Abstract
The East African soda lakes are known worldwide for their huge populations of lesser flamingos. Their phytoplankton community is often dominated by the cyanobacterium Limnospira fusiformis, the main food of lesser flamingos. In the early 2010s, the population of the cyanobacterium collapsed and the picoplanktic green alga Picocystis salinarum became dominant in Lake Nakuru. Consequently, lesser flamingos had to migrate to other lakes in search of food. To establish the reasons for the success of P. salinarum, photosynthesis measurements have been performed on monoalgal cultures of both species. The examined environmental variables (temperature, light intensity) were not responsible for the dominance of P. salinarum either alone or in their any combination. Moreover, photosynthetic activity of the cyanobacterium was higher by an order of magnitude during all light and temperature treatments. Co-cultivation of L. fusiformis and P. salinarum in a chemostat revealed that a possible reason for the Limnospira replacement can be a rapid and remarkable increase of conductivity, as P. salinarum showed higher level of tolerance to this rapid change. Shortly after returning to the initial conductivity levels, the population of L. fusiformis recovered quickly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inland saline lakes occur worldwide with a total volume almost equaling that of freshwater lakes (Shiklomanov, 1990; Williams, 1993). Alkaline saline lakes of East Africa are characterized by an ionic dominance of sodium, carbonate, and bicarbonate (Jirsa et al., 2013). The core soda lakes in the Kenyan part of the African Rift Valley are lakes Nakuru, Bogoria, Magadi, and Elmentaita. These lakes provide extreme habitats with high pH (9–11), conductivity (20–120 mS cm−1), water temperature (20–40°C), and high grazing pressure on the primary producers (Vareschi, 1982; Ballot et al., 2004; Oduor & Schagerl, 2007a; Schagerl & Burian, 2016). The Kenyan alkaline saline lakes are endorheic and are recharged mainly by rainfall, seasonal surface streams, and (mostly hot) springs (Oduor & Schagerl, 2007a; Renaut et al., 2017). As a result of their highly stochastic environmental dynamics, temporal fluctuations in the ionic components result in unexpected shifts in phytoplankton species composition (Vareschi, 1982; Melack, 1988; Oduor & Schagerl, 2007a; Krienitz & Kotut, 2010; Schagerl, 2016).
The East African alkaline saline lakes are worldwide famous for supporting huge populations of lesser flamingos (Phoeniconaias minor (Saint Hilaire, 1798)); the most famous being Lake Nakuru (Vareschi, 1978). These lakes are among the most productive ecosystems in the world owing to their high primary productivity provided by phytoplankton (Melack, 1981; Oduor & Schagerl, 2007b; Schagerl et al., 2015). Most of the time, phytoplankton is dominated by a spirally twisted, filamentous cyanobacterium, microplanktic (20–200 µm size class) Limnospira fusiformis (Voronichin) Nowicka-Krawczyk and Mühlsteinová & Hauer (syn. Arthrospira fusiformis (Voronichin) Komárek & Lund) (Cyanobacteria, Oscillatoriales) (Fig. 1a; Vareschi, 1978; Dadheech et al., 2010; Krienitz & Kotut, 2010; Kaggwa et al., 2013a; Schagerl et al., 2015; Krienitz, 2018). The key to the survival, success, and dominance of L. fusiformis is its fast growth and high photosynthetic rate (Talling et al., 1973; Melack & Kilham, 1974; Oduor & Schagerl, 2007b; Pálmai et al., 2013). Its size and spirally twisted form make it suitable for being sieved effectively by the special bill lamellae of lesser flamingos, hence L. fusiformis serves as the main food source for these birds. A strong relationship between the quantity of phytoplankton (dominated by L. fusiformis) and the number of the birds has been described (Jenkin, 1957; Vareschi, 1978; Vareschi & Vareschi, 1984; Krienitz & Kotut, 2010; Kaggwa et al., 2013b; Mgimwa et al., 2021). In the absence of their main food source, lesser flamingos survive by grazing on diatoms, other cyanobacteria, and algae of suitable size or migrate to other lakes (Tuite, 2000; Krienitz et al., 2016). Tuite (2000) described two distinct types of lesser flamingos’ distributions: the “clumped” distribution pattern, in which the majority of the total lesser flamingo population is concentrated at one or two lakes and the “dispersed” distribution pattern, in which the population is spread across all available habitats. These patterns are strongly related to the availability of L. fusiformis. Presence or absence of lesser flamingo clumps is strongly related to ecosystem services listed under the subsection Cultural services by the Millennium Ecosystem Assessment (2003, 2005) provided by African saline lakes on various ways (Naselli-Flores & Padisák, 2023a). They are considered as kind of firebird, the symbol of immortality by locals, moreover generate mass tourism thus establishing workplaces and contributing to national income of these regions (Krienitz, 2018).
An abrupt change in phytoplankton composition was observed by Krienitz & Kotut (2010): following the collapse of L. fusiformis’ population, the picoplanktic green alga Picocystis salinarum R.A. Lewin (Picocystophyceae) became dominant. Although P. salinarum was characterized by high abundance, their small cells with a diameter of only 2–3 µm (Fig. 1b; Lewin et al., 2000) were too small to be eaten by the flamingos. According to a similar phenomenon observed in Lakes Elmenteita and Nakuru, the occurrence of P. salinarum is presumable since 1973–1974. As Melack (1988) described, a rapid increase in the salinity of the lakes was followed by the collapse of the population of L. fusiformis and the green algae dominated nanoplankton became dominant. Though the dominant species was not identified at that time it could have been the two decades later described P. salinarum. Interestingly, P. salinarum usually occurs in temperate alkaline saline waters, but is also able to form blooms under ice (Table 1). Since the identification of P. salinarum in the year 2000, various studies have been carried out on this eukaryote (Roesler et al., 2002; Fanjing et al., 2009; Ben Ali et al., 2017, 2021; Ben Ouada et al., 2018a, b; Delgado et al., 2021; Phillips et al., 2021; Singh et al., 2022, 2023). It has been reported to be a good food source for invertebrates (Roesler et al., 2002), tolerates heavy metal solutions quite well and has the ability to remove bisphenol forms (Ben Ali et al., 2017, 2021; Ben Ouada et al., 2018a, b). The ability to metabolize inorganic arsenic was reported for a Picocystis strain from Mono Lake (Glabonjat et al., 2020). P. salinarum was found in several hypersaline habitats and its biomass productivity and photosynthetic activity were enhanced in very high dissolved inorganic carbon concentration and salinity (salinity ≈ 150‰) and found to be suitable for bicarbonate-based carbon capturing (Singh et al., 2022, 2023).
Both L. fusiformis and P. salinarum prefer alkaline saline habitats; however, the number of documented co-occurrences is low (Table 1). The co-occurrence of Picocystis and Limnospira was first described in Lake Nakuru (Krienitz & Kotut, 2010; Krienitz et al., 2012) and later in Lake Dziani Dzaha (Cellamare et al., 2018; Bernard et al., 2019). In both studies, the huge dominance of L. fusiformis in the phytoplankton biomass was observed. However, in contrast to the East African soda lakes, in Lake Dziani Dzaha, the cyanobacterium was not replaced by P. salinarum. Hence, both species were dominant within their taxonomic and ecological groups in Lake Dziani Dzaha: L. fusiformis was responsible for 99.99% (8,249,182 sequences) of Cyanobacteria abundance, while P. salinarum accounted also for 99.99% (1,480,251 sequences) of eukaryotic phytoplankton species (Bernard et al., 2019).
To our knowledge, no study on any Kenyan strain of P. salinarum has been conducted so far except our previous work (Pálmai et al. 2020). Although L. fusiformis is a well-known species and has been the target of many ecological and biotechnological studies (Cifferi, 1983; Affan et al., 2015; Castro et al., 2015; Ronga et al., 2019; Shao et al., 2019), no laboratory experiment focusing on the co-existence of L. fusiformis and P. salinarum has been published. In the current study, we focused on the effects of three important abiotic environmental factors: temperature, light intensity, and conductivity. First, we determined the photosynthetic activity of L. fusiformis in a wide range of temperature and light intensity in a monoculture. Thereafter, we examined the effect of rapid conductivity changes on a mixed culture of the two species in a chemostat to reveal whether it can explain dominance shift between the two species.
Materials and methods
Strains and cultivating
Photosynthesis and co-cultivation experiments were carried out with Limnospira fusiformis (KR 2005/117) and Picocystis salinarum (KR 2010/2) strains from the collection of Leibniz-Institute of Freshwater Ecology and Inland Fisheries. Both strains were collected from Lake Nakuru, Kenya. Their taxonomic identities were confirmed by molecular phylogenetic analyses (Dadheech et al., 2010; Krienitz et al., 2012). The sequences of 16S-23S ITS and cpc BA IGS of the Limnospira fusiformis strain were stored at the National Center for Biotechnology Information (NCBI) under the accession numbers FJ001900 and FJ001933. The sequence of the small subunit (SSU) rRNA gene of the Picocystis salinarum strain was stored at NCBI under the accession number HM990668.
Monoalgal stock cultures of the two species were held in M0 medium with the following ingredients: 15 g l−1 NaHCO3, 4 g l−1 Na2CO3, 0.1 g l−1 NaCl, 0.08 g l−1 Na2-EDTA, 0.01 g l−1 FeSO4 × 7H2O, 0.2 g l−1 MgSO4 × 7H2O, 0.5 g l−1 K2HPO4, 2.5 g l−1 NaNO3, 0.04 g l−1CaCl2, and 1 ml l−1 of A5-micronutrients, with the conductivity of 19.6 mS cm−1 (salinity ~ 10.5‰) and pH 9.8 (Shafik et al., 2014; Pálmai et al., 2020). The cultures were maintained at 20 ± 1°C and 65 µmol photons m−2 s−1 in 12:12 light:dark cycle in the Alga Culturing Laboratory of Research Group of Limnology (University of Pannonia, Veszprém, Hungary).
Photosynthesis experiments
The photosynthetic characteristics of L. fusiformis were examined during exponential growth phase of monoalgal culture in M0 medium. We compared our results on the photosynthetic characteristic of L. fusiformis with previously published findings on P. salinarum (Pálmai et al., 2020). We investigated the photosynthetic activity of the species in 63 combinations of temperature and light intensity within the variable ranges of the two species’ natural habitats. We used the protocol in a photosynthetron system that was previously described by Üveges et al. (2012), Pálmai et al. (2013, 2020), and Lengyel et al. (2015). Measurements were carried out between 10 and 40°C with 5°C increments. The measuring temperature was provided by a Neslab RTE-211 circulating water bath. Tungsram F74 daylight tubes provided the following nine light intensities: 0; 15; 55; 130; 250; 360, 680; and 1480 and 1900 µmol m−2 s−1. Biomass-specific photosynthetic activity was determined by oxygen yield measurements: the culture was homogenized in a 15-L vessel and then divided into Karlsruhe flasks (~ 250 ml). The first measuring was carried out at 10°C. We started the process with a 1-h pre-incubation in the dark. As the photosynthetic activity of the species was followed by measuring dissolved oxygen (DO) concentration (IntelliCAL LDO101 sensor, Hach Lange), DO was measured at the beginning of the experiment (t = 0 h), then after 1 h (t = 1 h), and after 2 h (t = 2 h) (Pálmai et al. 2020). Respiration, net-, and gross photosynthesis were calculated using the formula of Wetzel & Likens (2000). Subsequently, photosynthetic activity–light intensity (P–I) curves were fitted and the photosynthetic parameters (PBmax: biomass-specific maximum photosynthetic production, Ik: onset of saturation, α: light utilization parameter, β: photoinhibition parameter, RB: biomass-specific respiration) calculated according to Webb et al. (1974) in the absence and to Platt et al. (1980) in the presence of photoinhibition, using GraFit 7.0 software (Leatherbarrow, 2009).
Co-cultivation experiment
Growth and co-cultivation of the two species were examined in a continuous culture in a chemostat developed by Shafik et al. (2001). The culturing vessel, with the approximate volume of 1 L, was placed into an aquarium filled up with distilled water, at constant temperature of 29 ± 1°C provided by a Thermo Scientific AC150-A25 circulating bath. The light intensity on the outer surface of the culturing vessel was 200 µmol m−2 s−1 (which decreased rapidly after entering the culturing vessel because of the high cell density) with a 12:12 light:dark cycle provided by daylight tubes of Tungsram (F74). The values of temperature and light intensity were selected based on previous field observations (Vareschi, 1982; Krienitz & Kotut, 2010; Jirsa et al., 2013) and our photosynthesis measurements (Fig. 2a, b, c, d; Table 2; Pálmai et al., 2020) to provide preferable conditions for both species’ growth. The aquarium was illuminated from one side and the walls of the other three parts of the aquarium were covered by mirrors to provide homogeneous illumination. The mixed culture was aerated with sterilized air (obtained by passing air through a Millipore membrane filter with 0.2 µm pore size) from the bottom of the cultivating vessels to avoid the effect of the different sinking rates of the species. That is, the air supply was not only responsible for the supply of CO2 but also for the continuous mixing of the culture. Culturing medium was continuously added by a Masterflex L/S Variable-Speed Drive at a flow rate of 285 ± 18 ml d−1. We applied chemostat system to avoid any kind of nutrient limitation, but as we studied a mixture of two species with very different growth rates, steady state (when the dilution rate is equal to the species growth rate) was not reached for both species in all phases.
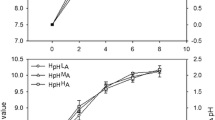
Photosynthetic parameters of Limnospira fusiformis (KR 2005/117) (empty bars) and Picocystis salinarum (KR 2010/2) (black bars) strains isolated from Lake Nakuru in M0 medium at different temperatures, and the error bars represent the standard deviations. Data of Picocystis salinarum were obtained from Pálmai et al. (2020), a Biomass-specific maximal gross photosynthetic activity (PBmax), b Onset of saturation (Ik), c Light utilization (α), and d Biomass-specific respiration (RB). PBmax value of L. fusiformis at 45°C on a is calculated from the fitted curve on the measuring data in the 10–40°C range
In the co-cultivation experiment, we applied the mixture of the above described monoalgal cultures. The initial biomass concentration ratio in the mixed culture was 90:10 L. fusiformis:P. salinarum µg chlorophyll a l−1 in a cultivating chamber with a volume of 1 L. The initial chlorophyll a concentration of L. fusiformis was 2000 µg l−1 and for P. salinarum, it was 220 µg l−1. We applied 90:10 ratio in order to represent the naturally high dominance of L. fusiformis in the phytoplankton. Samples were taken three times a week to estimate population sizes by counting individual numbers according to Utermöhl (1958). For microscopic analyses, 10 ml subsample was preserved in Lugol’s solution and 1 ml of HCl (1N) was added to the samples to avoid the decomposition of the cells in the alkaline environment. Settling units were counted in a 3 ml counting chamber after at least 12 h of settling, as a settling unit on filament was considered in the case of L. fusiformis and one cell in the case of P. salinarum.
To examine the effect of the rapidly changing environment on the co-existence of the two species, a 90-day experiment was run. We modified the conductivity of the initial M0 medium by increasing the concentration of NaHCO3 to 60 g l−1 and the concentration of Na2CO3 to 16 g l−1. The conductivity of the initial medium was at a similar level to that reported by Krienitz & Kotut (2010) to be the minimum, while the conductivity of the medium with increased level of carbonates was higher than the median conductivity of Lake Nakuru reported by the same authors. The experiment consisted of three phases: in phase I, the mixed culture was grown in M0 medium for 30 days, where the conductivity was 19.66 ± 0.15 mS cm−1 (salinity ~ 10.5‰) and the pH was 9.84 ± 0.05. In phase II, the conductivity of the medium was increased to 52.53 ± 1.47 mS cm−1 (salinity ~ 31.1‰) and the pH decreased to 9.5 ± 0.05; the mixed culture was grown in this modified medium for another 30 days. For the last 30 days, in phase III, the conductivity was returned to the initial level (20.41 ± 0.81 mS cm−1; salinity ~ 11.0‰) along with an increase of pH (9.84 ± 0.06). During the 90-day experiment, we monitored the changes in the conductivity and the pH of the medium at each sampling time. Conductivity of the medium was measured by an HQ40d Hach Lange multimeter equipped with an Intellical CDC401 sensor and the pH with an Intellical PHC201 sensor.
Results
Photosynthesis measurements
We recorded increasing photosynthetic activity of L. fusiformis between 10 and 40°C. The highest biomass-specific maximal gross photosynthetic activity (PBmax = 47.7 µg O2 µg−1 Chl a h−1) of L. fusiformis was obtained at 40°C (Fig. 2a; Table 2). Similarly, temperature positively influenced the onset of saturation (Ik) of the species. L. fusiformis had high optimum light intensity at all measuring temperatures and its highest Ik was 335 µmol m−2 s−1 at 40°C (Fig. 2b; Table 2). Good light utilization (α) was observed along the applied temperature gradient: α values of the species varied between 0.14 and 0.176 (µg O2 µg−1 Chl a h−1) (µmol m−2 s−1)−1 (Fig. 2c; Table 2). Photoinhibition occurred at the temperature range of 10–25°C. The biomass-specific respiration (RB) values of the species were about the same level in the 10–20°C temperature range. However, with a further increase of the temperature we found a remarkable increase in the RB values at the 25–40°C temperature range (Fig. 2d; Table 2). The small RB values coupled with very high level of PBmax resulted in very high PBmax/RB values (Table 2).
We recorded the lowest, but still considerable photosynthetic activity for L. fusiformis in the light intensity range 15–130 µmol m−2 s−1. At the lowest two light intensities only a slight effect of temperature was observed. At the light intensity range of 130–250 µmol m−2 s−1, the highest photosynthetic activity of the species was recorded at about 30°C (Fig. 3). The rapid increase in photosynthesis with the increasing light intensity confirms the good light utilization of the species. Alongside an increase in photosynthetic activity at higher light intensity range, a slight increase in temperature optima was observed. At high light intensities, photosynthetic activity of the species increased with the increasing temperature, with the highest photosynthetic activity being recorded at the highest temperature.
Co-cultivation experiment
We examined the effect of rapid shifts in conductivity on the growth of mixed culture of L. fusiformis and P. salinarum in two culturing media characterized by different conductivity values, obtained by altering NaHCO3 and Na2CO3 concentrations.
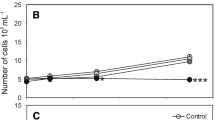
In phase I, an increase in the biomass of both species (Fig. 4) was observed in the initial medium. This increase in biomass was continuous and in a straight line for L. fusiformis until a shift to the high conductivity medium. This is in contrast to P. salinarum, which almost reached a steady state by the end of phase I.
Growth curves of Limnospira fusiformis (a, empty circles) and Picocystis salinarum (b, black circles) in continuous culture in phase I (dashed line in M0 medium, pH 9.84 ± 0.05, conductivity = 19.66 ± 0.15 mS cm−1, salinity ~ 10.5‰), phase II (solid line, in modified medium, pH 9.5 ± 0.05, conductivity = 52.53 ± 1.47 mS cm−1, salinity ~ 31.1‰), and phase III (dashed line in M0 medium, pH 9.84 ± 0.06, conductivity = 20.41 ± 0.81 mS cm−1, salinity ~ 11.0‰). Dots represent the counted settling units per liter; vertical lines indicate the change of the medium on day 31 and 75
Following the shift in medium, the biomass of both species began to decrease. In phase II, the biomass of L. fusiformis decreased during the entire high conductivity phase. In case of P. salinarum, a remarkable decrease in biomass was recorded; however, the green alga was able to adapt to the changes in the conductivity and from the middle of phase II (~ 2 weeks after the medium change) its biomass began to grow. At the end of phase II, P. salinarum had reached a steady state again. On return to the initial medium in phase III, the biomass of both species began to increase following an initial stationary state. In phase III, L. fusiformis remained at an exponential growth phase all the way to the end of the experiment. In contrast, P. salinarum reached steady state again. The growth of the two species did not reach the level of the initial section in phase I.
Discussion
The East African alkaline saline lakes are highly productive ecosystems (Melack, 1979, 1981; Oduor & Schagerl, 2007b; Schagerl et al., 2015). The very high primary production is often caused by a single phytoplankton species, Limnospira fusiformis, serving as essential food source for the lesser flamingos (Jenkin, 1957; Vareschi, 1978; Krienitz & Kotut, 2010; Krienitz, 2018). In these extreme habitats, phytoplankton composition is affected by several environmental variables including a number of abiotic factors such as nutrient availability, temperature, light intensity, conductivity, and also by biotic factors, like inter- and intraspecific competition and viral infections (Vareschi, 1979, 1982; Vareschi & Vareschi, 1984; Jirsa et al., 2013; Peduzzi et al., 2014; Krienitz et al., 2016; Schagerl & Burian, 2016).
Limnospira fusiformis is considered to prefer higher temperatures: the positive correlation between temperature and photosynthetic activity of the species is well known and has been confirmed by previous studies as well as by our recent results (Vonshak, 1997; Pálmai et al., 2013; Table 2). In previous studies, P. salinarum was found to have a photosynthetic activity lower by an order of magnitude or even more compared to L. fusiformis which is in line with our results (Roesler et al., 2002; Pálmai et al., 2020). The photosynthetic activity of the green alga showed a strong temperature dependence with photoinhibition occurring over a wide range of temperatures (Pálmai et al., 2020), while in case of L. fusiformis, we found photoinhibition only at lower temperatures (Table 2). This huge difference between the PBmax of the two species was also recorded in our previous study in Cl−-dominated medium (Pálmai et al. 2013). Similar levels of biomass-specific respiration of P. salinarum and L. fusiformis were recorded along a wide range of temperature, but the cyanobacterium had high PBmax and these huge differences resulted in an extremely high PBmax/RB ratio. This observation has also been described for other cyanobacteria species (Van Liere & Mur, 1980; Vonshak, 1997). In contrast, P. salinarum had a moderate ratio along the temperature gradient with PBmax/RB values similar to those recorded by Humphrey (1975) for several algal species.
Light availability in the East African region is quite good; however, the high turbidity caused by both wind and bioturbation by a huge population of birds and shading by high phytoplankton crop results in a high light attenuation within the water column (Vareschi, 1982; Oduor & Schagerl, 2007b). These light conditions coupled with high temperature create suitable habitat for both species. According to our results L. fusiformis can utilize higher light intensity, at least for shorter periods of time without photoinhibition even at high temperature but also performs well under lower illumination (Vonshak, 1997; Pálmai et al., 2013; Table 2). In contrast, the turbid and light limited water column offers favorable conditions for both P. salinarum and L. fusiformis, as they have good light utilization (Kebede & Ahlgren, 1996; Roesler et al., 2002; Fanjing et al. 2009; Pálmai et al., 2020; Table 2). The pigment composition of the two species as described by Bernard et al. (2019) also supports our findings on the difference in the light tolerance ranges. Although, photoinhibition in P. salinarum was recorded over a wide range of temperature (Pálmai et al., 2020) in its natural environment, the species can avoid the negative effect of high light intensity of the surface layer by occupying the deeper parts of the water column characterized by lower light availability (this sometimes means only 20–30 cm below the surface), hence avoiding the surface layer (Vareschi, 1982; Oduor & Schagerl, 2007b).
Since both photosynthetic activity and growth of P. salinarum are far below the values of L. fusiformis’ (Kebede & Ahlgren, 1996; Roesler et al., 2002; Fanjing et al., 2009; Pálmai et al., 2013, 2020; Table 2), we assumed that another abiotic environmental factor or changes in this factor could be the reason for the dominance change between the two species in the Kenyan soda lakes. Krienitz & Kotut (2010), Schagerl et al. (2015) and Krienitz (2018) attributed the dominance of P. salinarum in the soda lakes of East Africa to the rapid changes in salinity.
Although there are no previous experiments on the growth of the Kenyan strains in mixed cultures, some studies have revealed that the two species remarkably differ in salt tolerance. Increasing sodium salt (Na2SO4, NaCl, NaHCO3) concentrations has a negative effect on the growth of L. fusiformis and also alters the morphology of the cyanobacterium (Kebede, 1997). Kebede (1997) recorded a negative correlation between the concentration of three sodium salts and the growth rate of L. fusiformis, with the highest growth occurring at a salinity of 13.2‰. A salinity range of 10–25‰ was found to be optimal for the growth of L. fusiformis in different media (Chen, 2011), which was also confirmed by our observations. The negative effect of high salinity (high NaCl concentration) was also recorded for P. salinarum; however, the eukaryote species has a higher salinity tolerance range than L. fusiformis (Kebede & Ahlgren, 1996; Kebede, 1997; Roesler et al., 2002; Fanjing et al., 2009; Schagerl & Burian, 2016; Pálmai et al., 2020; Singh et al., 2023).
Previous studies have confirmed that the populations of L. fusiformis collapse from time to time. The population collapse has been associated with a high turbidity and/or salinity periods of the lake (Melack, 1988; Schagerl et al., 2015). Empirical studies (Krienitz & Kotut, 2010; Schagerl et al., 2015; Krienitz, 2018) found a close relation between L. fusiformis biomass and salinity. Krienitz et al. (2012) observed that P. salinarum became dominant in Lake Nakuru following a drastic decrease in water level, accompanied by rapid and drastic changes in salinity, which was confirmed by in our current experimental study under laboratory conditions.
Beside the abiotic factors, two main biotic factors also affect the population size of L. fusiformis: intensive grazing pressure and virus infections that may result in a collapse of the cyanobacteria population and indirectly that of the entire food web as well (Peduzzi et al., 2014). Vareschi (1978) estimated the food requirements for an adult flamingo to be 70 g d−1 of dry mass. This means that there could be a strong pressure on L. fusiformis population even under favorable environmental conditions if a clumped distribution (Tuite, 2000) pattern of the flamingos occurs. A drastic change in the lake level of the observed cases was followed by a crash in the resident population of L. fusiformis. A lack of tolerance for this kind of change coupled with a high grazing pressure and viral infections can easily lead to the temporary disappearance of L. fusiformis. Following the collapse of the cyanobacterium, the lesser flamingos migrate to other lakes resulting in a dispersed distribution pattern (Tuite, 2000) and a reduction in grazing pressure, which allows the recovery of L. fusiformis population. According to Vareschi (1979) and Vareschi & Vareschi (1984) grazing by fish or zooplankton is not considered as main threat to L. fusiformis population. Tilapia graham Boulenger, 1912 shifted to filtering the cyanobacterium in Lake Nakuru, but it is implausible that it could significantly reduce the biomass of L. fusiformis (Vareschi, 1979). Zooplankton has a higher indirect impact on the phytoplankton due to its nutrient recycling activity compared to its direct grazing effect. The absence of this nutrient recycling can result in a nutrient limitation but it was usually observed in parallel with or after the decrease of L. fusiformis (Vareschi, 1978; Vareschi & Jacobs, 1984; Melack, 1988).
Due to the generally good nutrient supply in the East African lakes and based on our observations that the Kenyan strains’ light requirements only partially overlap (see Ik values in Table 2), resource competition for nutrients or light between the two species can be excluded. Even though, in previous cultivation studies usually higher Ik values have been recorded under laboratory conditions, compared to field measurements (Kebede & Ahlgren, 1996; Oduor & Schagerl, 2007b; Schagerl & Burian, 2016), the remarkable differences between the Ik values of the examined to species (Table 2) indicate considerable differences in their light preference. However, sometimes nitrogen limitation occurs in these habitats (Oduor & Schagerl, 2007a, 2007b), but this limitation affects the growth of both species negatively (Delgado et al., 2021; Schagerl et al., 2022) since they are not N2 fixers under natural (aerobic) conditions. The latter may explain the “silent” and chiefly subdominant co-occurrence of the diazotrophic Anabaenopsis in these lakes (Krienitz et al., 2016). The co-occurrence implies that P. salinarum and L. fusiformis can exist in the same habitat and dominate the phytoplankton together; however, the green alga is not able to overgrow L. fusiformis under stable environmental conditions. Whereas, the biomass of L. fusiformis in the soda lakes of East Africa is usually measured in tens to hundreds of mg l−1, the highest biomass of P. salinarum in Lake Nakuru ranges from 7.1 to 7.4 mg l−1 (Vareschi, 1982; Krienitz et al., 2012, 2016). These data suggest that P. salinarum benefits more from the environmental changes, hence becoming an active competitor for L. fusiformis.
In a wider context, the African lakes where L. fusiformis and/or P. salinarum may occur or co-occur are typical examples of extreme environments, in many cases constrained by multiple stressors; salinity being one of the most important ones (Padisák & Naselli-Flores, 2021). In these extreme environments, species are not selected by their “best capacities” (see difference in maximum production rates of L. fusiformis and P. salinarum) but by their physiological tolerance to the prevailing “extreme.” As to salinity tolerance, there are a number of mechanisms, mostly physiological, making it possible to exist in inland saline habitats where the salt content may well exceed that of the seawater (Stenger-Kovács et al., 2023). Under such circumstances, it is not the species’ trait affiliations (Padisák & Naselli-Flores 2021; Naselli-Flores & Padisák, 2023b) but their physiological and evolutionary adaptations that may lead to “success.” At present and with the lack of exact physiological studies, we do not know the biochemical instruments that enable the small, less productive green alga P. salinarum to outcompete L. fusiformis.
Nevertheless, according to our experiments, the periodic collapse of L. fusiformis might, therefore, be strongly associated with the combination of rapid environmental changes (Vareschi, 1982; Melack, 1988; Kebede, 1997; Schagerl et al., 2015; Oduor & Kotut, 2016) and biotic factors, such as cyanophage attacks, grazing, or interspecific competition (Peduzzi et al., 2014; Schagerl et al., 2015). Even though L. fusiformis covers a high range of salinity, it is sensitive to rapid changes in the physical environment that predicts the possibility of systematic collapses of the cyanobacterium population in future as a result of the rapid changes in water level in between the dry and flood periods in the East African alkaline saline lakes (Oduor & Kotut, 2016; Bett et al., 2019), especially under the increasing frequency of extreme events driven by the ongoing climate change (Jentsch et al., 2007; Coumou & Rahmstorf, 2012; Reichstein et al., 2013; Costa et al, 2023). Such an incident was experienced in the early 2010’s when L. fusiformis was replaced verifiably by P. salinarum (Krienitz & Kotut, 2010; Oduor & Kotut, 2016). After taking into account all the factors cited above as being responsible for the dominance changes, our experiments demonstrated that the rapid salinity changes are most likely the driver of this process.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Affan, M.-A., D.-W. Lee, S. M. Al-Harbi, H.-J. Kim, N. I. Abdulwassi, S.-J. Heo, C. Oh, H.-S. Park, C. W. Ma, H.-Y. Lee & D.-H. Kang, 2015. Variation of Spirulina maxima biomass production in different depths of urea-used culture medium. Brazilian Journal of Microbiology 46: 991–1000. https://doi.org/10.1590/S1517-838246420140188.
Ballot, A., L. Krienitz, K. Kotut, C. Wiegand, J. S. Metcalf, G. A. Codd & S. Pflugmacher, 2004. Cyanobacteria and cyanobacterial toxins in three alkaline Rift Valley lakes of Kenya-Lakes Bogoria, Nakuru and Elmenteita. Journal of Plankton Research 26: 925–935. https://doi.org/10.1093/plankt/fbh084.
Ballot, A., L. Krienitz, K. Kotut, C. Wiegand & S. Pflugmacher, 2005. Cyanobacteria and cyanobacterial toxins in the alkaline crater lakes Sonachi and Simbi, Kenya. Harmful Algae 4: 139–150. https://doi.org/10.1016/j.hal.2004.01.001.
Barinova, S. & M. Tavassi, 2009. Study of seasonal influences on algal biodiversity in the River Yarqon (central Israel) by bio-indication and canonical correspondence analysis (CCA). Turkish Journal of Botany 33: 353–372. https://doi.org/10.3906/bot-0812-12.
Barinova, S. S., O. V. Anissimova, E. Nevo, M. M. Jarygin & S. P. Wasser, 2004. Diversity and ecology of algae from the Nahal Qishon River, northern Israel. Plant Biosystems 138: 245–259. https://doi.org/10.1080/11263500400006985.
Ben Ali, R., S. Ben Ouada, L. Chouchene, I. Messaoudi, H. Ben Ouada & A. Othmane, 2017. Cadmium effect on physiological responses of the tolerant Chlorophyta species Picocystis sp. isolated from Tunisian wastewaters. Environmental Science and Pollution Research 24: 1803–1810. https://doi.org/10.1007/s11356-016-7950-0.
Ben Ali, R., S. Ben Ouada, C. Leboulanger, J. Ammar, S. Sayadi & H. Ben Ouada, 2021. Bisphenol A removal by the Chlorophyta Picocystis sp.: optimization and kinetic study. International Journal of Phytoremediation 23: 818–828. https://doi.org/10.1080/15226514.2020.1859985.
Ben Ouada, S., R. Ben Ali, C. Leboulanger, H. Ben Ouada & S. Sayadi, 2018a. Effect of bisphenol A on the extremophilic microalgal strain Picocystis sp. (Chlorophyta) and its high BPA removal ability. Ecotoxicology and Environmental Safety 158: 1–8. https://doi.org/10.1016/j.ecoenv.2018.04.008.
Ben Ouada, S., R. Ben Ali, C. Leboulanger, H. Zaghden, S. Choura, H. Ben Ouada & S. Sayadi, 2018b. Effect and removal of bisphenol A by two extremophilic microalgal strains (Chlorophyta). Journal of Applied Phycology 30: 1765–1776. https://doi.org/10.1007/s10811-017-1386-x.
Bernard, C., A. Escalas, N. Villeriot, H. Agogué, M. Hugoni, C. Duval, C. Carré, P. Got, G. Sarazin, D. Jézéquel, C. Leboulanger, V. Grossi, M. Ader & M. Troussellier, 2019. Very low phytoplankton diversity in a tropical saline-alkaline lake, with co-dominance of Arthrospira fusiformis (Cyanobacteria) and Picocystis salinarum (Chlorophyta). Microbial Ecology 78: 603–617. https://doi.org/10.1007/s00248-019-01332-8.
Bett, B., F. T. Otieno & F. Murithi, 2019. Climate change and disease dynamics: predicted changes in ecological niches for Rift Valley fever in East Africa. Encyclopedia of Food Security and Sustainability 3: 469–476. https://doi.org/10.1016/B978-0-08-100596-5.21574-6.
Castro, G. F. P., R. F. Rizzo, T. S. Passos, B. N. C. dos Santos, D. S. Dias, J. R. Domingues & K. G. L. Araújo, 2015. Biomass production by Arthrospira platensis under different culture conditions. Food Science and Technology (campinas) 35: 18–24. https://doi.org/10.1590/1678-457X.6421.
Cellamare, M., C. Duval, Y. Drelin, C. Djediat, N. Touibi, H. Agogué, C. Leboulanger, M. Ader & C. Bernard, 2018. Characterization of phototrophic microorganisms and description of new cyanobacteria isolated from the saline-alkaline crater-lake Dziani Dzaha (Mayotte, Indian Ocean). FEMS Microbiology Ecology 94: 1–25. https://doi.org/10.1093/femsec/fiy108.
Chen, Y. C., 2011. The effect of shifts in medium types on the growth and morphology of Spirulina platensis (Arthrospira platensis). Journal of Marine Science and Technology 19: 565–570. https://doi.org/10.51400/2709-6998.2171.
Ciferri, O., 1983. Spirulina, the edible microorganism. Microbiological Reviews 47: 551–578. https://doi.org/10.1128/mr.47.4.551-578.1983.
Costa, N. B., M. A. Kolman, A. Giani & P. Moisander, 2016. Cyanobacteria diversity in alkaline saline lakes in the Brazilian Pantanal wetland: a polyphasic approach. Journal of Plankton Research 38: 1389–1403. https://doi.org/10.1093/plankt/fbw066.
Costa, M. R. A., M. M. L. Cardoso, G. B. Selmeczy, J. Padisák & V. Becker, 2023. Phytoplankton functional response induced by extreme hydrological events in a tropical reservoir. Hydrobiologia. https://doi.org/10.1007/s10750-023-05241-3.
Coumou, D. & S. Rahmstorf, 2012. A decade of weather extremes. Nature Climate Change 2: 491–496. https://doi.org/10.1038/nclimate1452.
Dadheech, P. K., A. Ballot, P. Casper, K. Kotut, E. Novelo, B. Lemma, T. Pröschold & L. Krienitz, 2010. Phylogenetic relationship and divergence among planktonic strains of Arthrospira (Oscillatoriales, Cyanobacteria) of African, Asian and American origin deduced by 16S–23S ITS and phycocyanin operon sequences. Phycologia 49: 361–372. https://doi.org/10.2216/09-71.1.
Delgado, R. T., M. S. Guarieiro, P. W. Antunes, S. T. Cassini, H. M. Terreros & O. V. Fernandes, 2021. Effect of nitrogen limitation on growth, biochemical composition, and cell ultrastructure of the microalga Picocystis salinarum. Journal of Applied Phycology 33: 2083–2092. https://doi.org/10.1007/s10811-021-02462-8.
Fanjing, K., J. Qinxian, E. Jia & Z. Mianping, 2009. Characterization of a eukaryotic picoplankton alga, strain DGN-Z1, isolated from a soda lake in inner Mongolia, China. Natural Resources and Environmental Issues 15: 185–189.
Fott, B. & A. G. A. Karim, 1973. Spirulina plankton community in a lake in Jebel Marra, Sudan. Archiv Für Protistenkunde 115: 408–418.
Fužinato, S., A. Fodora & G. Subakov-Simić, 2010. Arthrospira fusiformis (Voronichin) Komárek et Lund (Cyanoprokaryota)—a new species for Europe. Algological Studies 134: 17–24. https://doi.org/10.1127/1864-1318/2010/0134-0017.
Girma, M. B., D. Kifle & H. Jebessa, 2012. Deep underwater seismic explosion experiments and their possible ecological impact—the case of Lake Arenguade-Central Ethiopian highlands. Limnologica 42: 212–219. https://doi.org/10.1016/j.limno.2011.12.002.
Glabonjat, R. A., J. S. Blum, L. G. Miller, S. M. Webb, J. F. Stolz, K. A. Francesconi & R. S. Oremland, 2020. Arsenolipids in cultured Picocystis strain ML and their occurrence in biota and sediment from Mono Lake, California. Life 10: 93. https://doi.org/10.3390/life10060093.
Hamad, G. M., N. Abd El-Baky, M. M. Sharaf & A. A. Amara, 2023. Volatile compounds, fatty acids constituents, and antimicrobial activity of cultured Spirulina (Arthrospira fusiformis) isolated from Lake Mariout in Egypt. The Scientific World Journal 2023: 1–9. https://doi.org/10.1155/2023/9919814.
Hamisi, M. I., C. Lugomela, T. J. Lyimo, B. Bergman & B. Díez, 2017. Plankton composition, biomass, phylogeny and toxin genes in Lake Big Momela, Tanzania. African Journal of Aquatic Science 42: 109–121. https://doi.org/10.2989/16085914.2017.1334621.
Hammer, U. T., 1986. Saline Lake Ecosystems of the World, Springer, New York:
Harper, D. M., R. B. Childress, M. M. Harper, R. R. Boar, P. H. Hickley, S. C. Mills, N. Otieno, T. Drane, E. Vareschi, O. Nasirwa, E. E. Mwatha, J. P. E. C. Darlington & X. Escuté-Gasulla, 2003. Aquatic biodiversity and saline lakes: lake Bogoria National Reserve, Kenya. Hydrobiologia 500: 259–276. https://doi.org/10.1023/A:1024722821407.
Hill, L. M., W. W. Bowerman, J. C. Roos, W. C. Bridges & M. D. Anderson, 2013. Effects of water quality changes on phytoplankton and lesser flamingo Phoeniconaias minor populations at Kamfers Dam, a saline wetland near Kimberley. South Africa. African Journal of Aquatic Science 38: 287–294. https://doi.org/10.2989/16085914.2013.833889.
Hindák, F., 1985. Morphology of trichomes in Spirulina fusiformis Voronichin from Lake Bogoria, Kenya. Archiv für Hydrobiologie, Supplement Volumes 71. Algological Studies 38–39: 201–218.
Humphrey, G. F., 1975. The photosynthesis: respiration ratio of some unicellular marine algae. Journal of Experimental Marine Biology and Ecology 18: 111–119. https://doi.org/10.1016/0022-0981(75)90068-4.
Iltis, A., 1969. Phytoplancton des eaux natronées du Kanem (Tchad): 1. Les lacs permanents à spirulines. Cahiers ORSTOM. Série Hydrobiologie 3: 29–44.
Iltis, A., 1971. Phytoplancton des eaux natronées du Kanem (Tchad): 5. Les lacs mésohalins. Cahiers ORSTOM. Série Hydrobiologie 5: 73–84.
Jenkin, P. M., 1957. The filter-feeding and food of flamingoes (Phoenicopteri). Philosophical Transactions of the Royal Society of London Series b, Biological Sciences 240: 401–493. https://doi.org/10.1098/rstb.1957.0004.
Jentsch, A., J. Kreyling & C. Beierkuhnlein, 2007. A new generation of climate-change experiments: events, not trends. Frontiers in Ecology and the Environment 5: 315–324. https://doi.org/10.1890/1540-9295(2007)5[365:ANGOCE]2.0.CO;2.
Jirsa, F., M. Gruber, A. Stojanovic, S. O. Omondi, D. Mader, W. Körner & M. Schagerl, 2013. Major and trace element geochemistry of Lake Bogoria and Lake Nakuru, Kenya, during extreme draught. Geochemistry 73: 275–282. https://doi.org/10.1016/j.chemer.2012.09.001.
Kaggwa, M. N., A. Burian, S. O. Oduor & M. Schagerl, 2013a. Ecomorphological variability of Arthrospira fusiformis (Cyanoprokaryota) in African soda lakes. MicrobiologyOpen 2: 881–891. https://doi.org/10.1002/mbo3.125.
Kaggwa, M. N., M. Gruber, S. O. Oduor & M. Schagerl, 2013b. A detailed time series assessment of the diet of lesser flamingos: further explanation for their itinerant behaviour. Hydrobiologia 710: 83–93. https://doi.org/10.1007/s10750-012-1105-1.
Kebede, E., 1997. Response of Spirulina platensis (=Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. Journal of Applied Phycology 9: 551–558. https://doi.org/10.1023/A:1007949021786.
Kebede, E. & G. Ahlgren, 1996. Optimum growth conditions and light utilization efficiency of Spirulina platensis (=Arthrospira fusiformis) (Cyanophyta) from Lake Chitu, Ethiopia. Hydrobiologia 332: 99–109. https://doi.org/10.1007/BF00016689.
Kihwele, E. S., C. Lugomela & K. M. Howell, 2014. Temporal changes in the lesser flamingos population (Phoenicopterus minor) in relation to phytoplankton abundance in Lake Manyara, Tanzania. Open Journal of Ecology 04: 145–161. https://doi.org/10.4236/oje.2014.43016.
Krienitz, L., 2018. Lesser Flamingos Descendants of Phoenix, Springer, Berlin: https://doi.org/10.1007/978-3-662-58163-6.
Krienitz, L. & K. Kotut, 2010. Fluctuating algal food populations and the occurrence of lesser flamingos (Phoeniconaias minor) in three Kenyan Rift Valley Lakes. Journal of Phycology 46: 1088–1096. https://doi.org/10.1111/j.1529-8817.2010.00915.x.
Krienitz, L., C. Bock, K. Kotut & W. Luo, 2012. Picocystis salinarum (Chlorophyta) in saline lakes and hot springs of East Africa. Phycologia 51: 22–32. https://doi.org/10.2216/11-28.1.
Krienitz, L., P. K. Dadheech & K. Kotut, 2013. Mass developments of a small sized ecotype of Arthrospira fusiformis in Lake Oloidien, Kenya, a new feeding ground for lesser flamingos in East Africa. Fottea 13: 215–225. https://doi.org/10.5507/fot.2013.017.
Krienitz, L., D. Krienitz, P. K. Dadheech, T. Hübener, K. Kotut, W. Luo, K. Teubner & W. D. Versfeld, 2016. Food algae for lesser flamingos: a stocktaking. Hydrobiologia 775: 21–50. https://doi.org/10.1007/s10750-016-2706-x.
Kumssa, T. & A. Bekele, 2014. Feeding ecology of lesser flamingos (Phoeniconaias minor) in Abijata-Shalla Lakes National Park (ASLNP) with special reference to lakes Abijata and Chitu, Ethiopia. Asian Journal of Biological Sciences 7: 57–65. https://doi.org/10.3923/ajbs.2014.57.65.
Leatherbarrow, R. J., 2009. GraFit Data Analysis Software for Windows, Erithacus Software Ltd., Horley:
Lengyel, E., A. W. Kovács, J. Padisák & C. Stenger-Kovács, 2015. Photosynthetic characteristics of the benthic diatom species Nitzschia frustulum (Kützing) Grunow isolated from a soda pan along temperature-, sulfate- and chloride gradients. Aquatic Ecology 49: 401–416. https://doi.org/10.1007/s10452-015-9533-4.
Lewin, R. A., L. Krienitz, R. Goericke, H. Takeda & D. Hepperle, 2000. Picocystis salinarum gen. et sp. nov. (Chlorophyta)—a new picoplanktonic green alga. Phycologia 39: 560–565. https://doi.org/10.2216/i0031-8884-39-6-560.1.
Lugomela, C., H. B. Pratap & Y. D. Mgaya, 2006. Cyanobacteria blooms—a possible cause of mass mortality of lesser flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae 5: 534–541. https://doi.org/10.1016/j.hal.2005.10.001.
Luo, W., L. Huirong, K. Kotut & L. Krienitz, 2017. Molecular diversity of plankton in a tropical crater lake switching from hyposaline to subsaline conditions: Lake Oloidien, Kenya. Hydrobiologia 788: 2005–2229. https://doi.org/10.1007/s10750-016-2998-x.
Makeeva, E. G. & N. V. Osipova, 2022. Algae of the salt Lake Altaiskoye (Republic of Khakassia): taxonomic composition and ecological features. Inland Water Biology Pleiades Journals 15: 107–114. https://doi.org/10.1134/S1995082922020067.
Melack, J. M., 1979. Photosynthesis and growth of Spirulina platensis (Cyanophyta) in an equatorial lake (Lake Simbi, Kenya). Limnology and Oceanography 24: 753–760. https://doi.org/10.4319/lo.1979.24.4.0753.
Melack, J. M., 1981. 7. Photosynthetic activity of phytoplankton in tropical African soda lakes. Hydrobiologia 81: 71–85. https://doi.org/10.1007/BF00048707.
Melack, J. M., 1988. Primary producer dynamics associated with evaporative concentration in a shallow, equatorial soda lake (Lake Elmenteita, Kenya). Hydrobiologia 158: 1–14. https://doi.org/10.1007/BF00026264.
Melack, J. M. & P. Kilham, 1974. Photosynthetic rates of phytoplankton in East African alkaline, saline lakes. Limnology and Oceanography 19: 743–755. https://doi.org/10.4319/lo.1974.19.5.0743.
Melack, J. M., P. Kilham & R. Fisher, 1982. Responses of phytoplankton to experimental fertilization with ammonium and phosphate in an African soda lake. Oecologia 52: 321–326. https://doi.org/10.1007/BF00367954.
Mgimwa, E. F., J. R. John & C. V. Lugomela, 2021. The influence of physical–chemical variables on phytoplankton and lesser flamingo (Phoeniconaias minor) abundances in Lake Natron, Tanzania. African Journal of Ecology 59: 667–675. https://doi.org/10.1111/aje.12863.
Millennium Ecosystem Assessment, 2003. Ecosystems and Human Well-Being: A Framework for Assessment, Island Press, Washington, DC:
Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-Being: Synthesis, Island Press, Washington, DC:
Moustaka-Gouni, M., E. Michaloudi, M. Katsiapi & S. Genitsari, 2007. The coincidence of an Arthrospira-Anabaenopsis bloom and the mass mortality of birds in Lake Koronia. Harmful Algae News. 35: 6–7.
Mungoma, S., 1990. The alkaline, saline lakes of Uganda: a review. Hydrobiologia 208: 75–80. https://doi.org/10.1007/BF00008445.
Naselli-Flores, L. & J. Padisák, 2023a. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia. https://doi.org/10.1007/s10750-022-04795-y.
Naselli-Flores, L. & J. Padisák, 2023b. Analysis of morphological traits as a tool to identify the realized niche of phytoplankton populations: what do the shape of planktic microalgae, Anna Karenina and Vincent van Gogh have in common? Hydrobiologia. https://doi.org/10.1007/s10750-023-05195-6.
Oduor, S. O. & M. Schagerl, 2007a. Temporal trends of ion contents and nutrients in three Kenyan Rift Valley saline–alkaline lakes and their influence on phytoplankton biomass. Hydrobiologia 584: 59–68. https://doi.org/10.1007/s10750-007-0605-x.
Oduor, S. O. & M. Schagerl, 2007b. Phytoplankton primary productivity characteristics in response to photosynthetically active radiation in three Kenyan Rift Valley saline-alkaline lakes. Journal of Plankton Research 29: 1041–1050. https://doi.org/10.1093/plankt/fbm078.
Oduor, S. O. & K. Kotut, 2016. Soda lakes of the East African Rift System: the past, the present and the future. In Schagerl, M. (ed), Soda Lakes of East Africa. Springer, Cham. https://doi.org/10.1007/978-3-319-28622-8_15.
Ogato, T. & D. Kifle, 2014. Morphological variability of Arthrospira (Spirulina) fusiformis (Cyanophyta) in relation to environmental variables in the tropical soda lake Chitu, Ethiopia. Hydrobiologia 738: 21–33. https://doi.org/10.1007/s10750-014-1912-7.
Padisák, J. & L. Naselli-Flores, 2021. Phytoplankton in extreme environments: importance and consequences of habitat permanency. Hydrobiologia 848: 157–176. https://doi.org/10.1007/s10750-020-04353-4.
Pálmai, T., V. Üveges, L. Krienitz & J. Padisák, 2013. Az Arthrospira fusiformis és a Picocystis salinarum fotoszintézisének karakterisztikái különböző fényintenzitásokon és hőmérsékleten. Hidrológiai Közlöny 93: 64–66.
Pálmai, T., B. Szabó, K. Kotut, L. Krienitz & J. Padisák, 2020. Ecophysiology of a successful phytoplankton competitor in the African flamingo lakes: the green alga Picocystis salinarum (Picocystophyceae). Journal of Applied Phycology 32: 1813–1825. https://doi.org/10.1007/s10811-020-02092-6.
Peduzzi, P., M. Gruber, M. Gruber & M. Schagerl, 2014. The virus’s tooth: cyanophages affect an African flamingo population in a bottom-up cascade. The ISME Journal 8: 1346–1351. https://doi.org/10.1038/ismej.2013.241.
Phillips, A. A., D. R. Speth, L. G. Miller, X. T. Wang, F. Wu, P. M. Medeiros, D. R. Monteverde, M. R. Osburn, W. M. Berelson, H. L. Betts, R. S. Wijker, S. W. Mullin, H. A. Johnson, V. J. Orphan, W. W. Fischer & A. L. Sessions, 2021. Microbial succession and dynamics in meromictic Mono Lake, California. Geobiology 19: 376–393. https://doi.org/10.1111/gbi.12437.
Platt, T., C. L. Gallegos & W. G. Harrison, 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research 38: 687–701.
Reichstein, M., M. Bahn, P. Ciais, D. Frank, M. D. Mahecha, S. I. Seneviratne, J. Zscheischler, C. Beer, N. Buchmann, D. C. Frank, D. Papale, A. Rammig, P. Smith, K. Thonicke, M. van der Velde, S. Vicca, A. Walz & M. Wattenbach, 2013. Climate extremes and the carbon cycle. Nature 500: 287–295. https://doi.org/10.1038/nature12350.
Renaut, R. W., R. B. Owen & J. K. Ego, 2017. Geothermal activity and hydrothermal mineral deposits at southern Lake Bogoria, Kenya Rift Valley: Impact of lake level changes. Journal of African Earth Sciences 129: 623–646. https://doi.org/10.1016/j.jafrearsci.2017.01.012.
Rich, F., 1932. Reports on the Percy Sladen Expedition to some Rift Valley Lakes in Kenya in 1929.—IV. Phytoplankton from the Rift Valley Lakes in Kenya. Annals and Magazine of Natural History 10: 233–262. https://doi.org/10.1080/00222933208673571.
Ridley, M. W., B. L. Moss & L. R. C. Percy, 1955. The food of flamingos in Kenya Colony. Journal of the East African Natural History Society. 22: 147–158.
Roesler, C. S., C. W. Culbertson, S. M. Etheridge, R. Goericke, R. P. Kiene, L. G. Miller & R. S. Oremland, 2002. Distribution, production, and ecophysiology of Picocystis strain ML in Mono Lake, California. Limnology and Oceanography 47: 440–452. https://doi.org/10.4319/lo.2002.47.2.0440.
Ronga, D., E. Biazzi, K. Parati, D. Carminati, E. Carminati & A. Tava, 2019. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9: 192. https://doi.org/10.3390/agronomy9040192.
Samylina, O. S., L. M. Gerasimenko & N. V. Shadrin, 2010. Comparative characteristic of the phototroph communities from the mineral lakes of Crimea (Ukraine) and Altai Region (Russia). International Journal on Algae 12: 142–158. https://doi.org/10.1615/InterJAlgae.v12.i2.40.
Schagerl, M., 2016. Soda lakes of East Africa. Springer, Cham. https://doi.org/10.1007/978-3-319-28622-8.
Schagerl, M. & A. Burian, 2016. The ecology of African soda lakes: driven by variable and extreme conditions. In Schagerl, M. (ed), Soda Lakes of East Africa. Springer, Cham. https://doi.org/10.1007/978-3-319-28622-8_12.
Schagerl, M. & S. O. Oduor, 2008. Phytoplankton community relationship to environmental variables in three Kenyan Rift Valley saline-alkaline lakes. Marine and Freshwater Research 59: 125. https://doi.org/10.1071/MF07095.
Schagerl, M., A. Burian, M. Gruber-Dorninger, S. O. Oduor & M. N. Kaggwa, 2015. Algal communities of Kenyan soda lakes with a special focus on Arthrospira fusiformis. Fottea 15: 245–257. https://doi.org/10.5507/fot.2015.012.
Schagerl, M., R. Angel, U. Donabaum, A. M. Gschwandner & D. Woebken, 2022. Limnospira fusiformis harbors dinitrogenase reductase (nifH)-like genes, but does not show N2 fixation activity. Algal Research 66: 102771. https://doi.org/10.1016/j.algal.2022.102771.
Shafik, H. M., S. Herodek, M. Présing & L. Vörös, 2001. Factors effecting growth and cell composition of cyanoprokaryote Cylindrospermopsis raciborskii (Wołoszyńska) Seenayya et Subba Raju. Algological Studies/archiv Für Hydrobiologie 103: 75–93. https://doi.org/10.1127/algol_stud/103/2001/75.
Shafik, H. M., T. Pálmai & J. Padisák, 2014. Módosított tápoldat egy trópusi sós tóból izolált Arthrospira fusiformis és Picocystis salinarum algafajok számára. Hidrológiai Közlöny 94: 43–45.
Shao, W., R. Ebaid, M. El-Sheekh, A. Abomohra & H. Eladel, 2019. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): an overview. Grasas y Aceites 70: 292. https://doi.org/10.3989/gya.0690181.
Shiklomanov, I. A., 1990. Global water resources. Nature and Resources 26: 34–43.
Sili, C., G. Torzillo & A. Vonshak, 2012. Arthrospira (Spirulina). In Whitton, B. A. (ed), Ecology of Cyanobacteria II. Springer, Berlin.
Singh, J., C. Maharana & D. W. Dhar, 2022. Alkalihalophilic alga Picocystis salinarum SLJS6 from Sambhar Salt Lake: potential for bicarbonate-based biomass production and carbon capture. Bioresource Technology Reports 20: 101252. https://doi.org/10.1016/j.biteb.2022.101252.
Singh, J., S. Kaushik, C. Maharana, G. D. Jhingan & D. W. Dhar, 2023. Elevated inorganic carbon and salinity enhances photosynthesis and ATP synthesis in picoalga Picocystis salinarum as revealed by label free quantitative proteomics. Frontiers in Microbiology 14: 1059199. https://doi.org/10.3389/fmicb.2023.1059199.
Stenger-Kovács, C. & V. B. Béres, K. Buczkó, J. T. Al-Imari, D. Lázár, J. Padisák & E. Lengyel, 2023. Review of phenotypic response of diatoms to salinization with biotechnological relevance. Hydrobiologia. https://doi.org/10.1007/s10750-023-05194-7.
Talling, J. F., R. B. Wood, M. V. Prosser & R. M. Baxter, 1973. The upper limit of photosynthetic productivity by phytoplankton: evidence from Ethiopian soda lakes. Freshwater Biology 3: 53–76. https://doi.org/10.1111/j.1365-2427.1973.tb00062.x.
Tarazona Delgado, R., H. M. Terreros, M. M. Astocóndor & E. M. Huatuco, 2017. Picocystis salinarum (Prasinophyceae, Chlorophyta) en las Salinas de Chilca, Lima, primer registro para el Perú. Arnaldoa 24: 557–566.
Tuite, C. H., 1981. Standing crop densities and distribution of Spirulina and benthic diatoms in East African alkaline saline lakes. Freshwater Biology 11: 345–360. https://doi.org/10.1111/j.1365-2427.1981.tb01266.x.
Tuite, C. H., 2000. The distribution and density of lesser flamingos in East Africa in relation to food availability and productivity. Waterbirds: the International Journal of Waterbird Biology 23: 52–63. https://doi.org/10.2307/1522147.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. SIL Communications 9: 1–38. https://doi.org/10.1080/05384680.1958.11904091.
Üveges, V., K. Tapolczai, L. Krienitz & J. Padisák, 2012. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 698: 263–272. https://doi.org/10.1007/s10750-012-1103-3.
Van Liere, L. & L. R. Mur, 1980. Occurrence of Oscillatoria agardhii and some related species, a survey. In Barica, J. & L. R. Mur (eds), Hypertrophic Ecosystems Developments in Hydrobiology, Vol. 2. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-9203-0_8.
Vareschi, E., 1978. The ecology of Lake Nakuru (Kenya)—I. Abundance and feeding of the lesser flamingo. Oecologia 32: 11–35. https://doi.org/10.1007/BF00344687.
Vareschi, E., 1979. The ecology of Lake Nakuru (Kenya)—II. Biomass and spatial distribution of fish (Tilapia grahami Boulenger=Sarotherodon alcalicum grahami Boulenger). Oecologia 37: 321–335. https://doi.org/10.1007/BF00347909.
Vareschi, E., 1982. The ecology of Lake Nakuru (Kenya)—III Abiotic Factors and Primary Production. Oecologia 55: 81–101. https://doi.org/10.1007/BF00386722.
Vareschi, E. & J. Jacobs, 1984. The ecology of Lake Nakuru (Kenya)—V. Production and consumption of consumer organisms. Oecologia 61: 83–98. https://doi.org/10.1007/BF00379092.
Vareschi, E. & A. Vareschi, 1984. The ecology of Lake Nakuru (Kenya)—IV. Biomass and distribution of consumer organisms. Oecologia 61: 70–82. https://doi.org/10.1007/BF00379091.
Vonshak, A., 1997. Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. CRC Press, London. https://doi.org/10.1201/9781482272970.
Wang, S., W. Lambert, S. Giang, R. Goericke & B. Palenik, 2014. Microalgal assemblages in a poikilohaline pond. Journal of Phycology 50: 303–309. https://doi.org/10.1111/jpy.12158.
Webb, W. L., M. Newton & D. Starr, 1974. Carbon dioxide exchange of Alnus rubra - A mathematical model. Oecologia 17: 281–291. https://doi.org/10.1007/BF00345747.
Wetzel, R. G. & G. E. Likens, 2000. Limnological analyses. Springer, Nova York. https://doi.org/10.1007/978-1-4757-3250-4.
Williams, W. D., 1993. Conservation of salt lakes. Hydrobiologia 267: 291–306. https://doi.org/10.1007/BF00018809.
Acknowledgements
We thank Dávid Németh for his technical assistance in the laboratory works during the pilot study.
Funding
Open access funding provided by ELKH Centre for Agricultural Research. This work was supported by the ÚNKP-18-IV-PE-12 New National Excellence Program of the Ministry of Human Resources. JP and EL were supported by the NKFIH KKP 144068 during manuscript writing. TP was supported by the National Research, Development and Innovation Office ‘OTKA’ K 128575 and TKP2021-NKTA-06 during manuscript writing.
Author information
Authors and Affiliations
Contributions
KK and LK studied phytoplankton communities in soda lakes and came up with the idea of testing the behavior of competing major players under controlled experimental laboratory conditions; they collected field samples, isolated the species, and established pure cultures. TP, BSz, EL, and JP conceived and designed the experiments. TP, BSz, and EL kept up the cultures, performed the experiments, and made photos. TP, BSz, and EL analyzed the data. TP wrote the first draft and all other authors improved the manuscript. All authors have read the submitted version of the manuscript and approved its submission.
Corresponding author
Ethics declarations
Conflict of interest
All Authors declare that they have no competing interest.
Ethical approval
Not Applicable.
Additional information
Handling editor: John M. Melack
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pálmai, T., Szabó, B., Lengyel, E. et al. Growth response of the picoplanktic Picocystis salinarum and the microplanktic Limnospira (Arthrospira) fusiformis strains from Lake Nakuru (Kenya) to rapidly changing environmental conditions. Hydrobiologia 851, 1873–1889 (2024). https://doi.org/10.1007/s10750-023-05397-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05397-y