & E. A. Malek, 1986. Freshwater mollusks of the Dominican Republic. Nautilius 100: 130–134.
Haag, W. R. & J. D. Williams, 2013. Biodiversity on the brink: an assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735: 45–60.
Haas, F., 1969. Superfamilia Unionacea. Das Tierreich, Vol. 88. De Gruyter, Berlin.
Hadfield, C. A. & L. A. Clayton, 2011. Fish quarantine: current practices in public zoos and aquaria. Journal of Zoo and Wildlife Medicine 42: 641–650. https://doi.org/10.1638/2011-0034.1.
Hamidah, A., 2006. The glochidia (Anodonta woodiana Lea) viability on host fish. Biota 11: 185–189 (in Indonesian with English abstract).
Hamidah, A., 2012. Parasitic duration of kijing taiwan (Anodonta woodiana Lea) glochidia on fish host. Jurnal Penelitian Universitas Jambi 14: 45–48 (in Indonesian with English abstract).
Hastuti, Y. P., K. Nirmala & T. Setioaji, 2013. Nitrogen and phosphorus absorption capability in environmental culture by Taiwan gravestone Anadonta woodiana Lea. Jurnal Akuakultur Indonesia 11: 86–95.
Haubrock, P. J., R. N. Cuthbert, A. Ricciardi, C. Diagne & F. Courchamp, 2022. Economic costs of invasive bivalves in freshwater ecosystems. Diversity and Distributions 28: 1010–1021. https://doi.org/10.1111/ddi.13501.
He, J. & Z. Zhuang, 2013. The freshwater bivalves of China, ConchBooks, Harxheim:
Hliwa, P., B. Zdanowski, G. J. Dietrich, A. Andronowska, J. Król & A. Ciereszko, 2015. Temporal changes in gametogenesis of the invasive Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) from the Konin Lakes system (Central Poland). Folia Biologica 63: 175–185. https://doi.org/10.3409/fb63_3.175.
Huang, X.-C., J.-H. Su, J.-X. Ouyang, S. Ouyang, C.-H. Zhou & X.-P. Wu, 2019. Towards a global phylogeny of freshwater mussels (Bivalvia: Unionida): species delimitation of Chinese taxa, mitochondrial phylogenomics, and diversification patterns. Molecular Phylogenetics and Evolution 130: 45–59. https://doi.org/10.1016/j.ympev.2018.09.019.
Huber, V. & J. Geist, 2017. Glochidial development of the freshwater swan mussel (Anodonta cygnea, Linnaeus 1758) on native and invasive fish species. Biological Conservation 209: 230–238. https://doi.org/10.1016/j.biocon.2017.02.030.
Huber, V. & J. Geist, 2019. Reproduction success of the invasive Sinanodonta woodiana (Lea 1834) in relation to native mussel species. Biological Invasions 21: 3451–3465.
Ilarri, M. I., A. T. Souza, V. Modesto, L. Guilhermino & R. Sousa, 2015. Differences in the macrozoobenthic fauna colonising empty bivalve shells before and after invasion by Corbicula fluminea. Marine and Freshwater Research 66: 549–558. https://doi.org/10.1071/MF14004.
Izzatullaev, Z. I. & C. T. Boymurodov, 2016. The results of the pearl’s growing of bivalve freshwater mollusks (Bivalvia: Unionidae, Anodontinae) of Uzbekistan. Bulletin of Moscow Society of Naturalists 121: 16–19 (in Russian with English abstract).
Jing, X., K. Min, H. Jiang & X. Yuan, 2011. Experiment on water purification of intensive culturing fishpond using Anodonta woodiana. Journal of Southern Agriculture 42: 450–452 (in Chinese with English abstract).
Jing, W., L. Lang, Z. Lin, N. Liu & L. Wang, 2019. Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere 219: 321–327. https://doi.org/10.1016/j.chemosphere.2018.12.033.
Kamburska, L., R. Lauceri & N. Riccardi, 2013. Establishment of a new alien species in Lake Maggiore (Northern Italy): Anodonta (Sinanodonta) woodiana (Lea, 1834) (Bivalvia: Unionidae). Aquatic Invasions 8: 111–116. https://doi.org/10.3391/ai.2013.8.1.13.
Kappes, H. & P. Haase, 2012. Slow, but steady: Dispersal of freshwater molluscs. Aquatic Sciences 74: 1–14.
Karatayev, A. Y. & L. E. Burlakova, 2022. What we know and don’t know about the invasive zebra (Dreissena polymorpha) and quagga (Dreissena rostriformis bugensis) mussels. Hydrobiologia 1–74.
Kondakov, A. V., D. M. Palatov, Z. P. Rajabov, M. Y. U. Gofarov, E. S. Konopleva, A. A. Tomilova, I. V. Vikhrev & I. N. Bolotov, 2018. DNA analysis of a non-native lineage of Sinanodonta woodiana species complex (Bivalvia: Unionidae) from Middle Asia supports the Chinese origin of the European invaders. Zootaxa 4462: 511–522. https://doi.org/10.11646/zootaxa.4462.4.4.
Kondakov, A. V., Y. V. Bespalaya, I. V. Vikhrev, E. S. Konopleva, M. Y. Gofarov, A. A. Tomilova, M. V. Vinarski & I. N. Bolotov, 2020a. The Asian pond mussels rapidly colonize Russia: successful invasions of two cryptic species to the Volga and Ob rivers. BioInvasions Records 9: 504–518. https://doi.org/10.3391/bir.2020.9.3.07.
Kondakov, A. V., E. S. Konopleva, I. V. Vikhrev, Y. V. Bespalaya, M. Y. Gofarov, M. V. Kabakov, A. A. Tomilova, M. V. Vinarski & I. N. Bolotov, 2020b. Phylogeographic affinities, distribution and population status of the non-native Asian pond mussels Sinanodonta lauta and S. woodiana in Kazakhstan. Ecologica Montenegrina 27: 22–34.
Konečný, A., O. P. Popa, V. Bartáková, K. Douda, J. Bryja, C. Smith, L. O. Popa & M. Reichard, 2018. Modelling the invasion history of Sinanodonta woodiana in Europe: tracking the routes of a sedentary aquatic invader with mobile parasitic larvae. Evolutionary Applications 11: 1975–1989. https://doi.org/10.1111/eva.12700.
Konieczny, P., J. Tomaszewska-Gras, W. Andrzejewski, B. Mikołajczak, M. Urbańska, J. Mazurkiewicz & J. Stangierski, 2016. DSC and electrophoretic studies on protein denaturation of Anodonta woodiana (Lea, 1834). Journal of Thermal Analysis and Calorimetry 126: 69–75. https://doi.org/10.1007/s10973-016-5707-0.
Konieczny, P., W. Andrzejewski, T. Yang, M. Urbańska, J. Stangierski, Ł Tomczyk & B. Mikołajczak, 2022. Selected quality attributes of freshwater mussel powder as a promising ingredient for pet food. Animals 12: 90. https://doi.org/10.3390/ani12010090.
Koroh, P. A., & C. Lumenta, 2014. Pakan suspensi daging kekerangan bagi pertumbuhan benih sidat (Anguilla bicolor). Budidaya Perairan 2: 7–13. (in Indonesian with English abstract). https://doi.org/10.35800/bdp.2.1.2014.3787
Kraszewski, A., 2007. The continuing expansion of Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) in Poland and Europe. Folia Malacologica 15: 65–69.
Kraszewski, A. & B. Zdanowski, 2007. Sinanodonta woodiana (Lea, 1834) (Mollusca)—a new mussel species in Poland: occurrence and habitat preferences in a heated lake system. Polish Journal of Ecology 55: 337–356.
Labecka, A. M. & M. Czarnoleski, 2021. Patterns of growth, brooding and offspring size in the invasive mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) from an anthropogenic heat island. Hydrobiologia 848: 3093–3113. https://doi.org/10.1007/s10750-019-04141-9.
Labecka, A. M. & J. Domagala, 2018. Continuous reproduction of Sinanodonta woodiana (Lea, 1824) females: an invasive mussel species in a female-biased population. Hydrobiologia 810: 57–76.
Labecka, A. M. & J. Domagala, 2019. Two pathways for spermatogenesis in Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). Journal of Molluscan Studies 85: 300–310.
Lajtner, J. & P. Crnčan, 2011. Distribution of the invasive bivalve Sinanodonta woodiana (Lea, 1834) in Croatia. Aquatic Invasions 6(1): 119–124.
Lea, I., 1837. Observations on the Naïades; and descriptions of new species of that and other families. Transactions of the American Philosophical Society 5: 23–119. https://doi.org/10.2307/1004939.
Liu, J., B. Gu, J. Bian, X. Cheng, Q. Ke & H. Yan, 2008. Antitumor activities of liposome-incorporated aqueous extracts of Anodonta woodiana (Lea, 1834). European Food Research and Technology 227: 919–924. https://doi.org/10.1007/s00217-007-0806-6.
Liu, H., J. Yang & J. Gan, 2010. Trace element accumulation in bivalve mussels Anodonta woodiana from Taihu Lake, China. Archives of Environmental Contamination and Toxicology 59: 593–601. https://doi.org/10.1007/s00244-010-9521-6.
Liu, Y., A. Hao, Y. Iseri, T. Kuba & Z. Zhang, 2014. A Comparison of the mussel Anodonta woodiana’s acute physiological responses to different algae diets. Journal of Clean Energy Technologies 2: 126–131. https://doi.org/10.7763/JOCET.2014.V2.106.
Liu, X., Y. Liu, R. Wu, D. T. Zanatta, M. Lopes-Lima, D. V. Gonçalves, A. E. Bogan, S. Ouyang & X. Wu, 2022. Systematics, distribution, biology, and conservation of freshwater mussels (Bivalvia: Unionida) in China. Aquatic Conservation: Marine and Freshwater Ecosystems 32: 859–895. https://doi.org/10.1002/aqc.3799.
Lopes-Lima, M., P. Lima, M. Hinzmann, A. Rocha & J. Machado, 2014. Selective feeding by Anodonta cygnea (Linnaeus, 1771): the effects of seasonal changes and nutritional demands. Limnologica 44: 18–22.
Lopes-Lima, M., L. E. Burlakova, A. Y. Karatayev, K. Mehler, M. Seddon & R. Sousa, 2018. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810: 1–14.
Lopes-Lima, M., A. Hattori, T. Kondo, J. H. Lee, K. S. Kim, A. Shirai, H. Hayashi, T. Usui, K. Sakuma, T. Toriya, Y. Sunamura, H. Ishikawa, N. Hoshino, Y. Kusano, H. Kumaki, Y. Utsugi, S. Yabe, Y. Yoshinari, H. Hiruma, A. Tanaka, K. Sao, T. Ueda, I. Sano, J. I. Miyazaki, D. V. Gonçalves, O. K. Klishko, E. S. Konopleva, I. V. Vikhrev, A. V. Kondakov, M. Y. Gofarov, I. N. Bolotov, E. M. Sayenko, M. Soroka, A. Zieritz, A. E. Bogan & E. Froufe, 2020. Freshwater mussels (Bivalvia: Unionidae) from the Rising Sun (Far East Asia): phylogeny, systematics, and distribution. Molecular Phylogenetics and Evolution 146: 106755. https://doi.org/10.1016/j.ympev.2020.106755.
Lorencová, E., L. Beran, V. Horsáková & M. Horsák, 2015. Invasion of freshwater molluscs in the Czech Republic: time course and environmental predictors. Malacologia 59: 105–120.
Mabrouki, Y., & A. Fouzi Taybi, 2022. The first record of the invasive Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) in the African continent. Natura Croatica: Periodicum Musei Historiae Naturalis Croatici, 31, 393–398. https://doi.org/10.20302/NC.2022.31.28
Mangkapa A., C. Lumenta & J. F. Mokolensang, 2017. Efficiency of Taiwanese mussel (Anodonta woodiana) powder-supplemented feed on growth of carp, Cyprinus caprio L. Budidaya Perairan 5: 36–43. (in Indonesian with English abstract) https://doi.org/10.35800/bdp.5.3.2017.17875
Marić, J. J., M. Kračun-Kolarević, S. Kolarević, K. Sunjog, J. Kostić-Vuković, B. Deutschmann, H. Hollert, D. Tenji, M. Paunović & B. Vuković-Gačić, 2020. Selection of assay, organism, and approach in biomonitoring significantly affects the evaluation of genotoxic potential in aquatic environments. Environmental Science and Pollution Research 27: 33903–33915. https://doi.org/10.1007/s11356-020-09597-0.
McDowell, W. G. & R. Sousa, 2019. Mass mortality events of invasive freshwater bivalves: current understanding and potential directions for future research. Frontiers in Ecology and Evolution 7: 331. https://doi.org/10.3389/fevo.2019.00331.
Methling, C., K. Douda & M. Reichard, 2019. Intensity-dependent energetic costs in a reciprocal parasitic relationship. Oecologia 191: 285–294. https://doi.org/10.1007/s00442-019-04504-y.
Modesto, V., M. Ilarri, A. M. Labecka, N. Ferreira-Rodríguez, N. E. Coughlan, X. Liu & R. Sousa, 2023. What we know and do not know about the invasive Asian clam Corbicula fluminea. Hydrobiologia. https://doi.org/10.1007/s10750-023-05280-w.
Monaco, P., F. Famiani & F. La Iacona, 2016. Bulldozing and resting traces of freshwater mussel Anodonta woodiana and substrate characteristics in lake-margin and river settings of Umbria, Italy. Rivista Italiana di Paleontologia e Stratigrafia (Research in Paleontology and Stratigraphy) 122: 53–60. https://doi.org/10.13130/2039-4942/6912
Müller, T., M. Czarnoleski, A. M. Labecka, A. Cichy, K. Zajac & D. Dragosz-Kluska, 2015. Factors affecting trematode infection rates in freshwater mussels. Hydrobiologia 742: 59–70. https://doi.org/10.1007/s10750-014-1965-7.
Ng, T. H., S. K. Tan, W. H. Wong, R. Meier & S. Chan, 2016. Molluscs for sale: assessment of freshwater gastropods and bivalves in the ornamental pet trade. PLoS One 11: e0161130. https://doi.org/10.1371/journal.pone.0161130.
Niero, I., 2003. Sulla presenza in Veneto e centro Italia di Anodonta woodiana woodiana (Lea, 1834)(Mollusca, Bivalvia). Bollettino Del Museo Civico Di Storia Naturale Di Venezia 54: 29–33 ((in Italian)).
O’Connell, M. T. & R. J. Neves, 1999. Evidence of immunological responses by a host fish (Ambloplites rupestris) and two non-host fishes (Cyprinus carpio and Carassius auratus) to glochidia of a freshwater mussel (Villosa iris). Journal of Freshwater Ecology 14: 71–78.
Padilla, D. K. & S. L. Williams, 2004. Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Frontiers in Ecology and the Environment 2: 131–138.
Patoka, J., O. Kopecký, V. Vrabec & L. Kalous, 2017. Aquarium molluscs as a case study in risk assessment of incidental freshwater fauna. Biological Invasions 19: 2039–2046.
Paunovic, M., B. Csányi, V. Simic, B. Stojanovic & P. Cakic, 2006. Distribution of Anodonta (Sinanodonta) woodiana (Lea, 1834) in inland waters of Serbia. Aquatic Invasions 1: 154–160.
Putro, S. P., A. Suherman & R. Hariyati, 2010. Development of aquaculture environmental management for the improvement of production capacity for sustainable aquaculture activities. Proceeding the International Conference on Management of Innovation & Technology: 978–979. Diponegoro University, Indonesia.
Popa, O., & D. Murairu, 2009. Freshwater bivalve molluscs invasive in Romania. In: Pyšek P., J. Pergl (eds). Biological Invasions: Towards a Synthesis. Neobiota 8: 123–133.
Poznańska-Kakareko, M., K. Wiśniewski, D. Szarmach, A. Witkowska, T. Kakareko, Ł Jermacz & J. Kobak, 2021. Importance of substratum quality for potential competitive niche overlap between native and invasive unionid mussels in Europe. Science of the Total Environment 799: 149345. https://doi.org/10.1016/j.scitotenv.2021.149345.
Prié, V., 2023. How was France invaded? 170 years of colonisation of metropolitan France by freshwater mussels. Hydrobiologia. https://doi.org/10.1007/s10750-023-05274-8.
Prié, V., A. Valentini, M. Lopes-Lima, E. Froufe, M. Rocle, N. Poulet, P. Taberlet & T. Dejean, 2021. Environmental DNA metabarcoding for freshwater bivalves biodiversity assessment: methods and results for the Western Palearctic (European sub-region). Hydrobiologia 848: 2931–2950. https://doi.org/10.1007/s10750-020-04260-8.
Prié, V., A. Danet, A. Valentini, M. Lopes-Lima, P. Taberlet, A. Besnard, N. Roset, O. Gargominy & T. Dejean, 2023. Conservation assessment based on large-scale monitoring of eDNA: Application to freshwater mussels. Biological Conservation 283: 110089. https://doi.org/10.1016/j.biocon.2023.110089.
Puillandre, N., S. Brouillet & G. Achaz, 2021. ASAP: assemble species by automatic partitioning. Molecular Ecology Resources 21: 609–620. https://doi.org/10.1111/1755-0998.13281.
Rahayu S. Y. S. & Rachman B, 2015. Budidaya kijing taiwan (Anodonta woodiana, lea) dengan cara pemeliharaan berbeda. Ekologia 15: 6–13 (in Indonesian with English abstract) https://doi.org/10.33751/ekol.v15i1.205
Rahayu, S. Y. S., D. D. Solihin, W. Manalu & R. Affandi, 2013. Nucleus pearl coating process of freshwater mussel Anodonta woodiana (Unionidae). HAYATI Journal of Biosciences 20: 24–30. https://doi.org/10.4308/hjb.20.1.24.
Rahayu, S. Y. S., A. Fadila, & M. R. Fahmi, 2023. Identification of metallothionein protein in Anodonta woodiana as a biomarker of mercury (Hg) contamination. Nusantara Bioscience 15: 90–94. https://doi.org/10.13057/nusbiosci/n150111
Ramayani, F., U. M. Tang, & Rusliadi, 2012. Utilization of Taiwanese clam (Anadonta woodiana, Lea) as biofilter in culturing system of silais (Ompok hypopthalmus). Riau University. (in Indonesian with English abstract)
Ratnasingham, S. & P. D. N. Hebert, 2013. A DNA-Based registry for all animal species: the Barcode Index Number (BIN) system. PLoS ONE 8: e66213. https://doi.org/10.1371/journal.pone.0066213.
Reichard, M., M. Ondrackova, M. Przybylski, H. Liu & C. Smith, 2006. The costs and benefits in an unusual symbiosis: experimental evidence that bitterling fish (Rhodeus sericeus) are parasites of unionid mussels in Europe. Journal of Evolutionary Biology 19: 788–796.
Reichard, M., M. Vrtílek, K. Douda & C. Smith, 2012. An invasive species reverses the roles in a host-parasite relationship between bitterling fish and unionid mussels. Biology Letters 8: 601–604.
Reichard, M., K. Douda, M. Przybylski, O. P. Popa, E. Karbanova, K. Matasova, K. Rylkova, M. Polacik, R. Blazek & C. Smith, 2015. Population-specific responses to an invasive species. Proceedings of the Royal Society B 282: 1063.
Reichard, M., K. Douda, R. Blažek & A. Janovská, 2023. Increased plasma cortisol level as acute response to glochidia parasitism. Environmental Biology of Fishes 106: 101–106. https://doi.org/10.1007/s10641-022-01379-6.
Sahidin, A., G. Muhammad, Z. Hasan, M. C. W. Arief, R. M. Marwoto & A. Komaru, 2021. Indonesian freshwater bivalves: a meta-analysis of endemicity, ecoregion distributions, and conservation status. Aquaculture, Aquarium, Conservation & Legislation 14: 3750–3775.
Sahidin, A., G. Muhammad, Z. Hasan, M. C. W. Arief & A. Komaru, 2022. Color profile and microstructure of the nacre shell of an invasive freshwater mussel, Sinanodonta woodiana, at different elevations in West Java. Indonesia. Aquaculture 553: 738068.
Saiful I. N., & C. Lumenta, 2014. Proximate analysis of Kijing Taiwan carcass (Anodonta woodiana) in wet and dry forms. Budidaya Perairan 2: 45–53 (in Indonesian with English abstract) https://doi.org/10.35800/bdp.2.3.2014.5702
Saiful, N. I., C. Lumenta & J. Sampekalo, 2019. Kijing Taiwan flour substitution in 3–5 cm tilapia fish feed formulation (Substusi Tepung Kijing Taiwan Dalam Formulasi Pakan Ikan Nila Ukuran 3–5 cm). Budidaya Perairan 3: 19–26 (in Indonesian with English abstract). https://doi.org/10.35800/bdp.3.1.2015.6926
Sano, I., T. Saito, S. Ito, B. Ye, T. Uechi, T. Seo, V. Tu Do, K. Kimura, T. Hirano, D. Yamazaki, A. Shirai, T. Kondo, O. Miura, J. Miyazaki & S. Chiba, 2022. Resolving species-level diversity of Beringiana and Sinanodonta mussels (Bivalvia: Unionidae) in the Japanese archipelago using genome-wide data. Molecular Phylogenetics and Evolution 175: 107563. https://doi.org/10.1016/j.ympev.2022.107563.
Sárkány-Kiss, A., I. Sîrbu & O. Hulea, 2000. Expansion of the adventive species Anodonta woodiana (Lea, 1834) (Mollusca, Bivalvia, Unionoidea) in central and eastern Europe. Acta Oecologica. University of Sibiu 7: 49–57.
Shiryaev, V. V., 1976. A method for assessment of molluscan biomass consumed by muskrat. Russian Journal of Ecology 3: 101–102 (in Russian).
Sicuro, B., B. Castelar, D. Mugetti, P. Pastorino, A. Chiarandon, V. Menconi, M. Galloni & M. Prearo, 2020. Bioremediation with freshwater bivalves: a sustainable approach to reducing the environmental impact of inland trout farms. Journal of Environmental Management 276: 111327. https://doi.org/10.1016/j.jenvman.2020.111327.
Šlapanský, L., P. Jurajda & M. Janáč, 2016. Early life stages of exotic gobiids as new hosts for unionid glochidia. Freshwater Biology 61(6): 979–990.
Slavík, O., P. Horký, K. Douda, J. Velíšek, J. Kolářová & P. Lepič, 2017. Parasite-induced increases in the energy costs of movement of host freshwater fish. Physiology and Behavior 171: 127–134.
Sotka, E. E. & J. E. Byers, 2019. Not so fast: promoting invasive species to enhance multifunctionality in a native ecosystem requires strong (er) scrutiny. Biological Invasions 21: 19–25. https://doi.org/10.1007/s10530-018-1822-0.
Sousa, R., J. L. Gutierrez & D. C. Aldridge, 2009. Non-indigenous invasive bivalves as ecosystem engineers. Biological Invasions 11: 2367–2385.
Sousa, R., S. Varandas, R. Cortes, A. Teixeira, M. Lopes-Lima, J. Machado & L. Guilhermino, 2012. Massive die-offs of freshwater bivalves as resource pulses. Annales De Limnologie-International Journal of Limnology 48: 105–112. https://doi.org/10.1051/limn/2012003.
Sousa, R., A. Novais, R. Costa & D. L. Strayer, 2014. Invasive bivalves in fresh waters: impacts from individuals to ecosystems and possible control strategies. Hydrobiologia 735: 233–251. https://doi.org/10.1007/s10750-012-1409-1.
Sousa, R., D. Halabowski, A. M. Labecka, K. Douda, O. Aksenova, Y. Bespalaya, I. Bolotov, J. Geist, H. A. Jones, E. Konopleva, M. W. Klunzinger, C. A. Lasso, I. Lewin, X. Liu, M. Lopes-Lima, J. Mageroy, M. Mlambo, K. Nakamura, M. Nakano, M. Osterling, J. Pfeiffer, V. Prie, L. R. P. Paschoal, N. Riccardi, R. Santos, S. Shumka, A. K. Smith, M. O. Son, A. Teixeira, F. Thielen, S. Torres, S. Varandas, I. V. Vikhrev, X. Wu, A. Zieritz & J. G. Nogueira, 2021. The role of anthropogenic habitats in freshwater mussel conservation. Global Change Biology 27: 2298–2314. https://doi.org/10.1111/gcb.15549.
Spyra, A., M. Strzelec, I. Lewin, M. Krodkiewska, A. Michalik-Kucharz & M. Gara, 2012. Characteristics of Sinanodonta woodiana (Lea, 1834) populations in fish ponds (Upper Silesia, Southern Poland) in relation to environmental factors. International Review of Hydrobiology 97: 12–25. https://doi.org/10.1002/iroh.201111425.
Stangierski, J., R. Rezler, B. Grześ, W. Andrzejewski & P. Konieczny, 2021. Physicochemical characteristics of surimi-like material made from the muscle tissues of freshwater mussels (Sinanodonta woodiana Lea, 1834). Journal of Food Measurement and Characterization 15: 2161–2172. https://doi.org/10.1007/s11694-020-00801-w.
Strayer, D. L., & D. R. Smith, 2003. A guide to sampling freshwater mussel populations. Monograph No. 8. American Fisheries Society, Bethesda, Maryland.
Szlauer-Łukaszewska, A., W. Andrzejewski, H. Gierszal & M. Urbańska, 2017. Co-occurrence of Sinanodonta woodiana with native Unionidae in the lower Oder. Oceanological and Hydrobiological Studies 46: 244–248. https://doi.org/10.1515/ohs-2017-0025.
Tajer, J. C., 2020. New site of the Chinese pond mussel Sinanodonta woodiana (Bivalvia: Unionidae) in the fish pond near Milicz. Przegląd Przyrodniczy XXX I: 4.
Talarico, L., A. Bryjová, D. Čížková, K. Douda & M. Reichard, 2022. Individual copy number variation and extensive diversity between major MHC-DAB1 allelic lineages in the European bitterling. Immunogenetics 74: 497–505. https://doi.org/10.1007/s00251-021-01251-4.
Taskinen, J., T. Mäkelä & E. T. Valtonen, 1997. Exploitation of Anodonta piscinalis (Bivalvia) by trematodes: Parasite tactics and host longevity. Annales Zoologici Fennici 34: 37–46.
Taskinen, J., M. Urbańska, F. Ercoli, W. Andrzejewski, M. Ożgo, B. Deng, J. M. Choo & N. Riccardi, 2021. Parasites in sympatric populations of native and invasive freshwater bivalves. Hydrobiologia 848: 3167–3178. https://doi.org/10.1007/s10750-020-04284-0.
Tonguthai, K., 1997. Control of freshwater fish parasites: a Southeast Asian perspective. International Journal for Parasitology 27: 1185–1191. https://doi.org/10.1016/S0020-7519(97)00116-1.
Urbańska, M. & W. Andrzejewski, 2019. An invasion in progress—Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) in Poland. Folia Malacologica 27: 327–335.
Urbańska, M., W. Andrzejewski, A. Łakomy & H. Gierszal, 2013. Predation on alien species: a case of oystercatcher (Haematopus ostralegus) foraging on Sinanodonta woodiana – an alien pond mussel. Polish Journal of Ecology 61: 175–177.
Urbańska, M., W. Andrzejewski, H. Gierszal & J. Golski, 2019a. Are there differences in the fouling of the native and invasive Unionidae by Dreissena polymorpha? Inland Waters 9: 73–77. https://doi.org/10.1080/20442041.2018.1502985.
Urbańska, M., M. Kirschenstein, K. Obolewski & M. Ożgo, 2019b. Silent invasion: Sinanodonta woodiana successfully reproduces and possibly endangers native mussels in the north of its invasive range in Europe. International Review of Hydrobiology 104: 127–136. https://doi.org/10.1002/iroh.201801971.
Urbańska, M., A. Kamocki, M. Kirschenstein & M. Ożgo, 2021. The Chinese pond mussel Sinanodonta woodiana demographically outperforms European native mussels. Scientific Reports 11: 17058. https://doi.org/10.1038/s41598-021-96568-1.
Vaughn, C. C., 2010. Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. Bioscience 60: 25–35. https://doi.org/10.1525/bio.2010.60.1.7.
von Proschwitz, T., 2008. The Chinese giant mussel—Sinanodonta woodiana (Lea, 1834) (Bivalvia, Unionidae)—an unwelcome addition to the Swedish fauna. Basteria 72: 307–311.
Wächtler, K., M. C. Dreher-Mansur & T. Richter, 2001. Larval types and early postlarval biology in naiads (Unionoida). In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoida Springer, Berlin: 93–125.
Ward, J. M. & A. Ricciardi, 2007. Impacts of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Diversity and Distributions 13: 155–165. https://doi.org/10.1111/j.1472-4642.2007.00336.x.
Watters, G. T., 1997. A synthesis and review of the expanding range of the Asian freshwater mussel Anodonta woodiana (Bivalvia: Unionidae). Veliger 40: 152–156.
Wen, H., G. Xu & D. Hua, 2006. Preliminary observation on parasitic metamorphosis development of Anodonta woodiana pacifica. Journal of Shanghai Ocean University 15: 252–255 ((in Chinese with English abstract)).
White, J. D., S. K. Hamilton & O. Sarnelle, 2015. Heat-induced mass mortality of invasive zebra mussels (Dreissena polymorpha) at sublethal water temperatures. Canadian Journal of Fisheries and Aquatic Sciences 72: 1221–1229. https://doi.org/10.1139/cjfas-2015-0064.
Wijayanti, D. A., A. M. S. Hertika & B. Yanuwiadi, 2017. The perceptions of community on the role of Kijing Taiwan (Anodonta woodiana) in freshwater aquaculture ponds. Indonesian Journal of Environment and Sustainable Development 8: 49–52.
Wojton, A., I. Kasprzyk, P. Kosciolek & K. Pilch, 2012. The occurrence of the protected swan mussel Anodonta cygnea (Linnaeus, 1758) and the invasive alien Chinese mussel Sinanodonta woodiana (Lea, 1834) in the fish ponds in the Wislok River Basin (SE Poland). Folia Malacologica 20: 135–138. https://doi.org/10.2478/v10125-012-0018-y.
Wu, Q., Y. Chen & Z. Liu, 2005. Filtering capacity of Anodonta woodiana and its feeding selectivity on phytoplankton. Chinese Journal of Applied Ecology 16: 2423–2427 (in Chinese with English abstract).
Wu, R. W., Y. T. Liu, S. Wang, X. J. Liu, D. T. Zanatta, K. J. Roe, W. L. Song, C. T. An & X. P. Wu, 2018. Testing the utility of DNA barcodes and a preliminary phylogenetic framework for Chinese freshwater mussels (Bivalvia: Unionidae) from the middle and lower Yangtze River. PloS One 13: e0200956. https://doi.org/10.1371/journal.pone.0200956.
Xiaojun, J., M. Kuan-hong, J. Haizhou & Y. Xinhua, 2011. Experiment on water purification of intensive culturing fishpond using Anodonta woodiana. Journal of Southern Agriculture 42: 450–452 (in Chinese with English abstract).
Yan, J., L. Kewu, L. Xiaowen, M. Lie, S. Anjing, Q. Andong, L. Jing & S. Qizhi, 2002. Analysis of protein, amino acids and some elements in mantellum of four kinds of freshwater mussel. Natural Product Research and Development 14: 45–49.
Yang, J., H. Harino, H. Liu & N. Miyazaki, 2008. Monitoring the organotin contamination in the Taihu Lake of China by bivalve mussel Anodonta woodiana. Bulletin of Environmental Contamination and Toxicology 81: 1641–1668. https://doi.org/10.1007/s00128-008-9464-z.
Yermoshyna, T., & O. Pavliuchenko, 2021. Population structure and symbiotic relationships of the invasive species Sinanodonta woodiana (Lea, 1834) in water bodies of Ukraine. In E3S Web of Conferences (Vol. 280, p. 06006). EDP Sciences.
Yin, F., Q. Gong, Y. Li, X. Dan, P. Sun, Q. Gao, Z. Shi, S. Peng & A. Li, 2014. Effects of Cryptocaryon irritans infection on the survival, feeding, respiratory rate and ionic regulation of the marbled rockfish Sebastiscus marmoratus. Parasitology 141: 279–286. https://doi.org/10.1017/S0031182013001613.
Yu, J., M. Xia, H. He, E. Jeppesen, B. Guan, Z. Ren, J. J. Elser & Z. Liu, 2020. The host mussel Sinanodonta woodiana alleviates negative effects of a small omnivorous fish (Acheilognathus macropterus) on water quality: a mesocosm experiment. Freshwater Science 39: 752–761. https://doi.org/10.1086/711295.
Yuryshynets, V. & N. Krasutska, 2009. Records of the parasitic worm Aspidogaster conchicola (Baer 1827) in the Chinese pond mussel Sinanodonta woodiana (Lea 1834) in Poland and Ukraine. Aquatic Invasions 4: 491–494. https://doi.org/10.3391/ai.2009.4.3.9.
Zieritz, A. & D. Aldridge, 2011. Sexual, habitat-constrained and parasite-induced dimorphism in the shell of a freshwater mussel (Anodonta anatina, Unionidae). Journal of Morphology 272: 1365–1375.
Zieritz, A., M. Lopes-Lima, A. E. Bogan, R. Sousa, S. Walton, K. A. Rahim, J. J. Wilson, P. Y. Ng, E. Froufe & S. McGowan, 2016. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Science of the Total Environment 571: 1069–1078. https://doi.org/10.1016/j.scitotenv.2016.07.098.
Zieritz, A., A. E. Bogan, E. Froufe, O. Klishko, T. Kondo, U. Kovitvadhi, S. Kovitvadhi, J. H. Lee, M. Lopes-Lima, J. M. Pfeiffer, R. Sousa, T. Van Do, I. Vikhrev & D. T. Zanatta, 2018a. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 810: 29–44. https://doi.org/10.1007/s10750-017-3104-8.
Zieritz, A., A. E. Bogan, K. A. A. Rahim, R. Sousa, L. Jainih, S. Harun, N. F. A. Razak, B. Gallardo, S. McGowan, R. Hassan & M. Lopes-Lima, 2018b. Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biological Conservation 219: 126–137. https://doi.org/10.1016/j.biocon.2018.01.012.
Zieritz, A., F. N. Mahadzir, W. N. Chan & S. McGowan, 2019. Effects of mussels on nutrient cycling and bioseston in two contrasting tropical freshwater habitats. Hydrobiologia 835: 179–191. https://doi.org/10.1007/s10750-019-3937-4.
Zieritz, A., H. Taha, M. Lopes-Lima, J. Pfeiffer, K. W. Sing, Z. Sulaiman, S. McGowan & K. A. A. Rahim, 2020. Towards the conservation of Borneo’s freshwater mussels: rediscovery of the endemic Ctenodesma borneensis and first record of the non-native Sinanodonta lauta. Biodiversity and Conservation 29: 2235–2253. https://doi.org/10.1007/s10531-020-01971-1.
Zieritz, A., W. N. Chan, S. McGowan & C. Gibbins, 2021. High rates of biodeposition and N-excretion indicate strong functional effects of mussels (Bivalvia: Unionida) in certain anthropogenic tropical freshwater habitats. Hydrobiologia 848: 3153–3166. https://doi.org/10.1007/s10750-020-04464-y.
Zieritz, A., R. Sousa, D. C. Aldridge, K. Douda, E. Esteves, N. Ferreira-Rodriguez, J. H. Mageroy, D. Nizzoli, M. Osterling, J. Reis, N. Riccardi, D. Daill, C. Gumpinger & A. S. Vaz, 2022. A global synthesis of ecosystem services provided and disrupted by freshwater bivalve molluscs. Biological Reviews 97: 1967–1998. https://doi.org/10.1111/brv.12878.
Funding
Open access publishing supported by the National Technical Library in Prague. This publication is based upon work from COST Action CA18239, supported by COST (European Cooperation in Science and Technology). Support to KD, BV and JM came from the Czech Science Foundation [No. 19–05510S]. INB was supported by the RSF Grant No 21-17-00126. EF and MLL were supported by the Portuguese Science Foundation (CEECINST/00027/2021/CP2789/CT0003) and (2020.03608.CEECIND), respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling editor: Sidinei M. Thomaz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Manuel P. M. Lopes-Lima, Lyubov E. Burlakova, Ting Hui Ng, Alexandra Zieritz & Ronaldo G. Sousa / Biology and Impact of Invasive Freshwater Molluscs
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Douda, K., Zieritz, A., Vodáková, B. et al. Review of the globally invasive freshwater mussels in the genus Sinanodonta Modell, 1945. Hydrobiologia (2024). https://doi.org/10.1007/s10750-023-05457-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-023-05457-3