Abstract
Aquatic organisms are constantly exposed to effluents which often contain microplastics. Microplastics adversely affect aquatic organisms as a result of mechanical damage during ingestion or intoxication by chemicals adsorbed on the microplastics. Sub-optimal temperatures may acerbate the adverse effects of microplastics on aquatic biota. Brachionid rotifers, such as Brachionus havanaensis and Brachionus calyciflorus, are common in tropical freshwaters. They are generalist filter feeders capable of consuming microplastic particles of a wide size range 0.05 to 40 µm, which can eventually affect growth and competitive interactions among species. Here, we evaluated the effect of 30 µm beads of polystyrene microplastics at 10 and 20 mg l−1 on the population growth and competition of B. havanaensis and B. calyciflorus at 20 and 25 °C using 0.5 × 106 cells ml−1 Chlorella vulgaris as food. Population growth decreased in treatments with microplastics at both temperatures as compared to the controls. The population growth rates ranged between 0.21 and 0.38 d−1 for B. calyciflorus, and between 0.27 and 0.48 d−1 for B. havanaensis. The presence of the competing species significantly lowered the population growth rate for B. calyciflorus but not for B. havanaensis. On the other hand, while the presence of the microplastics had little impact on the population growth rate of B. calyciflorus in the presence of the competitor, the reverse was true for B. havanaensis, especially at 20 °C. Our results show that the presence of microplastics may affect rotifer community structure in natural water bodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mass production, consumption, and inadequate treatment of waste water, have resulted in the contamination of waterbodies with plastics and microplastics (MPs). Microplastics are found as filaments and amorphous particles in freshwater and, plastispheres are becoming quite common in various oceans and seas around the world (Zhao et al., 2014; Mason et al., 2016; Amaral-Zettler et al., 2021). Microplastic density in different lakes is reported to range from 10–3 to 10 particles m−3 (Nava et al., 2023). The density of these particles in Pearl river, China is reported between 379 and 7924 particles m−3 (Lin et al., 2018). Freshwaters are also simultaneously exposed to complex mixtures of inorganic and organic pollutants (Altshuler et al., 2011; Guilhermino et al., 2021). Their mixtures and the continuous exposure could increase the adverse effects on aquatic microfauna (Wagner & Lambert, 2018; Guilhermino et al., 2021; Pestana et al., 2021).
The effects of the presence of multiple stressors, their interactions, and the acute and chronic exposure to aquatic organisms can be diverse, from mechanical damage by ingestion to intoxication associated with chemicals, with effects on demographic variables and species interactions or behaviour (Preston & Snell, 2001). Most studies on ecotoxicology with zooplankton have focused on testing the adverse effects on ecological variables such as survivorship, growth, and reproduction (Preston, 2002). Other characteristics, sensitive to the presence of contaminants, are feeding behaviour, escape mechanisms, swimming speed, and ecological interactions especially competition, and predation (Snell & Joaquim-Justo, 2007).
Aquatic communities usually are exposed to multiple stressors, and their interaction could have antagonist or synergistic effects (Sinclair & Arnott, 2015). The ingestion of MPs by aquatic organisms such as cladocerans (Nugnes et al., 2022), copepods (Setälä et al., 2018), and rotifers (Drago & Weithoff, 2021; Xue et al., 2021) has been demonstrated and attributed to their nonselective behaviour (Monakov, 2003; Sun et al., 2019). Early studies, however, indicated that rotifers can be selective in their prey preferences (Gilbert & Starkweather, 1977) and that large biotic and abiotic particles interfere in prey ingestion by metazoans (Moyo, 2022).
Rotifers are important in aquatic food webs as primary consumers linking lower and higher trophic levels (Wallace, 2002), with high sensitivity to environmental conditions and contamination (Wallace et al., 2006). They are sensitive bioindicators of water quality (Ejsmont-Karabin, 2012; Moreno-Gutiérrez et al., 2018) and are frequently used in toxicity tests due to their biological attributes (Sarma et al., 2006). There is also the potential for bioaccumulation or biotransference of microplastics to higher trophic levels if the size of the particles is very small (< 10 μm) and are actually consumed by the rotifers (Gambardella et al., 2018; Sun et al., 2019). Adverse conditions from multiple stressors can eventually modify ecological interactions, such as intra- and inter-specific competition (Gao et al., 2021).
Rotifers such as Brachionus havanaensis and Brachionus calyciflorus are common in freshwaters of pantropical regions (Koste, 1978). Many brachionids have similar food requirements (Dumont, 1977). However, there are few studies on the effects of microplastics on freshwater biota, and even less data available on the interactions between freshwater zooplankton, especially in the presence of multiple stressors. Species with low threshold food concentrations and high population growth rates often win in competition scenarios (Sarma et al., 1996). Several factors influence the population growth rates of rotifers; these include environmental variables such as temperature, food concentration and type, and rotifer characteristics such as their growth rates, capacity to withstand starvation and threshold food concentration (Kirk, 2002).
There are few studies that indicate the size of the microplastic particles in freshwaters. One such study by Eo et al. (2019) indicates that the size of most of the microplastics spheres in the Nakdong river in Korea ranges between 50 and 150 µm. Most studies indicate that the dominant microplastics in water and sediments are fibres and amorphous particles (Su et al., 2016; Nava et al., 2023). Brachionids are known to ingest particles in the 5–45 µm size range (Gilbert, 2022). Hence our choice for the selection of the microplastic particle size is within this range. In this work we evaluated the effect of 30 µm-sized beads of polystyrene microplastics at two concentrations (10 and 20 mg l−1) and at two temperatures (20 and 25 ºC) on population growth and competition of B. havanaensis and B. calyciflorus using 0.5 × 106 cells ml−1 of Chlorella vulgaris as food. We hypothesized that the higher MPs concentration would adversely affect the growth rate of the rotifers. Also, Brachionus calyciflorus, due to its higher growth rate, would be able to outcompete Brachionus havanaensis.
Material and methods
Culture of alga and rotifers
We obtained the rotifers Brachionus calyciflorus, Pallas, 1766 and Brachionus havanaensis, Rousselet, 1911 from a permanent waterbody, Lake Xochimilco (Mexico City, Mexico) and separately established a clonal population for each species under laboratory conditions. We used re-constituted moderately hardwater (here after called the EPA medium) for culturing rotifers. The EPA medium was prepared daily by dissolving 96 mg NaHCO3, 60 mg CaSO4, 60 mg MgSO4, and 4 mg KCl in 1 L of distilled water (Weber, 1993). The cultures of each species were maintained separately at 20 and 25 °C before using them for testing. The single-celled green alga Chlorella vulgaris which was used as food for rotifers, was batch-cultured on a defined medium (Bold’s basal) (Borowitzka & Borowitzka, 1988). Log phase alga was harvested, centrifuged and resuspended in a small volume of distilled water and stored at 4 °C until use. The algal density was quantified using a haemocytometer and required concentrations (0.5 × 106 or 1.0 × 106 cells.ml−1) were obtained using EPA medium for dilution for maintaining the cultures or the test individuals in the experiments (0.5 × 106 cells ml−1).
Microplastics
We used 30 µm diameter analytical grade polystyrene microplastic particles (SIGMA-ALDRICH laboratories). A stock solution of 80 mg l−1 was prepared in EPA medium. The stock solution was then sonicated at a frequency of 20 kHz at 10 watts for 3 min to separate the plastic beads.
Population growth
The experiments were set up in glass vials containing 20 ml of EPA medium with three concentrations; control group (0 mg l−1), 10 and 20 mg l−1 of polystyrene beads plus 0.5 × 106 cells ml−1 of C. vulgaris as food. We also set up negative controls with 20 mg l−1 of MPs but without algal food. Each treatment was set up with 4 replicates at two temperatures, 20 and 25 °C for Brachionus calyciflorus and Brachionus havanaensis cultured alone and together. Each test jar received 20 individuals of a mixed—age population, with juveniles (48–72 h old) in equal proportions of either rotifer species.
Following the start of population growth experiments, at every 24 h interval, the number of living individuals of either rotifer species was counted, individually, not by aliquots, and transferred to fresh medium with corresponding MPs and alga. The growth experiments were discontinuted after the populations in each test jar declined.
From the experiments we obtained the peak population density and day to achieve it at each treatment. The rate of population increase (r) was calculated based on a regression between the log natural-transformed population density vs time for each replicate (Princeé, 2016).
We statistically quantified the differences among the treatments for each variable using a two-way ANOVA, followed by Tukey post-hoc tests using SigmaPlot (ver. 11).
Results
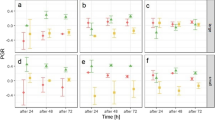
Population growth of Brachionus calyciflorus grown at 20 °C and 25 °C in controls and in the presence of microplastics and in competition with B. havanaensis showed typical growth curves of a short initial phase, a long (ca. 7 days) exponential phase followed by a declinin the population (< a week) (Fig. 1). The presence of the competitor greatly reduced the population growth of B. calyciflorus. An adverse effect of the presence of microplastics was also evident.
Brachionus calyciflorus had a population growth rate of 0.20 to 0.38 d−1 in the presence of the competitor and the microplastics. There was a significant impact of the presence of microplastics, the competitor, and the interaction of both of these factors on the population growth rate of B. calyciflorus at 20 °C (P < 0.01, 2-way ANOVA, Table 1). At 25 °C, there was a significant impact of the presence of the microplastics (P < 0.01, 2-way ANOVA, Table 1) but not on the presence of the competitor (P > 0.05, 2-way ANOVA, Table 1). There was a significant decrease (P < 0.05, Tukey posthoc test) in the population growth of B. calyciflorus in the presence of microplastics as compared to controls when cultured alone at both test temperatures (Fig. 2).
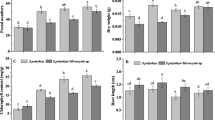
Population growth rate (d−1), peak population density (ind. ml−1), and day of peak population density of Brachionus calyciflorus alone (black bars) and with Brachionus havanaensis as competitor (gray bars) with different microplastic beads concentrations (10 and 20 mg l.−1) and controls without microplastics at 20 and 25 °C. Data bars carrying different alphabets represent significant differences among treatments (P < 0.05, two-way ANOVA and Tukey post-hoc tests)
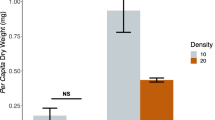
The peak population density of B. calyciflorus ranged between 5 and 9 ind. ml−1 at 20 °C and between 6 and 11 ind. ml−1 at 25 °C (Fig. 2). This variable was significantly lower, at both test temperatures, in the presence of microplastics and the competitor (P < 0.01, 2-way ANOVA, Table 1) with the exception of 20 mg L−1 microplastics at 20 °C where no significant difference was observed (P > 0.05, Tukey post-hoc test).
The day at which the peak population density (Fig. 2) was observed ranged between 7 and 11 at 20 °C and was not significantly different due to the presence of the microplastics or the competitor (P > 0.05, 2-way ANOVA, Table 1). At 25 °C, however, B. calyciflorus reached a peak population density significantly earlier in the presence of the competitor and the microplastics (P < 0.05, Tukey post-hoc test).
As in the case of B. calyciflorus, B. havanaensis showed growth curves with the three standard phases. In the presence of the competitor (B. calyciflorus), the population growth of B. havanaensis decreased in most treatments (Fig. 3).
The growth rate of B. havanaensis ranged between 0.28 and 0.43 d−1 at 20 °C and 0.35 and 0.49 d−1 at 25 °C (Fig. 4). There was a significant impact (P < 0.01) due to the presence of the competitor but not the microplastics (P > 0.05, 2-way ANOVA, Table 2) at 20 °C. On the other hand, there was a significant effect of the presence of the competitor and the microplastics (P < 0.05, 2-way ANOVA, Table 2) at 25 °C. The combined effects of the MPs and the competitor resulted in significantly lower growth rates (P < 0.05, Tukey post-hoc test) only at 20 °C.
Population growth rate (d−1), peak population density (ind. ml−1), and day of peak population density of Brachionus havanaensis alone (black bars) and with Brachionus calyciflorus as competitor (gray bars) with two concentrations (10 and 20 mg l.−1) of microplastics and controls without microplastics at 20 and 25 °C. Data bars carrying different alphabets represent significant differences among treatments (P < 0.05, two-way ANOVA and Tukey post-hoc tests)
The peak population density of B. havanaensis at 20 °C and 25 °C ranged from 8 to 23 ind. ml−1, respectively (Fig. 4). There was a significant effect of the presence of the microplastics and the competitor on this variable at both the temperatures (P < 0.05, 2-way ANOVA, Table 2). The adverse impact of the presence of the competitor was more evident at 20 than at 25 °C (P < 0.05, Tukey post-hoc test).
The day at which B. havanaensis reached the peak population density ranged between 5 to 12 at the temperatures tested (Fig. 4). It was significantly different in relation to the presence of microplastics and the competitor at both test temperatures (P < 0.05, 2-way ANOVA, Table 2). The day at which the peak population density was observed was significantly later due to the presence of the competitor, especially at 25 °C (P < 0.05, Tukey post-hoc test).
Discussion
The size of the microplastic beads used in this study are within the size range that B. calyciflorus can ingest but larger than B. havanaensis can feed on (Monakov, 2003). In several tropical water bodies algal blooms are common. These are often dominated by prokaryotes and eukaryotes > 30 µm in size. Previous studies indicate that B. calyciflorus is also capable of ingesting cyanobacteria and algae of > 30 µm in size (Starkweather, 1981; Monakov, 2003; Soares et al., 2010). Here we observed that the adverse effect of competition was greater on B. calyciflorus while the adverse effect due to the presence of microplastics was greater on B. havanaensis.
Our study showed microplastics, larger than the preferred edible algal size, can affect rotifers without entering the zooplankton gut. We also found that the presence of microplastics resulted in different growth patterns, depending on the temperature, for either rotifer species. B. calyciflorus reached lower densities at 20 °C as compared to 25 °C. On the other hand, B. havanaensis was adversely affected due to microplastics at the higher test concentration only (20 mg l−1). These results probably reflect the effect of interference in grazing by the rotifers due to the presence of the microplastics. Zooplankton feeding is often inhibited due the presence of large sized phytoplankton in the medium. Gliwicz and Siedlar (1980) show that cladocerans reduce their carapace gape in the presence of large phytoplankton. The response of rotifers to the presence of large sized interfering particles is known to vary. Gilbert (1990) showed that the feeding of small rotifers such as Keratella and Synchaeta on Cryptomonas was unaffected due to the presence of the filamentous cyanobacterium Anabaena affinis. We too observed that the population growth rate of the small B. havanaensis was unaffected due to the presence of the microplastics. On the other hand, Rothhaupt (1990) has shown that rotifer feeding is inhibited in the presence of large (> 18 μm) particles. Drago and Weithoff (2021) also found that B. calyciflorus and B. fernandoi did not actually ingest microplastics > 5 μm, but the presence of these particles affected the growth rates of rotifers especially at lower food concentrations.
Our findings indicate that larger B. calyciflorus was more adversely affected than the smaller B. havanaensis. Field studies show that in the presence of large, interefering particles such as cyanobacterial colonies, B. havananensis is more abundant than B. calyciflorus (Gayosso-Morales et al., 2017). Microplastics in natural systems are rarely rounded particles, as tested here; they are more often fibrous or amorphous. It is therefore, quite likely that the presence of these particles will favour the presence of smaller rather than larger zooplankton.
In this study B. calyciflorus reached lower population densities than B. havanaensis. Previous studies also report that smaller bodied rotifers have lower threshold food concentrations and can reach higher population densities, as compared to larger taxa (Sarma et al., 2008; Gilbert, 2022). However, in competiton experiments, due to their capacity to better exploit the resources, in short term observations, larger species outcompete smaller ones. Here we observed that the percentage decline, with regard to temperature (20 and 25 °C), in the population of B. havanaensis was less (9 and 10%, respectively) than that of B. calyciflorus (37 and 25%, respectively) in the presence of the competitor. The increased susceptibility of B. calyciflorus to the presence of microplastics may also be due to the greater energy consumption of the species with higher swimming speeds as compared to B. havanaensis; further studies are needed to corroborate these hypotheses.
The adverse effects of lower concentration of the microplastics was more evident at 20 °C for both species. Our observations indicate that more than 90% of the microplastics sedimented within 8 h of setting up the experiment. Nevertheless, at the higher concentration of MPs, B. calyciflorus was not affected adversely, while B. havanaensis was. Our results show that the presence of microplastics may alter the outcome of competition among small and large zooplankton in natural communites, and thereby interfering with the size structure of planktonic communities.
The effect of environmental variables on the metabolic rates of rotifers are rarely studied (Miracle & Serra, 1989). However, inferences can be made from the available literature on the effect of biotic and abiotic factors on the demographic patterns of rotifers. In general, and especially for B. calyciflorus, several factors show a Q10 ≥ 2 which indicates an increased metabolic activity with a rise in temperature (Halsey et al., 2015). Here too, we observed an adverse effect due to the presence of the microplastics, competitor or both on the growth of either test species; the effect was greater on B. calyciflorus than on B. havanaensis. The time taken for the population to double the initial density was more in the case of B. calyciflorus than for B. havanaensis, especially in the presence of the competitor. Our personal observations also indicate that B. calyciflorus withstands starvation for 4 days at 20 °C and for 5 days at 25 °C; on the other hand, B. havanaensis can tolerate starvation for a longer period, 8 and 9 days, respective to the temperature. These factors explain the improved tolerance of B. havanaensis to the presence of the microplastics and the competitor. Thus, a synergistic effect of contaminants and interspecific interactions will result in changes in the community structure. Further studies on the effect of abiotic and biotic factors on the metabolic rates of rotifers will improve our understanding of these results.
Conclusions
Our study showed that microplastics caused adverse effects on the population growth and competitive outcome between two brachionid rotifers. Microplastics interacted with temperature and further caused adverse effects on the peak population density and rate of population increase of B. calyciflorus and B. havanaensis, separately and in mixed cultures. The adverse effects of microplastics were more severe for B. havanaensis than for B. calyciflorus, while competition had a greater adverse effect on B. calyciflorus. This study indicates that multiple stressors have different effects on species and will induce changes in zooplankton community structure in aquatic ecosystems.
References
Altshuler, I., B. Demiri, S. Xu, A. Constantin, N. D. Yan & M. E. Cristescu, 2011. An integrated multi-disciplinary approach for studying multiple stressors in freshwater ecosystems: Daphnia as a model organism. Integrative and Comparative Biology 51: 623–633. https://doi.org/10.1093/icb/icr103.
Amaral-Zettler, L. A., T. Ballerini, E. R. Zettler, A. A. Asbun, A. Adame, R. Casotti, B. Dumontet, V. Donnarumma, J. C. Engelmann, L. Frère, J. Mansui, M. Philippon, L. Pietrelli & M. Sighicelli, 2021. Diversity and predicted inter- and intra-domain interactions in the Mediterranean plastisphere. Environmental Pollution 286: 117439.
Borowitzka, M. A. & L. J. Borowitzka, 1988. Micro-algal biotechnology, Cambridge University Press, London:
Drago, C. & G. Weithoff, 2021. Variable fitness response of two rotifer species exposed to microplastics particles: the role of food quantity and quality. Toxics 9: 1–13.
Ejsmont-Karabin, J., 2012. The usefulness of zooplankton as lake ecosystem indicators: rotifer trophic state index. Polish Journal of Ecology 60: 339–350.
Gambardella, C., S. Morgana, M. Bramini, A. Rotini, L. Manfra, L. Migliore, V. Piazza, F. Garaventa & M. Faimali, 2018. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Marine Environmental Research 141: 313–321. https://doi.org/10.1016/j.marenvres.2018.09.023.
Gao, Y., Q. F. Liu, Y. T. Zheng, Z. N. Lai, C. Wang, Y. Y. Zeng & W. L. Yang, 2021. Effects of temperature, food concentration, and initial rotifer density on the interspecific competition between two rotifer species. Applied Ecology and Environmental Research 19: 4151–4168.
Gayosso-Morales, M. A., S. Nandini, F. F. Martínez-Jeronimo & S. S. S. Sarma, 2017. Effect of organic and inorganic turbidity on the zooplankton community structure of a shallow waterbody in Central Mexico (Lake Xochimilco, Mexico). Journal of Environmental Biology 38: 1183–1196.
Gilbert, J. J., 1990. Differential effects of Anabena affinis on cladocerans and rotifers: mechanisms and implications. Ecology 71: 1727–1740.
Gilbert, J. J., 2022. Food niches of planktonic rotifers : diversification and implications. Limnology and Oceanography 67: 1–34.
Gilbert, J. J. & P. L. Starkweather, 1977. Feeding in the rotifer Brachionus calyciflorus - I Regulatory mechanisms. Oecologia 28: 125–131.
Gliwicz, Z. & E. Siedlar, 1980. Food size limitation and algae interfering with food collection in Daphnia. Arch Hydrobiol 88: 155–177.
Guilhermino, L., A. Martins, S. Cunha & J. O. Fernandes, 2021. Long-term adverse effects of microplastics on Daphnia magna reproduction and population growth rate at increased water temperature and light intensity: combined effects of stressors and interactions. Science of the Total Environment 784: 147082. https://doi.org/10.1016/j.scitotenv.2021.147082.
Halsey, L. G., P. G. Matthews, E. L. Rezende, L. Chauvaud & A. A. Robson, 2015. The interactions between temperature and activity levels in driving metabolic rate: theory, with empirical validation from contrasting ectotherms. Oecologia 177: 1117–1129. https://doi.org/10.1007/s00442-014-3190-5.
Kirk, K. L., 2002. Competition in variable environments: experiments with planktonic rotifers. Freshwater Biology 47: 1089–1096.
Krebs, C. J., 1985. Ecology: The Experimental Analysis of Distribution and Abundance, Addison-Wesley, Boston, USA:
Lin, L., L. Z. Zuo, J. P. Peng, L. Q. Cai, L. Fok, Y. Yan, H. X. Li & X. R. Xu, 2018. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Science of the Total Environment 644: 375–381. https://doi.org/10.1016/j.scitotenv.2018.06.327.
Mason, S. A., L. Kammin, M. Eriksen, G. Aleid, S. Wilson, C. Box, N. Williamson & A. Riley, 2016. Pelagic plastic pollution within the surface waters of Lake Michigan, USA. Journal of Great Lakes Research 42: 753–759. https://doi.org/10.1016/j.jglr.2016.05.009.
Miracle, M. R. & M. Serra, 1989. Salinity and temperature influence in rotifer life history. Hydrobiologia 186: 81–102.
Monakov, A.V., 2003. Feeding of freshwater invertebrates. Kenobi productions, Ghent, Belgium
Moreno-Gutiérrez, R. M., S. S. S. Sarma, A. S. Sobrino-figueroa & S. Nandini, 2018. Population growth of rotifers from a high altitude eutrophic waterbody, Madín reservoir (State of Mexico, Mexico): the importance of seasonal sampling. Journal of Limnology 77(3): 441–451.
Moyo, S., 2022. An enigma: a meta-analysis reveals the effect of ubiquitous microplastics on different taxa in aquatic systems. Frontiers in Environmental Science 10: 1–17.
Nava, V., S. Chandra, J. Aherne, et al., 2023. Plastic debris in lakes and reservoirs. Nature 619: 317–322. https://doi.org/10.1038/s41586-023-06168-4.
Nugnes, R., M. Lavorgna, E. Orlo, C. Russo & M. Isidori, 2022. Toxic impact of polystyrene microplastic particles in freshwater organisms. Chemosphere 299: 134373. https://doi.org/10.1016/j.chemosphere.2022.134373.
Pestana, C. J., D. S. Moura, J. Capelo-Neto, C. Edwards, D. Dreisbach, B. Spengler & L. A. Lawton, 2021. Potentially poisonous plastic particles: microplastics as a vector for cyanobacterial toxins microcystin-LR and microcystin-LF. Environmental Science and Technology 55: 15940–15949.
Preston, B. L., 2002. Indirect effects in aquatic ecotoxicology: implications for ecological risk assessment. Environmental Management 29: 311–323.
Preston, B. L. & T. W. Snell, 2001. Direct and indirect effects of sublethal toxicant exposure on population dynamics of freshwater rotifers: a modeling approach. Aquatic Toxicology 52: 87–99.
Princée, F. P. G., 2016. Exploring Studbooks for Wildlife Management and Conservation, Springer, Cham:, 291.
Rothhaupt, K. O., 1990. Differences in particle size-dependent feeding efficiencies of closely related rotifer species. Limnology and Oceanography 35: 16–23.
Sarma, S. S. S., F. Martínez-Jerónimo, T. Ramírez-Pérez & S. Nandini, 2006. Effect of cadmium and chromium toxicity on the demography and population growth of Brachionus calyciflorus and Brachionus patulus (Rotifera). Journal of Environmental Science and Health - Part A Toxic/hazardous Substances and Environmental Engineering 41: 543–558.
Sarma, S. S. S., J. L. Franco-Téllez & S. Nandini, 2008. Effect of algal food (Chlorella vulgaris) concentration and inoculation density on the competition among three planktonic Brachionidae (Rotifera: Monogononta). Hidrobiologica 18: 123–132.
Setälä, O., M. Lehtiniemi, R. Coppock & M. Cole, 2018. Chapter 11. Microplastics in marine food webs. In Zeng, E. Y. (ed), Microplastic Contamination in Aquatic Environments An Emerging Matter of Environmental Urgency Elsevier, Amsterdam: 339–363.
Sinclair, J. S. & S. E. Arnott, 2015. Effects of an invasive consumer on zooplankton communities are unaltered by nutrient inputs. Freshwater Biology 60: 161–173.
Snell, T. W. & C. Joaquim-Justo, 2007. Workshop on rotifers in ecotoxicology. Hydrobiologia 593: 227–232.
Soares, M. C. S., M. Lürling & V. L. M. Huszar, 2010. Responses of the rotifer Brachionus calyciflorus to two tropical toxic cyanobacteria (Cylindrospermopsis raciborskii and Microcystis aeruginosa) in pure and mixed diets with green algae. Journal of Plankton Research 32: 999–1008. https://doi.org/10.1093/plankt/fbq042.
Eo, S., S.H. Hong, Y. K. Song, Han, G. M. & Shim, W. J., 2019. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Research 160: 228–237. https://doi.org/10.1016/j.watres.2019.05.053.
Starkweather, P. L., 1981. Trophic relationships between the rotifer Brachionus calyciflorus and the blue-green alga Anabaena flos-aquae. Verh Int Ver Limnol 21: 1507–1514.
Su, L., X. Yingang, L. Lingyun, Y. Dongqi, P. Kolandhasamy, D. Li & H. Shi, 2016. Microplastics in Taihu Lake, China. Environmental Pollution 216: 711–719. https://doi.org/10.1016/j.envpol.2016.06.036.
Sun, Y., W. Xu, Q. Gu, Y. Chen, Q. Zhou, L. Zhang, L. Gu, Y. Huang, K. Lyu & Z. Yang, 2019. Small-sized microplastics negatively affect rotifers: changes in the key life-history traits and rotifer- Phaeocystis population dynamics. Environmental Science and Technology 53: 9241–9251.
Wagner, M. & S. Lambert, 2018. Freshwater Microplastics. Emergening Environmental Contaminants? Handbook of Environmental Chemistry, Vol. 58. Springer, New York:
Wallace, R. L., 2002. Rotifers: exquisite metazoans. Integrative and Comparative Biology 42: 660–667.
Weber, C. I., 1993. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater Marine Organisms. Environmental Protection Agency, Cincinnati, Ohio. EPA/600/4–90/027 4th edn.
Xue, Y. H., Z. X. Sun, L. S. Feng, T. Jin, J. C. Xing & X. L. Wen, 2021. Algal density affects the influences of polyethylene microplastics on the freshwater rotifer Brachionus calyciflorus. Chemosphere 270: 128613.
Zhao, S., L. Zhu, T. Wang & D. Li, 2014. Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Marine Pollution Bulletin 86: 562–568. https://doi.org/10.1016/j.marpolbul.2014.06.032.
Acknowledgements
MCRS thanks Posgrado en Ciencias del Mar y Limnología, UNAM and CONAHCyT for the PhD scholarship CVU 621703; SN and SSSS thank CONAHCyT (20520 and 18723, respectively).
Funding
This work was supported by PAPIIT IG200820, IN208023 and IN208223.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Authors declare that they have no competing interests and funding.
Ethical approval
Authors declare that the work presented here fully obeys the ethical guidelines established by our university.
Additional information
Guest editors: Maria Špoljar, Diego Fontaneto, Elizabeth J. Walsh & Natalia Kuczyńska-Kippen / Diverse Rotifers in Diverse Ecosystems
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reyes-Santillán, M.C., Nandini, S. & Sarma, S.S.S. Combined effects of microplastics and temperature on the competition between Brachionus havanaensis and Brachionus calyciflorus (Rotifera). Hydrobiologia 851, 3199–3211 (2024). https://doi.org/10.1007/s10750-023-05410-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05410-4