Abstract
Here, we studied the influence of changes of aquatic phases (standing and flowing phases) and human-induced habitat variability (natural and artificial) on the composition and diversity of benthic diatom assemblages in a small lowland stream in the Pannonian Ecoregion. Significant differences in composition were hypothesized between phases and habitats. Lower diversity was hypothesized in the flowing phase and in the artificial habitat. In addition, worser ecological status were assumed in the artificial habitat and in the standing phase than in the others. Our results only partially supported our hypotheses. While there was no significant difference in the composition of the assemblages between the natural and concreted habitats, the alteration in flow conditions resulted in a significant change. No significant differences in diversity were found between aquatic phases. In contrast, biodiversity was higher in the artificial habitat than in the natural one. While the anthropogenic impact, i.e., concreted streambed has no significant influence on diatom-based ecological status, values of diatom indices were significantly higher in the flowing phase. Our results highlight that extreme weather events play a major role in shaping diatom assemblages even during a short period, which should be taken into account in water management and nature conservation measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monitoring the inevitable changes in ecosystems caused by climatic extremes and actively responding to the effect of these extremes are one of the most important ecological, economic and social challenges of the century. Freshwater ecosystems are especially threatened by the consequences of climate change, such as flash floods, drastic decrease in water levels, even drying up of watercourses (Várbíró et al., 2020; Messager et al., 2021; Tornés et al., 2021). It has now been proved that intermittent watercourses, i.e., those that are dry for at least one day a year, occur on Earth as often as permanent streams (Messager et al., 2021). Worldwide, approximately 50–60% of watercourses can be considered intermittent (Messager et al., 2021), in the case of small streams, however, this value can exceed 70% (Datry et al., 2014) and their numbers are predicted to increase globally in the coming decades (Pumo et al., 2016).
Benthic diatoms play a pivotal role in the primary production of small watercourses (B-Béres et al., 2022a), which is especially true for intermittent streams in extremely low water level or drying conditions (Robson et al., 2008; Robson & Matthews, 2004). However, drying results in significant structural changes in the phytobenthic community (Tornés et al., 2021). Benthic diatom composition occurring under different hydrological conditions of intermittent streams, such as flowing, pool/standing, and drying phases (Gallart et al., 2017), is strongly dependent on the duration of drought (no precipitation) and drying (no water in the streambed) periods (Acuña et al., 2017; Sabater et al., 2017; Tornés et al., 2021). As flow and water level decrease, lentic taxa appear more and more often within the assemblages (Stubbington et al., 2017), and their abundance is controlled by the duration of low flow conditions before the actual drying (Tornés et al., 2021). In addition, changes in assemblages during the pool phase are mainly influenced by factors such as taxa composition before the drought period, the lifespan of low- or no-flow pools (Magand et al., 2020; Várbíró et al., 2020; Novais et al., 2020), grazing pressure by macroinvertebrates (Fisher & Dunbar, 2007), and the alternation of physical and chemical environments (e.g., increasing temperature and conductivity; decreasing pH and dissolved oxygen; Magand et al., 2020). In the drying phase, water scarcity is obviously the main factor in shaping of biofilm composition and results in increasing number of taxa, which are able to survive these harsh conditions (e.g., terrestrial species) (Timoner et al., 2014; Sabater et al., 2016, 2017; Stubbington et al., 2017).
The structural changes occurring during water scarcity also have a significant impact on biodiversity. However, our knowledge of the relationship between diversity and water intermittency is controversial. To resolve the contradictions, the spatial and temporal extent of diversity change must be examined. Recent studies highlight that biodiversity decreases, if the drying lasts for a long time (> 100 days; Tornés et al., 2021). The longer the dry period and the more watercourses are affected in the catchments, the more likely it is that the negative local effects will manifest at regional and then global level (Pumo et al., 2016; Acuña et al., 2017). It is also important to mention that biodiversity does not decrease linearly during drying. In the standing phase, the appearance of lentic species can even increase the diversity of the assemblages. In addition, newly immigrating aerophilic taxa can also contribute to higher diversity at the beginning of the drying phase (Sabater et al., 2016, 2017). However, recent studies point out that the longer the drying lasts, the higher the number of taxa and traits that disappear from the assemblages (Stubbington et al., 2017; Crabot et al., 2021; Tornés et al., 2021).
The diatom indices currently used to assess the diatom-based ecological status of watercourses were developed for permanent waters and are used to evaluate the quality of intermittent streams during flowing phase in many countries (e.g., EU member states) (Stubbington et al., 2019; Magand et al., 2020). Nowadays, however, there is an urgent need to decide whether these indices are also suitable for assessing the ecological status of streams during pool or dry phases. Up-to-date studies suggest that they can evaluate the effect of drying up and the accompanying environmental changes on the diatom-based ecological status of streams (Novais et al., 2020; B-Béres et al., 2022a, b).
There are a total of ~ 10,000 watercourses in Hungary, of which only nearly 10% are regularly examined within the framework of the National Monitoring Program (Stubbington et al., 2018; B-Béres et al., 2022b). At the same time, more than 30% of these waters already belong to the intermittent category, but currently only small streams can be classified into this type. The areas most affected by drought and drying are situated in the Great Hungarian Plain (River Basin Management Plan Report Suppl.1.1, 2022). These small streams, however, are not only threatened by drying, but are also affected by many other natural and artificial impacts that can result in short- or longer-term habitat changes. Artificial modifications can be found, for example, in streambed sections before and after bridges, which are fully concreted or covered with hollow concrete slabs. These streambed modifications can significantly affect hydrological conditions (e.g., water velocity), which can result in silt or sand deposition and ultimately changes in physical and chemical parameters (Gál et al., 2020) and the deterioration of ecological quality (Wemple et al., 2018). Our knowledge is, however, still limited of how these stream sections with partially or completely different habitats and environment contribute to the structural stability and biodiversity of aquatic biota (macroinvertebrates—Gál et al., 2020; fish—Wellman et al., 2000). As for diatom assemblages of intermittent streams, only changes caused by natural habitat differences are known so far (Witteveen et al., 2020).
The aim of our investigation was to study the structural and diversity changes in the benthic diatom assemblages of an intermittent lowland stream in the Great Hungarian Plain during different aquatic phases (flowing and standing phases) at two sampling sites, which are located very close to each other, but they differ significantly in their habitats (natural and artificial habitats). We also studied how changes in hydrological conditions and microhabitats influence the diatom-based ecological status of the stream.
We formulated the following hypotheses:
(H1)
Environmental variables—Aquatic phases and anthropogenic effects strongly influence the physical and chemical characteristics of the stream.
(H2)
Assemblages’ composition—Significant differences in composition are hypothesized between both aquatic phases and habitats.
(H3)
Biodiversity—Biodiversity is higher in standing phase than in flowing phase, while artificial habitat maintains less diversity than the natural one.
(H4)
Diatom indices and diatom-based quality—Both drought and anthropogenic impact result in decreasing diatom-based quality, thus significantly lower values of indices can be expected in standing phase and artificial habitat than in the other cases.
Materials and methods
Study area, sampling setup, and environmental parameters
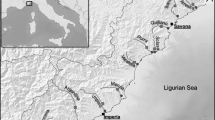
Altogether 24 diatom samples were collected from two sites of a small, intermittent lowland stream (Létai-ér) in the Pannonian Ecoregion (Eastern Hungary) (Fig. 1; Supplementary Fig. 1). The studied stream passes through mostly agricultural areas, pastures, and villages. Létai-ér belongs to the R04 Broad type (Solheim et al., 2019): the total length of this stream is 38.4 km, its catchment area is 157.4 km2, the bedrock is calcareous, and the sediment size is medium to fine (River Basin Management Plan Report Suppl.1.1, 2022). The stream shape is winding, the macrovegetation cover is significant, and the characteristic taxa are mostly Glyceria maxima (Hartm.) Holmb. (great manna grass), Lemna L. spp. (duckweed), Ceratophylum demersum L. (hornwort), Sparganium erectum L. (simplestem bur-reed), Berula erecta (Huds.) Coville (lesser water-parsnip), Phragmites australis (Cav.) Trin. ex Steud. (common reed), and Mentha L. spp. at the studied section.
The location of Hungary in Europe (a) and the location of study area (Létai-ér, Monostorpályi) in Eastern Hungary (b). The sampling sites (c): The solid line represents the natural part of the stream, and the black circle represents the sampling site here, while dashed line represents the artificial part of the stream and white square represents the sampling site here
Total annual precipitation was 568.85 mm in 2020 in the region (Supplementary Table 1), which corresponds to the value for the country (500–800 mm/year; The OMSZ’ General Review of the Climate of Hungary). However, there was a significant difference in the distribution of precipitation between months: While early/mid-summer (June and July) was the wettest period in 2020, April and November were the driest ones (Supplementary Table 1). But it has to be emphasized that the daily distribution of precipitation was not uniform in June and in July, more than 70% of the total monthly precipitation fell on the area in 5 days in June and 3 days in July. As for the air temperature, its average daily value was the lowest in January, while the highest in August (Supplementary Table 2). Based on these data it is clear there was a strong positive correlation between air temperature and monthly precipitation in the region (P = 0.037).
In 2020, benthic diatom and surface water samples were collected approximately every four weeks (from 06.01.2020 to 17.12.2020). Altogether 12 and 12 samples were taken from two different habitats, which are close to each other: one natural (47° 23′ 20.2″ N; 21° 46′ 23.7″ E) and one artificial (47° 23′ 19.6″ N; 21° 46′ 23.8″ E). The distance between the habitats is about 20–30 m (Fig. 1). In October, the water depth in the natural habitat was so low that surface water sample could not be taken, so the number of samples for measuring physical and chemical parameters is not equal in the two habitats (11 in natural habitat and 12 in artificial habitat). The features of the natural habitat, e.g., macrophyte cover, bedrock, sediment size, are the same as the general characteristics of the stream itself. In contrast, the artificial habitat is concreted, the sides of the streambed (littoral zone) are covered by hollow concrete slabs. The sediment is fine mud and pebbles. There are no emergent macrophytes in streambed, while duckweed and green algal filaments are common in summer.
To describe the hydrological conditions of the stream during the sampling year, we used the classification system introduced by Gallart et al. (2017). According to this system, two aquatic phases could be distinguished: flowing phase and standing/pool phase. The flowing phase is characterized by copious flow or at least scarce flow conditions, in contrast, there is no water flow in standing/pool phase (Magand et al., 2020). While 5 flowing phase and 7 standing phase events were observed in the natural habitat, the opposite was the case in artificial habitat (7 flowing phase events and 5 standing phase events) (Supplementary Table 3). It means that a total of 12 flowing phase and 12 standing phase events could be detected in 2020.
A total of 18 physical and chemical parameters were measured during samplings. Water temperature (T in °C), dissolved oxygen (O2 in mg L−1), oxygen saturation (O2%), pH, oxidation–reduction potential (ORP in mV), conductivity (COND in µS cm−1) were measured in the field with a portable multi-parameter digital meter (HQ30d, Germany). In addition, water samples were collected for further laboratory analyses. The samples were stored at 4 °C in a cooler bag during transportation to the laboratory. Total suspended solids (TSS in mg L−1—MSZ 12750-6, 1971) and total dissolved solids (TDS in mg L−1—Németh, 1998) were measured according to national standard and proposal. Biological oxygen demand (BOD in mg L−1—MSZ EN 1899-2, 2000), chemical oxygen demand (CODMn in g m−3—Felföldy, 1987), and chlorophyll-a content (Chl-a in µg L−1—Felföldy, 1987) were measured according to international standard and national proposal. Nutrient, silicate, and chloride contents were determined according to international and national standards (NO3−–N in mg L−1—MSZ 1484-13, 2009; NO2−–N in mg L−1—MSZ 1484-13, 2009; NH4+–N in mg L−1—MSZ ISO 7150-1, 1992; PO43−–P in mg L−1—MSZ EN ISO 6878, 2004; SO42− in mg L−1—MSZ 448-13, 1983; Si in mg L−1—ASTM D859-00, 2000 and Cl− in mg L−1—MSZ 1484-15, 2009). The assessment of water quality based on physical and chemical parameters was done according to the 3rd River Basin Management Plan Report (River Basin Management Plan Report Suppl.6.3, 2022).
Diatom sample collection, preparation, and identification
Sampling and preservation of benthic diatoms were done according to international standard (EN 13946, 2014). Benthic diatom samples were collected from submerged and emergent macrophytes in the natural habitat, while pebbles and green algal filaments (in summer) were used as substrate in the artificial (concreted) habitat. At least five stems (emergent) and five entire plants (submerged) or five pebbles were sampled. Diatom samples were preserved on the field with acetate-free Lugol’s solution. According to international standard (EN 13946, 2014), the hot hydrogen-peroxide method was used for digestion of organic matters and Naphrax resin was used for embedding. At least 400 valves per sample were recorded and identified at least to species level using Leica DMRB light microscope at 1000–1600× magnification (EN 14407, 2014). Literature used for the identification of taxa was Krammer and Lange-Bertalot (1997a, 1997b, 2004a, 2004b), Hofmann et al. (2006), Bey and Ector (2013), Potapova and Hamilton (2007) and Stenger-Kovács and Lengyel (2015). Names of taxa were updated using the database of AlgaeBase (Guiry & Guiry, 2023).
Data processing and analyses
Diatom taxa were classified into six traits with a total of 22 categories: length–width (L/W) ratio (6 categories) according to Stenger-Kovács et al. (2018), cell size (5 categories) according to Berthon et al. (2011), attachment types (3 categories) according to Lange et al. (2016), life forms, guilds, and pioneer character (3, 4, 1 categories, respectively) according to Rimet and Bouchez (2012) (Supplementary Table 4).
Taxa richness (Taxa_S), effective Shannon’s H (effSH; Jost, 2006; Chao et al., 2014) and Rao’s quadratic entropy (FDQ; Rao, 1982) were used to measure diversity. Taxa richness and Shannon’s H were calculated using Past software (version 4.12; Hammer et al., 2001). Effective Shannon’s H is the exponential of Shannon’s H. The functional diversity metric, Rao’s quadratic entropy, is a suitable metric for multi-trait analysis (Botta-Dukát, 2005). It was calculated using CANOCO 5.0 software package (Šmilauer & Lepš, 2014).
To assess the diatom-based ecological quality of the stream, four diatom indices were calculated: Specific Pollution Sensitivity index (IPS; Coste, 1982), Rott’s Saprobic Index (SID; Rott et al., 1997), Rott’s Trophic Index (TID; Rott et al., 1999), and the Hungarian Phytobenthos Metric (IPSITI; Várbíró et al., 2012). While diatom indices IPS, SID, and TID were calculated using the OMNIDIA software (Version 5.2; Lecointe et al., 2003), the ISPITI is the mean of the previously mentioned indices. The final value of these indices varies between 1to 20, where 1 indicates the worst and 20 represents the best quality.
Normality was tested by Shapiro–Wilk test. For testing significant differences in physical and chemical parameters between aquatic phases and habitats, unpaired t test (normal distribution data) or Mann–Whitney test (non-normal distribution data) were used. Monte Carlo permutation test (default 499 permutation) were performed to decide whether the detected pattern was significantly different from random. All these analyses were performed using Past software (version 4.12; Hammer et al., 2001).
To assess the impact of aquatic phases and habitats on the taxonomic and functional composition, non-metric multidimensional scaling (NMDS; Canoco 5.0; Šmilauer & Lepš, 2014) was applied using the relative abundance of taxa and the trait community weighted mean (CWM) matrices. To create the CWM matrix, average values of traits in the assemblages were weighted by the relative abundances of the taxa matrix. Permutational multivariate analysis of variance (PERMANOVA) was performed on the community matrix after NMDS analyses to test for statistically significant differences in terms of composition (Anderson, 2001). To identify the indicative species and traits of aquatic phases and habitats, indicator value (IndVal) analyses based on the relative abundances of taxa and CWM of traits were performed (Dufrêne & Legendre, 1997) using Past software (version 4.12; Hammer et al., 2001).
To compare the values of diversity metrics and diatom indices between aquatic phases and habitats, unpaired t test was used (Past software, version 4.12; Hammer et al., 2001). The fixed factors were the phases (flowing vs. standing) and the habitats (natural vs. artificial) and the dependent variables were Taxa_S, effSH, and FDQ as diversity metrics and IPS, SID, TID, and IPSITI as diatom indices.
Results
Environmental background
Altogether 18 environmental variables were involved in the analyses (Supplementary Table 5). Although the Létai-ér is in good condition based the annual average data of pH, conductivity, biological oxygen demand, dissolved oxygen, chloride, and inorganic nitrogen content, its phosphate concentration is very high (poor quality) (Table 1). The oxygen balance of the stream deteriorated significantly in the standing phase (mainly from mid-summer to late autumn); both dissolved oxygen and oxygen saturation were significantly lower during this phase (P < 0.001). Oxygen supply of the stream was strongly negatively related both to precipitation and air temperature (Supplementary Table 6). In contrast, temperature, chemical oxygen demand (COD), and phosphate-phosphorus content were significantly higher in standing phase (P < 0.005 and P < 0.05, respectively) (Table 1). Except the COD, these parameters positively correlated with air temperature and precipitation. It is important to stress again, that total amount of precipitation was high in early and mid-summer, however its distribution was clearly not uniform (Supplementary Table 1) due to the heavy rainy days in the region. As for natural and artificial, habitats, the concreting of the streambed did not result in significant differences in the physical and chemical variables (Table 1).
Taxa composition
A total of 151 diatom taxa were identified in the samples, all of them at least to species level (Supplementary Table 7). In flowing phase, altogether 123 taxa were recorded, while 117 taxa were found in standing phase. The number of diatom species that were identified in both phases were 88. As for habitats, a total of 96 and 134 taxa were found in the natural and artificial (concreted) habitats respectively, while altogether 79 taxa were found in both habitats.
According to the taxonomy-based NMDS analyses, the diatom composition showed clear separation between flowing and standing phases (Fig. 2a), while only a slight compositional separation was found between natural and artificial habitats (Fig. 2b). The PERMANOVA analyses revealed a significant difference (P = 0.0006) in taxa composition between phases, while no compositional difference between habitats was found (P = 0.3146). Altogether 16 diatoms, mainly Gomphonema Ehrenberg, Fragilaria Lyngbye and Surirella Turpin species and Meridion circulare (Greville) C.Agardh and Ulnaria danica (Kützing) Compère & Bukhtiyarova were indicative of the flowing phase (Supplementary Fig. 2a; Supplementary Table 8a). In contrast, a total of 9 diatom taxa, Navicula Bory and Nitzschia Hassall sensu lato ssp. and Cocconeis Ehrenberg species were significant indicators in standing phase (Supplementary Fig. 2a; Supplementary Table 8a). Although there were no significant differences in taxa composition between the natural and artificial (concreted) habitats, some species were identified as indicators of these habitats using IndVal analyses (3 and 19 species, accordingly; Supplementary Fig. 2b; Supplementary Table 8b).
Trait composition
Altogether 22 trait categories were involved in the analyses and 21 of them were found in both phases (flowing and standing) and habitats (natural and artificial). The filamentous category, however, was not present in standing phase and in natural habitat.
Similar to the taxa composition, trait-based analyses also revealed a clear separation in the flowing and standing phase composition (Fig. 3a), while there was a strong overlap between habitats (Fig. 3b). These phenomena were confirmed by PERMANOVA analyses, which showed a significant (P = 0.0013) difference in trait structure between phases but no difference (P = 0.5893) between habitats.
While large-sized (S4), rounded (LW2) and moderately elongated (LW4), moderately attached, colonial and high profile guild categories were indicative of flowing phase (Supplementary Fig. 3a, Supplementary Table 9a), round (LW1), slightly and greatly elongated (LW3, LW5), strongly attached, unicellular and low profile categories were characteristic in standing phase (Supplementary Fig. 3a, Supplementary Table 9a). The IndVal analyses did not reveal any indicator trait categories in natural habitat (Supplementary Fig. 3b, Supplementary Table 9b). In contrast, the most elongated category (LW6) was indicative of artificial habitat (Supplementary Fig. 3b, Supplementary Table 9b).
Biodiversity and diatom indices
Although the taxa and trait composition clearly separated due to hydrological conditions, no significant differences were detected in diversity metrics (Taxa S, effSH, FDQ: P > 0.05) (Fig. 4a–c). In contrast, taxa number and effective Shannon’s H were clearly higher in the artificial (concreted) habitat than in the natural ones (P = 0.0098 and P = 0.0375, respectively). Additionally, marginally significant differences were found in FDQ diversity metric, which was higher in artificial habitat (Fig. 4d–f).
In contrast to biodiversity patterns, hydrological regime caused significant differences in the diatom index values (P < 0.01, except of SID where P = 0.097) (Fig. 5a–c), while none of them differed significantly in habitats (P > 0.05) (Fig. 5d–f).
Discussion
Environmental changes
Low water level and low dilution can significantly change physical and chemical parameters in streams and rivers in drought period (extreme lack of precipitation) (Magand et al., 2020). Therefore, we hypothesized differences between flowing and standing phases in abiotic environment. Our results supported this hypothesis (H1). In our study, the water temperature, which is usually influenced by many factors (water depth, shading, TSS concentration, solar radiation, etc.—Brown & Hannah, 2008), was significantly higher in standing phase. The standing phase developed in May and lasted until December in Létai-ér. The lack of canopy cover in the sampling area, as well as the low water level (Magand et al., 2020) and the fact that the Indian summer in 2020 was prolonged in the region (The OMSZ’ Climate Data Series 1901–2020) explain the higher temperature value in standing phase. In contrast, dissolved oxygen and oxygen saturation were lower in this phase. Low flow conditions and higher water temperature reduce oxygen solubility (Padisák, 2005; Magand et al., 2020). In addition, the chemical oxygen demand (COD) was significantly higher in standing phase, which can also contribute to the lower oxygen concentration. As it was mentioned above, an extended macrophyte cover characterizes Létai-ér that results in accumulation of organic materials (OMs) in the streambed. The intense decomposition of these organic materials may be behind the increased COD level. The low or no flow conditions and low dilution capacity also provide optimal environment for the OMs’ accumulation (Magand et al., 2020). Furthermore, the presence of complex polymers can be presumed, since the biological oxygen demand (BOD) did not differ significantly between the aquatic phases. These polymers are difficult to degrade biologically but at the same time are chemically accessible.
Although nutrient contents may change even extremely during drying in intermittent streams (Magand et al., 2020; Novais et al., 2020), in our study only the phosphate concentration differed significantly between the aquatic phases (higher in the standing phase). The increase of phosphate content may be the result of low water levels due to increased evaporation. Other factors, such as less intensive activity of primary producers or surface or subsurface flow inputs, can be excluded because neither chlorophyll-a concentration and BOD values nor concentrations of nitrogen-forms and conductivity differed significantly between phases. Although human activities can also increase the phosphate content of surface waters, such as sewage discharge, we did not observe any signs of this during sampling, and the nearest sewage treatment plant is ~ 8–10 km from the sampling points (River Basin Management Plan Report Suppl.3.1, 2022). That is true, however, that the stream’ catchment is characterized by intensive agriculture and grazing and heavy rains in the early-, and mid-summer may wash nutrients from these areas into the stream.
Road-crossings or culverts have a significant effect on hydrological conditions of streams and greatly contribute to sediment formation. In addition, significantly less macrophyte cover can be characteristic in these artificially modified areas compared to the natural ones (Wemple et al., 2018; Gál et al., 2020). Therefore, we hypothesized a difference in the physical and chemical parameters of natural and artificial habitats. Our results, however, did not prove this assumption (rejects this part of H1). This was probably because both water phases (flowing and standing) were observed in both habitats (natural and artificial) during the study. These phases exerted more pressure on the physical and chemical parameters of the stream than the concreted streambed itself. However, no significant conclusions can be drawn from these results, due to the low sampling effort (one studied stream). In addition, the small distance (i.e., ~ 20–30 m) between the sampling sites may explain the similarity of the environmental parameters, since the flow of water from natural to artificial sites takes only ~ 25 min. (average velocity: 0.02 m s−1—River Basin Management Plan Report Suppl.1.1, 2022). More data are needed to assess the impact of concreting in intermittent streams.
Compositional changes
Extremes in water regime (floods, decreasing water level, drying up—B-Béres et al., 2014; Novais et al., 2020; Tornés et al., 2021) strongly disturb the aquatic assemblages, eventually resulting in the appearance or even dominance of taxa that favor or at least tolerate these altered conditions (Timoner et al., 2014; Sabater et al., 2016, 2017). Here, we hypothesized a significant compositional difference in the diatom assemblages between the aquatic phases and our results supported this hypothesis (H2). Flowing phase was characterized by large sized (S4) and/or rounded (LW2) or moderately elongated (LW4) and/or moderately attached and/or colonial and/or high profile guild taxa. This seems to contradict our previous knowledge, because these traits and taxa bearing them usually refer to intermittent streams in standing phase (Sabater et al., 2016, 2017). It should be emphasized, however, that the studied lowland stream is rich in emergent and submerged macrophytes. These plants provide a suitable substrate for these diatoms, allowing a thick biofilm to form. In addition, the water velocity is not too high in this lowland stream (average 0.02 m s−1—River Basin Management Plan Report Suppl.1.1, 2022), minimizing the physical disturbance caused by intense flow conditions. These environments (thick biofilm and low disturbance) favor colonial, elongated, and/or high profile taxa (Passy, 2007). The colonial, large sized Meridion circulare, which is the member of low profile guild, was also indicative in flowing phase, but was very abundant in the samples (rel. abundance > 5%) only from January to March. This species is known as a characteristic member of small streams in winter and early spring (Stenger-Kovács et al., 2013; B-Béres et al., 2022b). The elongated and planktic Ulnaria danica is probably settled from the water column, because the water level was not high even in this flowing phase (~ 10 cm in natural habitat; ~ 20 cm in artificial habitat).
In this study, standing phase was characterized by unicellular, slightly and greatly elongated (LW3 and LW5) taxa (motile Nitzschia spp. and Navicula s.l.), which are usually indicative for drying (McKew et al., 2011). As mentioned above, the water level was not high in the flowing phase, and it further decreased in the standing phase. Since the TSS content did not increase in standing phase (no shading effect), higher UV radiation reaching the biofilm strongly and negatively affected the resilience and resistance of the populations. Motile taxa, however, are able to migrate into the deeper sediment (McKew et al., 2011) and avoid high irradiation. Rounded (LW1), strongly attached, low profile Cocconeis species live in the lower layer of the biofilm (Passy, 2007). Low profile taxa are considered shade-tolerant (Liess et al., 2009; Stenger-Kovács et al., 2013; Tapolczai et al., 2016), but it seems that these species also survive in high light conditions (Leira et al., 2015; Kókai et al., 2019). At the same time, during the identification of the diatoms in the preparations, it is not possible to say whether there were living cells in the assemblages, or whether only dead diatoms were identified. Therefore, the light tolerance of large, rounded, low-profile species needs to be clarified so that these traits can be safely used to indicate aquatic phases in intermittent streams.
It is a well-known phenomenon, that habitat heterogeneity is reduced in streambed constructed by concrete (Wellman et al., 2000; Bouska et al., 2010). Significant compositional changes have already been observed in riverine macroinvertebrate (Gál et al., 2020) and fish assemblages (Wellman et al., 2000). In our study, we did not find any differences either in taxa or trait composition between the natural and artificial habitats (rejects this part of H2). Habitat change (from natural to artificial) does not seem to be as strong an environmental filter as the hydrological regime, either for taxa or for traits. This is also very surprising because different types of substrates were sampled in the two sites, i.e., macrophytes in the natural and pebbles and green algal filaments in the artificial habitats. It is known, that substrates can play an important factor controlling diatom composition (Cox, 1988; Cantonati & Spitale, 2009; Hájková et al., 2011; Cantonati et al., 2012). However, even in the same substrate type, the composition of diatoms can vary greatly depending on the current velocity and the specific location of the substrate in the streambed (Passy, 2001). But without a doubt, substrate strongly influences the structure of the assemblages and the relative abundance of taxa. Here, one possible reason could be the short distance between the sampling sites (see above). In this case, mass effect and/or species sorting may be the main organizing force allowing taxa to be present even at suboptimal environment or dispersed at any suitable sites (Soininen & Teittinen, 2019). It is especially true for motile taxa whose disperse may be more controlled by mass effect (Jamoneau et al., 2018). In this study, although no significant compositional difference was found, the IndVal analysis showed that the artificial (concreted) habitat was primarily indicated by motile taxa. Another reason for the similarity between the habitats may be the abundant macrophytes living next to the artificial site. Even though the streambed is concreted, this plant cover can play an important role, especially at higher water levels, in the formation of such similar diatom assemblages.
Biodiversity and ecological quality changes
It has been observed that lentic taxa, which appear during the formation of pools or the development of standing phase, can increase biodiversity of intermittent watercourses. Later, aerophile or even terrestrial taxa may further enhance biodiversity (Stubbington et al., 2017) up to a point (Tornés et al., 2021). Here, we found no difference in either taxonomic or in trait-based diversity metrics (rejects this part of H3). Probably because the Létai-ér was already rich in taxa and traits (e.g., colonial, high profile, moderately attached species), which are typical in mountainous or hilly streams during the fragmentation of surface water and drying up of stream (Falasco et al., 2016a).
Gál et al. (2020) found that biodiversity and the number of protected macroinvertebrate taxa decrease significantly at road crossings, while artificial streambed modification has a positive effect on the abundance of alien species. In our study, however, significantly higher species richness and effective Shannon’s H and marginally significantly higher functional diversity were found in the artificial habitat (rejects H3). This is probably not because the species that appeared in the artificial habitat were not previously the members of the Létai-ér' species pool. It is more likely, that the selection pressure on the diatom assemblages in the two habitats was not strong enough to result in structural changes, but was enough to change the relative abundance of the species. During the taxonomic identification, we found such species in the artificial (concreted) habitat (increasing Taxa S) that were hidden by the high relative abundance of the dominant taxa in the natural habitat. However, the sampling effort was low in our study, thus we can not draw general conclusions that would require more data from many more sampling sites. In addition to increasing the sampling effort, however, factors such as the distance between sampling sites, vegetation cover next to the streambed, must also be taken into account.
Despite the fact that the diatom indices were primarily developed for the detection of nutrient loads, according to our knowledge so far, they are robust enough to be included in the assessment of the ecological status of intermittent streams (Barthès et al., 2015; Falasco et al., 2016b; Novais et al., 2020; B-Béres et al., 2022b). Here, we hypothesized that drought significantly negatively affects water quality. Our results support this hypothesis: the shift in the hydrological regime (flowing to standing phase) created such a harsh environment (increased phosphate concentration, lack of shading, etc.) that was enough to induce compositional changes in diatom assemblages and contribute to an increase in the relative abundance of species (mainly Navicula and Nitzschia taxa), which favor these conditions but also indicate the deterioration of the ecological status. In contrast, we surprisingly did not find any differences in diatom indices between habitats (rejects this part of H4). Although it should be noted that the habitats did not differ in either their physical or chemical parameters (see explanation above).
Conclusion
Our results highlighted that even in the absence of direct drying, changes in the hydrological regimes have a significant assemblages-forming role in a lowland intermittent stream, even within a relatively short period of time (one year), which also influence the ecological status of the stream. Prolonged drought therefore significantly increase the vulnerability of these ecosystems. At the same time, our investigation also pointed out that characteristics typical of lowland streams, such as the extensive macrophyte cover even in the flowing phase, or the rapid sedimentation of inorganic particles during the intense decrease in water level, can slightly modify the generally accepted theories of diversity change related to hydrological regimes. In addition, our results also highlighted that streambed modifications (concreting) and the covering the littoral zone with hollow concrete slabs do not necessarily result in significant compositional changes in diatom assemblages. Even an increase in diversity can be predicted if the environmental conditions (nutrient supply, light intensity, etc.) do not deteriorate. However, this definitely requires further, much more extensive research than the study presented here. After all, an incalculable number of bridges cross watercourses, including intermittent streams and rivers worldwide, whose effect on the benthic algal assemblages is not yet known. However, without this knowledge, it is difficult to propose ecological action plans for nature conservation, water management, and restoration of intermittent watercourses.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acuña, V., M. Hunter & A. Ruhí, 2017. Managing temporary streams and rivers as unique rather than second-class ecosystems. Biological Conservation 211: 12–19. https://doi.org/10.1016/j.biocon.2016.12.025.
Anderson, M. J., 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences 58(3): 626–639. https://doi.org/10.1139/f01-004.
ASTM D859-00, 2000. ASTN Standard test method for siica in water. p. 5.
Barthès, A., J. Leflaive, S. Coulon, F. Peres, J. L. Rols & L. Ten-Hage, 2015. Impact of drought on diatom communities and the consequences for the use of diatom index values in the river Maureillas (Pyrénées-Orientales, France). River Research and Applications 31(8): 993–1002. https://doi.org/10.1002/rra.2793.
B-Béres, V., P. Török, Z. Kókai, E. T. Krasznai, B. Tóthmérész & I. Bácsi, 2014. Ecological diatom guilds are useful but not sensitive enough as indicators of extremely changing water regimes. Hydrobiologia 738: 191–204. https://doi.org/10.1007/s10750-014-1929-y.
B-Béres, V., Z. Kókai, G. Várbíró, G. Mustazhapova, Z. Csabai, B. Pernecker, G. Borics, I. Bácsi & P. Boda, 2022a. Flow intermittence drives the benthic algal composition, biodiversity and diatom-based quality of small hilly streams in the Pannonian ecoregion, Hungary. Frontiers in Ecology and Evolution 10: 834548. https://doi.org/10.3389/fevo.2022.834548.
B-Béres, V., C. Stenger-Kovács, K. Buczkó, J. Padisák, G. B. Selmeczy, E. Lengyel & K. Tapolczai, 2022b. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia. https://doi.org/10.1007/s10750-022-04984-9.
Berthon, V., A. Bouchez & F. Rimet, 2011. Using diatom lifeforms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south eastern France. Hydrobiologia 673: 259–271. https://doi.org/10.1007/s10750-011-0786-1.
Bey, M. Y., & L. Ector, 2013. Atlas des diatomées des cours d’eau de la région Rhône-Alpes. pp. 1182.
Botta-Dukát, Z., 2005. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. Journal of Vegetation Science 16(5): 533–540. https://doi.org/10.1111/j.1654-1103.2005.tb02393.x.
Bouska, W. W., T. Keane & C. P. Paukert, 2010. The effects of road crossings on prairie stream habitat and function. Journal of Freshwater Ecology 25: 499–506. https://doi.org/10.1080/02705060.2010.9664398.
Brown, L. E. & D. M. Hannah, 2008. Spatial heterogeneity of water temperature across an alpine river basin. Hydrological Processes: an International Journal 22(7): 954–967. https://doi.org/10.1002/hyp.6982.
Cantonati, M. & D. Spitale, 2009. The role of environmental variables in structuring epiphytic and epilithic diatom assemblages in springs and streams of the Dolomiti Bellunesi National Park (south-eastern Alps). Fundamental and Applied Limnology 174: 117–133. https://doi.org/10.1127/1863-9135/2009/0174-0117.
Cantonati, M., N. Angeli, E. Bertuzzi, D. Spitale & H. Lange-Bertalot, 2012. Diatoms in springs of the Alps: spring types, environmental determinants, and substratum. Freshwater Science 31(2): 499–524. https://doi.org/10.1899/11-065.1.
Chao, A., C.-H. Chiu & L. Jost, 2014. Unifying bers. Annual Review of Ecology, Evolution, and Systematics 45(1): 297–324. https://doi.org/10.1146/annurev-ecolsys-120213-091540.
Coste, M., 1982. CEMAGREF – Etude des méthodes biologiques d’appréciation quantitative de la qualité des eaux. Rapport Q. E. Lyon – Agence de l’Eau Rhône-Méditerranée-Corse.
Cox, E. J., 1988. Has the role of the substratum been underestimated for algal distribution patterns in freshwater ecosystems? Biofouling 1(1): 49–63. https://doi.org/10.1080/08927018809378095.
Crabot, J., C. P. Mondy, P. Usseglio-Polatera, K. M. Fritz, P. J. Wood, M. J. Greenwood, M. T. Bogan, E. I. Meyer & T. Datry, 2021. A global perspective on the functional responses of stream communities to flow intermittence. Ecography 44(10): 1511–1523. https://doi.org/10.1111/ecog.05697.
Datry, T., S. T. Larned, K. M. Fritz, M. T. Bogan, P. J. Wood, E. I. Meyer & A. N. Santos, 2014. Broad-scale patterns of invertebrate richness and community composition in temporary rivers: effects of flow intermittence. Ecography 37(1): 94–104. https://doi.org/10.1111/j.1600-0587.2013.00287.x.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67(3): 345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2.
EN 13946, 2014.Water quality. Guidance for the routine sampling and preparation of benthic diatoms from rivers and lakes.
EN 14407, 2014. Water quality. Guidance for the identification and enumeration of benthic diatom samples from rivers and lakes.
Falasco, E., E. Piano & F. Bona, 2016a. Diatom flora in Mediterranean streams: flow intermittency threatens endangered species. Biodiversity and Conservation 25: 2965–2986. https://doi.org/10.1007/s10531-016-1213-8.
Falasco, E., E. Piano & F. Bona, 2016b. Suggestions for diatom-based monitoring in intermittent streams. Knowledge & Management of Aquatic Ecosystems 417: 38. https://doi.org/10.1051/kmae/2016025.
Felföldy, L., 1987. A biológiai vízminősítés. 4. Javított és bővített kiadás. In: Felföldy (Ed.) Vízügyi Hidrobiológia 13. VGI. Budapest. p. 258. (in Hungarian).
Fisher, J. & M. J. Dunbar, 2007. Towards a representative periphytic diatom sample. Hydrology and Earth System Sciences 11(1): 399–407. https://doi.org/10.5194/hess-11-399-2007.
Gál, B., A. Weiperth, J. Farkas & D. Schmera, 2020. The effects of road crossings on stream macro-invertebrate diversity. Biodiversity and Conservation 29: 729–745. https://doi.org/10.1007/s10531-019-01907-4.
Gallart, F., N. Cid, J. Latron, P. Llorens, N. Bonada, J. Jeuffroy, S. M. Jiménez-Argudo, R. M. Vega, C. Solà, M. Soria, M. Bardina, A. J. Hernández-Casahuga, A. Fidalgo, T. Estrela, A. Munné & N. Prat, 2017. TREHS: An open-access software tool for investigating and evaluating temporary river regimes as a first step for their ecological status assessment. Science of the Total Environment 607: 519–540. https://doi.org/10.1016/j.scitotenv.2017.
Guiry, M. D. & G. M. Guiry, 2023. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org. accessed on 24 January 2023
Hájková, P., J. Bojková, M. Fránková, V. Opravilová, M. Hájek, K. Kintrová & M. Horsák, 2011. Disentangling the effects of water chemistry and substratum structure on moss-dwelling unicellular and multicellular micro-organisms in spring-fens. Journal of Limnology 70: 54–64. https://doi.org/10.4081/jlimnol.2011.s1.54.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST-palaeontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 9.
Hofmann, G., M. Werum & H. Lange-Bertalot, 2006. Diatomeen im Süßwasser-Benthos von Mitteleuropa. A.R.G. Gantner Verlag K.G.
Jamoneau, J., S. I. Passy, J. Soininen, T. Leboucher & J. Tison-Rosebery, 2018. Beta diversity of diatom species and ecological guilds: response to environmental and spatial mechanisms along the stream watercourse. Freshwater Biology 63: 62–73. https://doi.org/10.1111/fwb.12980.
Jost, L., 2006. Entropy and diversity. Oikos 113: 363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x.
Kókai, Z., G. Borics, I. Bácsi, Á. Lukács, B. Tóthmérész, E. Csépes, P. Török & V. B-Béres, 2019. Water usage and seasonality as primary drivers of benthic diatom assemblages in a lowland reservoir. Ecological Indicators 106: 105443. https://doi.org/10.1016/j.ecolind.2019.105443.
Krammer, K. & H. Lange-Bertalot, 1997a. Bacillariophyceae 1, Naviculaceae. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Elsevier, Heidelberg: 876.
Krammer, K. & H. Lange-Bertalot, 1997b. Bacillariophyceae 2, Bacillariaceae, Epithemiaceae, Surirellaceae. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Elsevier, Heidelberg: 596.
Krammer, K. & H. Lange-Bertalot, 2004a. Bacillariophyceae 3, Centrales, Fragilariaceae, Eunotiaceae. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Spektrum Akademischer Verlag, Heidelberg: 576.
Krammer, K. & H. Lange-Bertalot, 2004b. Bacillariophyceae 4, Achnanthaceae. kritische erganzungen zu Achnanthes s.l., Navicula str., Gomphonema. gesamt literaturverzeichnis teil 1–4. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Spektrum Akademischer Verlag, Heidelberg: 437.
Lange, K., C. R. Townsend & C. D. Matthaei, 2016. A trait based framework for stream algal communities. Ecology and Evolution 6: 23–36. https://doi.org/10.1002/ece3.1822.
Lecointe, C., M. Coste & J. Prygiel, 2003. Omnidia 3.2. Diatom index software including diatom database with taxonomic names, references and codes of 11645 diatom taxa. Hydrobiologia 269(270): 509–513. https://doi.org/10.1007/BF00028048.
Leira, M., M. L. Filippi & M. Cantonati, 2015. Diatom community response to extreme water-level fluctuations in two Alpine lakes: a core case study. Journal of Paleolimnology 53: 289–307. https://doi.org/10.1007/s10933-015-9825-7.
Liess, A., K. Lange, F. Schulz, J. J. Piggott, C. D. Matthaei & C. R. Townsend, 2009. Light, nutrients and grazing interact to determine diatom species richness via changes to productivity, nutrient state and grazer activity. Journal of Ecology 97: 326–336. https://doi.org/10.1111/j.1365-2745.2008.01463.x.
Magand C., M. H. Alves, E. Calleja, T. Datry, G. Dörflinger, J. England, F. Gallart, R. Gómez, D. Jordacaapdevilla, E. Marti, A. Munne, V. A. Pastor, R. Stubbington, I. Tziortzis & D. Von Schiller, 2020. Intermittent rivers and ephemeral streams: what water managers need to know. Technical report–Cost ACTION CA. https://doi.org/10.5281/zenodo.3888474.
McKew, B. A., J. D. Taylor, T. J. McGenity & J. C. G. Underwood, 2011. Resistance and resilience of benthic biofilm communities from a temperate saltmarsh to desiccation and rewetting. Multidisciplinary Journal of Microbial Ecology 5: 30–41. https://doi.org/10.1038/ismej.2010.91.
Messager, M. L., B. Lehner, C. Cockburn, N. Lamouroux, H. Pella, T. Snelder, K. Tockner, T. Trautmann, C. Watt & T. Datry, 2021. Global prevalence of non-perennial rivers and streams. Nature 594(7863): 391–397. https://doi.org/10.1038/s41586-021-03565-5.
MSZ 12750-6:1971, 1971. Testing of surface waters. Determination of all solved and floating matter content. (in Hungarian).
MSZ 12750-21, 1971. Testing of surface waters. Determination of oxygen assumption (demand for chemical oxygen). (in Hungarian).
MSZ 448-13, 1983. Ivóvízvizsgálat – Szulfátion meghatározás. (in Hungarian).
MSZ ISO 7150-1, 1992. Water quality. Determination of ammonium. Part 1: Manual spectrometric method. (in Hungarian).
MSZ EN 1899-2, 2000. Water quality. Determination of biochemical oxygen demand after n days (BODn) - Part 2: Method for undiluted samples (ISO 5815:1989, modified). (in Hungarian).
MSZ EN ISO 6878, 2004. Water quality. Determination of phosphorus. Ammonium molybdate spectrometric method.
MSZ 1484-13, 2009. Water quality. Part 12: Determination of nitrate and nitrite. Content by spectrophotometric method. (in Hungarian).
MSZ 1484-15, 2009. Water quality. Part 15: Determination of chloride content by argentometric titration method for the. (in Hungarian).
Németh, J., 1998. A Biológiai Vízminősítés Módszerei. Vízi Természet- és Környezetvédelem, Vol. 7. Budapest: Környezetgazdálkodási Intézet. (in Hungarian).
Novais, M. H., E. A. Morales, A. M. Penha, M. Potes, A. Bouchez, A. Barthes, M. J. Costa, R. Salgado, J. Santos & M. Morais, 2020. Benthic diatom community dynamics in Mediterranean intermittent streams: Effects of water availability and their potential as indicators of dry-phase ecological status. Science of the Total Environment 719: 137462. https://doi.org/10.1016/j.scitotenv.2020.137462.
Padisák, J., 2005. Általános limnológia. Budapest, ELTE Eötvös Kiadó. (in Hungarian).
Passy, S. I., 2001. Spatial paradigms of lotic diatom distribution: a landscape ecology perspective. Journal of Phycology 37: 370–378. https://doi.org/10.1046/j.1529-8817.2001.037003370.x.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany 86: 171–178. https://doi.org/10.1016/j.aquabot.2006.09.018.
Potapova, M. & P. B. Hamilton, 2007. Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. Journal of Phycology 43: 561–575. https://doi.org/10.1111/j.1529-8817.2007.00332.x.
Pumo, D., D. Caracciolo, F. Viola & L. V. Noto, 2016. Climate change effects on the hydrological regime of small non-perennial river basins. Science of the Total Environment 542: 76–92. https://doi.org/10.1016/j.scitotenv.2015.10.109.
Rao, C. R., 1982. Diversity and dissimilarity coefficients: a unified approach. Theoretical Population Biology 21: 24–43. https://doi.org/10.1016/0040-5809(82)90004-1.
River Basin Management Plan Report Suppl.1.1, 2022. https://vizeink.hu/vizgyujto-gazdalkodasi-terv-2019-2021/vgt3-elfogadott/#up01 Suppl.1.1. accessed on 8 March 2023.
River Basin Management Plan Report Suppl.3.1, 2022. https://vizeink.hu/vizgyujto-gazdalkodasi-terv-2019-2021/vgt3-elfogadott/#up01 Suppl.3.1. accessed on 1 June 2023.
River Basin Management Plan Report Suppl.6.3, 2022. https://vizeink.hu/vizgyujto-gazdalkodasi-terv-2019-2021/vgt3-elfogadott/#up01 Suppl.6.3. accessed on 8 March 2023.
Rimet, F. & A. Bouchez, 2012. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowledge and Management of Aquatic Ecosystems 406: 01. https://doi.org/10.1051/kmae/2012018.
Robson, B. J. & T. G. Matthews, 2004. Drought refuges affect algal recolonization in intermittent streams. River Research and Applications 20(7): 753–763. https://doi.org/10.1002/rra.789.
Robson, B. J., T. G. Matthews, P. R. Lind & N. A. Thomas, 2008. Pathways for algal recolonization in seasonally-flowing streams. Freshwater Biology 53(12): 2385–2401. https://doi.org/10.1111/j.1365-2427.2008.02061.x.
Rott, E., P. G. Hofmann, K. Pall, P. Pfister, & E. Pipp, 1997. Indikationslisten für Aufwuchsalgen in österreichischen Fliessgewässern, Teil 1: Saprobielle Indikation. Bundesministerium für Land-und Forstwirtschaft, Wasserwirtschaftskataster, Wien.
Rott, E., P. Pfister, H. Van Dam, E. Pipp, K. Pall, N. Binder & K. Ortler, 1999. Indikationslisten für Aufwuchsalgen in Österreichischen Fliessgewässern, Teil 2: Trophieindikation und autökologische Anmerkungen Bundesministerium für Land-und Forstwirtschaft, Wasserwirtschaftskataster, Wien:
Sabater, S., D. Barceló, N. De Castro-Català, A. Ginebreda, M. Kuzmanovic, M. Petrovic, Y. Picó, L. Ponsatí, E. Tornés & I. Muñoz, 2016. Shared effects of organic microcontaminants and environmental stressors on biofilms and invertebrates in impaired rivers. Environmental Pollution 210: 303–314. https://doi.org/10.1016/j.envpol.2016.01.037.
Sabater, S., X. Timoner, G. Bornette, M. De Wilde, J. C. Stromberg & J. C. Stella, 2017. The biota of intermittent rivers and ephemeral streams: Algae and vascular plants. In Intermittent rivers and ephemeral streams, 189–216. https://doi.org/10.1016/B978-0-12-803835-2.00016-4.
Šmilauer, P. & J. Lepš, 2014. Multivariate Analysis of Ecological Data using CANOCO 5, Cambridge University Press, Cambridge:
Soininen, J. & A. Teittinen, 2019. Fifteen importan questions in the spatial ecology of diatoms. Freshwater Biology 64: 2071–2083. https://doi.org/10.1111/fwb.13384.
Solheim, A. L., L. Globevnik, K. Austnes, P. Kristensen, S. J. Moe, J. Persson, G. Phillips, S. Poikane, W. van de Bund & S. Birk, 2019. A new broad typology for rivers and lakes in Europe: development and application for large-scale environmental assessments. Science of the Total Environment 697: 134043. https://doi.org/10.1016/j.scitotenv.2019.134043.
Stenger-Kovács, C. & E. Lengyel, 2015. Taxonomical and distribution guide of diatoms in soda pans of Central Europe. Studia Botanica Hungarica 46: 3–203. https://doi.org/10.17110/StudBot.2015.46.Suppl.3.
Stenger-Kovács, C., E. Lengyel, L. O. Crossetti, V. Üveges & J. Padisák, 2013. Diatom ecological guilds as indicators of temporally changing stressors and disturbances in the small Torna-stream, Hungary. Ecological Indicators 24: 138–147. https://doi.org/10.1016/j.ecolind.2012.06.003.
Stenger-Kovács, C., K. Körmendi, E. Lengyel, A. Abonyi, É. Hajnal, B. Szabó, K. Buczkó & J. Padisák, 2018. Expanding the trait-based concept of benthic diatoms: development of trait- and species-based indices for conductivity as the master variable of ecological status in continental saline lakes. Ecological Indicators 95: 63–74. https://doi.org/10.1002/ece3.5897.
Stubbington, R., J. England, P. J. Wood & C. E. Sefton, 2017. Temporary streams in temperate zones: recognizing, monitoring and restoring transitional aquatic-terrestrial ecosystems. Wiley Interdisciplinary Reviews: Water 4(4): 1223. https://doi.org/10.1002/wat2.1223.
Stubbington, R., R. Chadd, N. Cid, Z. Csabai, M. Miliša, M. Morais, A. Munné, P. Pařil, V. Pešić, I. Tziortzis, R. C. M. Verdonschot & T. Datry, 2018. Biomonitoring of intermittent rivers and ephemeral streams in Europe: current practice and priorities to enhance ecological status assessments. Science of the Total Environment 618: 1096–1113. https://doi.org/10.1016/j.scitotenv.2017.09.137.
Stubbington, R., A. Paillex, A. England, A. Barthès, A. Bouchez, F. Rimet, M. Sánchez- Montoya, C.G. Westwood & T.A. Datry, 2019. Comparison of biotic groups as dry-phase indicators of ecological quality in intermittent rivers and ephemeral streams. Ecological Indicators 97: 165–174. https://doi.org/10.1016/j.ecolind.2018.09.061.
Tapolczai, K., A. Bouchez, C. Stenger-Kovács, J. Padisák & F. Rimet, 2016. Trait-based ecological classifications for benthic algae: review and perspectives. Hydrobiologia 776(1): 1–17. https://doi.org/10.1007/s10750-016-2736-4.
The OMSZ’ Climate Data Series, 1901–2020. https://www.met.hu/eghajlat/magyarorszag_eghajlata/eghajlati_adatsorok/Debrecen/adatok/napi_adatok/index.php, accessed on 8 March 2023.
The OMSZ’ General Review of the Climate of Hungary. https://www.met.hu/eghajlat/magyarorszag_eghajlata/altalanos_eghajlati_jellemzes/csapadek/, accessed on 1 June 2023.
Timoner, X., C. M. Borrego, V. Acuna & S. Sabater, 2014. The dynamics of biofilm bacterial communities is driven by flow wax and wane in a temporary stream. Limnology and Oceanography 59(6): 2057–2067. https://doi.org/10.4319/lo.2014.59.6.2057.
Tornés, E., M. Colls, V. Acuña & S. Sabater, 2021. Duration of water flow interruption drives the structure and functional diversity of stream benthic diatoms. Science of the Total Environment 770: 144675. https://doi.org/10.1016/j.scitotenv.2020.144675.
Várbíró, G., G. Borics, B. Csányi, G. Fehér, I. Grigorszky, K. T. Kiss, A. Tóth & É. Ács, 2012. Improvement of the ecological water qualification system of rivers based on first results of the Hungarian phytobenthos surveillance monitoring. Hydrobiologia 695: 125–135. https://doi.org/10.1007/s10750-012-1120-2.
Várbíró, G., G. Borics, M. H. Novais, M. M. Morais, F. Rimet, A. Bouchez, K. Tapolczai, I. Bácsi, P. Usseglio-Polatera & V. B-Béres, 2020. Environmental filtering and limiting similarity as main forces driving diatom community structure in Mediterranean and continental temporary and perennial streams. Science of the Total Environment 741: 140459. https://doi.org/10.1016/j.scitotenv.2020.140459.
Wellman, J. C., D. L. Coms & S. B. Cook, 2000. Long-Term Impacts of Bridge and Culvert Construction or Replacement on Fish Communities and Sediment Characteristics of Streams. Journal of Freshwater Ecology 15: 317–328. https://doi.org/10.1080/02705060.2000.9663750.
Wemple, B. C., T. Browning, A. D. Ziegler, J. Celi, K. Pan, F. Jaramillo, N. K. Leite, S. J. Ramchunder, J. N. Negishi, X. Palomeque & D. Sawyer, 2018. Ecohydrological disturbances associated with roads: current knowledge, research needs, and management concerns with reference to the tropics. Ecohydrology 11: e1881. https://doi.org/10.1002/eco.1881.
Witteveen, N. H., A. Freixa & S. Sabater, 2020. Local and regional environmental factors drive the spatial distribution of phototrophic biofilm assemblages in Mediterranean streams. Hydrobiologia 847(10): 2321–2336. https://doi.org/10.1007/s10750-020-04258-2.
Acknowledgements
The project was financially supported by the National Research, Development and Innovation Office - NKFIH FK 132 142 (VBB), by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO-00458-20-8 (VBB) and by the ÚNKP-20-5 (VBB), ÚNKP-21-5 (VBB) and ÚNKP-22-5 (VBB) New National Excellence Program of the Ministry for Innovation and Technology from the Source of the National Research, Development and Innovation Fund. We are thankful to Tamás Bozóki for preparation of the map (Fig. 1).
Funding
Open access funding provided by ELKH Centre for Ecological Research. Funding were provided by Nemzeti Kutatási Fejlesztési és Innovációs Hivatal (Grant No. NKFIH FK 132 142), the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (Grant No. BO-00458-20-8), Innovációs és Technológiai Minisztérium (Grant No. ÚNKP-20-5), Innovációs és Technológiai Minisztérium (Grant No. ÚNKP-21-5) and Innovációs és Technológiai Minisztérium (Grant No. ÚNKP-22-5).
Author information
Authors and Affiliations
Contributions
VB-B developed the structure and drafted key issues of this paper; VB-B and SK wrote the manuscript. ZN-K proved the diatom data; IB and KM performed the physical and chemical analyses of samples in the laboratory; SK and ÁL carried out the statistical analyses; IB helped in writing of the manuscript; VB-B raised the topic; ÁL, ET-K, IB and VB-B collected samples. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
No human participants and animals were involved in the research.
Additional information
Handling editor: Sidinei M. Thomaz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Viktória B-Béres, Luigi Naselli-Flores, Judit Padisák & Gábor Borics / Trait-Based Approaches in Micro-Algal Ecology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiss, S., Nemes-Kókai, Z., Lukács, Á. et al. Aquatic phases have a stronger effect on lotic benthic diatoms than human-induced microhabitat variability. Hydrobiologia 851, 897–914 (2024). https://doi.org/10.1007/s10750-023-05405-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05405-1