Abstract
Long-term ecological research of deep Lake Mondsee covers over more than six decades of phytoplankton observation. According to our study, phytoplankton groups of various phenological traits are suitable to address the impact of two major environmental stressors: nutrient surplus by eutrophication from 1968 to 1998 and warming by climate change. Here, we focus on phytoplankton biovolume, phytoplankton assemblage structure, net changes rates, and phytoplankton biodiversity. Biweekly net change-dependent persistence of phytoplankton assemblages followed a dome-shaped relationship of observations. A short-term persistence of taxonomic traits along weeks is predominant and contributes to the structural stabilization of phytoplankton assemblages. This short-term persistence is interpreted by the benefit of lifetime adjustment of phytoplankton organisms. The long-term development phytoplankton structure is discussed as an alignment of organisms over generations. Single key taxa, as Planktothrix rubescens (De Candolle ex Gomont) Anagnostidis &Komárek 1988, which are omnipotent players in phytoplankton assemblages and occur during different environmental scenarios in the long-term, are most suitable for long-term ecological research. Our findings interpret that phytoplankton taxa are excellent organisms to track the impact of environmental constraints due to their short generation time (1), their lifetime adjustment (2), and the re-occurrence in the long-term over generations (3).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplankton organisms can adjust to environmental changes over time windows from seconds to days by regulation and acclimation (Raven & Geider, 2003) during their short life cycle but can built up large population densities lasting for weeks and months. The generation time of algae in the lab and field depends strongly on their physiological background and growth conditions with reported timespans from one day (shortest is few hours) to about one month (Feuillade & Krupka, 1986; Padisák et al. 1988; Halstvedt et al., 2007). Short-term responses during species lifetime are then mirrored by changes in phytoplankton dominance structure over weeks to months, mirrored in a seasonal pattern. Adaptation to different environmental conditions, such as eutrophication or global warming, over years is a dynamic process which may lead to a phenotypic change of thousands of generations after exposure to novel situations. Species shifts at longer timespans are thus accomplished by adjustments of their functional traits. Such modifications over generation time may be achieved by species persistence due to their phenotypic plasticity, inter-annual replacement of species due to directional selection, or long-term adaptation to environmental trends by genetic alterations (Zotina et al., 2003; Falkowski et al., 2004; Greisberger & Teubner 2007; Anneville et al., 2018).
Long-term investigations in phytoplankton therefore turn out to be essential to quantify temporal changes in assemblage structure and biomass, assessing phytoplankton trends through their response to the type and intensity of environmental traits over decades (Kamenir et al., 2006; Salmaso, 2010; Pomati et al., 2015; Morabito et al., 2018). Perturbations in long-term development of phytoplankton assemblages are often associated with multiannual or decadal environmental alterations because of eutrophication, oligotrophication mediated by restoration, climate warming and several additional impacts (Hajnal & Padisák, 2008; Ostrovsky et al., 2013; Teubner et al., 2018; Znachor et al., 2020).
Over an extended period, the response of phytoplankton to environmental modifications was studied in several large and deep peri-alpine lakes. Changes in cell size, species diversity, and composition appeared in Lago Maggiore when concentrations of total phosphorus declined below 15 µg l−1 (Ruggiu et al., 1998). Findings from Lake Geneva suggest that phytoplankton biomass is largely influenced by zooplankton during recovery from eutrophication (Anneville et al., 2019). The effect of regime shifts on the stability of phytoplankton populations during nutrient enrichment and decline in Lake Constance was linked to a loss in ability of algal groups to compensate changes (Jochimsen et al., 2012).
Information on Austrian lakes north of the Alps during the oligo-mesotrophic period in the first half of the twentieth century is fragmented (Brehm & Zederbauer, 1906; Ruttner, 1937). In the 1950s, untreated effluents from settlements and cyanobacterial blooms caused by P. rubescens presented a challenge (Liepolt, 1957) and expanded to many lakes in Austria including Mondsee. Dokulil & Skolaut (1991) and Dokulil (1993) analyzed the succession and response of phytoplankton to these changes in Mondsee. The limnological development and monitoring of Mondsee was outlined by Luger et al. (2021). An earlier overview compared Lake Mondsee with the head-water Lake Irrsee, sharing the same catchment as Mondsee (Achleitner et al., 2007). The progress of eutrophication and restoration since the 1950s indicating broad improvements in water quality was summarized for several peri-alpine lakes in Austria by Dokulil (2017, 2020). The course of (re)-oligotrophication as a result of reductions in nutrient loading to lakes was evaluated for 35 case studies by Jeppesen et al. (2005), which also included Mondsee. This study could show that phytoplankton biomass declined in response to reduced total phosphorus (TP) concentrations accompanied by shifts in assemblage structure across many lakes.
In our study, here we focus on the successive phytoplankton development for more than half a century in Mondsee. For decades of intensive sampling, we assess the short-term structural changes at biweekly intervals, in addition to those along long-term changes over decades. These two timescales are interpreted to cover the adjustment of organism’s during lifetime (1) and over generations (2). We postulate that phytoplankton groups of similar taxonomic traits are suitable entities to track structural shifts of phytoplankton assemblages at both timescales. We address phytoplankton biovolume, phytoplankton assemblage structure, net changes rates, and phytoplankton biodiversity.
Materials and methods
Site description
The peri-alpine lake Mondsee (481 m asl, maximum depth 68 m, area 13.8 km2) lies in Austria’s northern lake district, the Salzkammergut, east of the city of Salzburg (Longitude 13°22′29″, Latitude 47 49′24″). The lake belongs to the river Ager catchment. Head water lakes are Fuschlsee, Irrsee which both drain into Mondsee. The outflow then connects to Attersee and finally to the Ager River. Coordinates, morphometric data, and further information for the lakes can be obtained from BAW (2010). Average physical and chemical variables are summarized for a depth profile averaged for the years 2018 to 2020 (Table 1).
The long-term climatic trend at the meteorological station Mondsee is characterized by annual means for two decades of the observation period 1970 to 2020. Average near-surface air temperature increased from 8.9 ± 1.2 °C for the years 1970–1979 to 10.2 ± 0.9 °C (years 2010–2019). Average total solar radiation (TIR) rose from 37 ± 2 to 40 ± 2 J m−2 while mean sunshine duration increased from 1,555 ± 139 to 1,831 ± 121 h for the two decades, respectively (Kurmayer et al., 2022). Duration of ice-cover varied from 74 days in 1982 to no ice recently. In the twenty-first century, the lake was only ice-covered in the years 2002, 2006, and 2012. On average, the lake was ice-covered for 14 days in the period from 1982 to 2022.
Sampling and data sources
Phytoplankton has been regularly monitored since 1978. Quantitative data prior to 1978 were digitized from graphs in Findenegg (1959a, 1959b, 1965, 1969). Additional data for chlorophyll-a and phytoplankton biomass, particularly P. rubescens, were extracted from Jantsch (1977) and Müller-Jantsch (1979). Quantitative phytoplankton samples were compiled for the period 1977 to 2020 from several authors (Table 2).
Sampling intervals ranged from weekly (1982–1984) to monthly (1985–1987) were biweekly between 1987 and 2004 and monthly thereafter. Sampling depth changes somewhat over the years, but between autumn 1981 and 2020 integrated samples were taken from the upper 20 m on all occasions with an integrating sampler (Züllig Co.; Schröder, 1969). Samples for phytoplankton enumeration were preserved with 200 µl Lugol solution according to Utermöhl (1958) in brown 100-ml glass bottles added and stored in the dark at 4 °C till analysis.
Cell numbers were estimated with an inverted microscope using the sedimentation technique developed by Utermöhl (1958). From 1995 till 2004, a computerized counting program was used for enumeration and biovolume (BV) analysis (Hamilton, 1990). Cell dimensions were measured with an image analysis system (Lucia, V 3.1, Prague) and cell volumes calculated using simple geometric approximations (Rott, 1981). Species abundance was then converted to biovolume, identical to biomass when multiplied by cell density (Schagerl et al., 2022).
The dataset for analyzing phytoplankton structure refers to the BV fractions of functional taxonomic entities representing the whole phytoplankton composition. These are for the cyanobacteria: Chroococcales, Oscillatoriales (mainly P. rubescens) and Nostocales; for Bacillariophyceae: solitary centric diatoms, filamentous centric diatoms (Aulacoseira) and pennate diatoms, for Chlorophyceae: Volvovales and Chlorococcales, further charophytes: Desmidiales, Cryptophyceae, Chrysophyceae and dinophytes (dinoflagellates). Taxonomic references are reported in AlgaeBase [http://www.algaebase.org; searched on 18 Jan 2023].
Missing BV data for the year 2006 were converted from chlorophyll-a to total biovolume (r2 = 0.62) assuming a chlorophyll content of 0.5%. Algal classes for the year 2006 were approximated from mean algal class percentages before and after 2006.
Diversity changes in the phytoplankton community were assessed by the Shannon Index (H′) using biovolumes according to:
where pi is the proportion of species i-biovolume to the total biovolume of all species.
A simple technique for the detection of different periods is cumulative sums (CUSUM) where the minimum and maximum points on the curve mark the beginning and end of a period. Here, we used rescaled adjusted partial sums (RAPS), part of the CUSUM family similar to Z-scores, which can visualize trends, shifts and periodicities in data records (Garbrecht & Fernandez, 1994). The cumulative sum calculation subtracts the mean of the series from the data divided by the variance, and successive cumulation of the residuals (Ibanez et al., 1993). Successive negative residuals produce a decreasing slope, whereas successive positive residuals create an increasing slope (the value of the slope is proportional to the mean deviation). Values not very different from the mean show no slope.
Net change rates of chl-a were calculated from biweekly time intervals for 1982–2002 as follows:
where chl-at2 and chl-at1 are the chl-a concentrations at time t = t2 and t1, respectively, and Δt is the time-span in number of days (Teubner et al., 2003, 2006). Likewise, the persistence of phytoplankton composition at biweekly time intervals was calculated as Bray–Curtis similarity index (0–100) using the software package PRIMER 5 (further description of data treatment see Teubner et al., 2018). For analyzing the short-term compositional shifts of phytoplankton, aimed to fit to time-window of lifetime adjustment of phytoplankton organisms, the phytoplankton composition by Bray–Curtis similarity refers to sampling pairs of successive observations every second week. Assessing long-term compositional shifts in phytoplankton by Bray–Curtis similarity, the assemblage of 1982 was used as reference (start of our measuring campaign) and compared to the ongoing evolution of phytoplankton in time steps of 2 weeks during two decades. This assessment aims at assemblage shifts over generations of phytoplankton organisms. In both cases, Bray–Curtis similarity was calculated from BVs of taxonomic phytoplankton entities which are mentioned in the methods above.
Surface water temperatures (SWT) from 0.5 m depth were used as a proxy for climate change. The mean, maximum, and minimum depth of the euphotic zone (Zeu), either measured or calculated from Secchi-disk readings (SD), indicated variations in the underwater light field. A conversion factor of 3 was estimated by systematically combining UW-light measurements with Secchi depth. Total phosphorus (TP) concentrations were used as proxy for the trophic situation. TP for the years 1962 to 1977 has been approximated from paleolimnological diatom and cyanobacterial data (Dokulil & Teubner, 2005). Missing phytoplankton BV data for the years 1967 and 1973 to 1976 were approximated from a significant regression of measured TP concentrations versus plankton biovolume (BV = 0.318 + 0.0647TP, r2 = 0.49, n = 54, F = 50.52, P < 0.001). The thermal summer stratification refers to the time-span of thermal resistance to mixing (RTRM) indices > 8.5 (early summer onset with RTRM > 8.5 varies between Julian Day 112 and 149, autumn off-set with RTRM > 8.5 from day 289 to 316 for 1982 to 2002). Measurements of soluble reactive silica (SRSi) and N–NO3 refer to the sampling period from 1982 to 2002. Before 1985, temperature (°C) was determined at site with a mercury thermometer mounted within the sampler. In the laboratory, conductivity was measured with an electrode at reference temperature of 20 °C and oxygen concentrations were quantified by Winkler titration (ISO 7888, 1985; Winkler, 1888). From 1985 onward, temperature, pH, conductivity, and oxygen were measured using a Yellow Springs multiparameter Sonde 6920. Determination of phosphorus and nitrogen compounds used spectrometric methods (ISO 6878, 2004; ISO 11905-1, 1997).
Principal component (PCA) and canonical correspondence analysis (CCA) were performed in ‘Past’ Software, V4.11 (Hammer et al., 2001, https://www.nhm.uio.no/english/research/resources/past/). Variables were standardized prior to analysis (mean/stdev). In total, 73,448 data points were analyzed for biovolume and diversity. Normal distribution was done with the Kolmogorov–Smirnov test using ‘SigmaPlot’ V15 (Systat ©).
Results
Early phytoplankton observations
Data about early phytoplankton records were compiled in Table 3 concerning few species of dinoflagellates, chrysophytes, diatoms, and cyanobacteria. Phytoplankton species from lakes in the Mondsee area were first listed in a study on 18 lakes in Austria by Imhof (1885). Mondsee proved richest with four species of Dinobryon, one Peridinium and one Ceratium designated as ‘Protozoa,’ within ‘pelagic fauna’ at that time. Brehm & Zederbauer (1906) described Oscillaria rubescens (later named Oscillatoria, at present Planktothrix) at 0, 2 and 5 m in a sample from December 1901 as rare. The species was not detected in samples from the same depths in March 1902. The authors compered their finding to observations in other lakes and stated ‘Oscillaria rubescens is very abundant in winter [in Zellersee] and displaces all other forms, but is completely absent in summer, so it shows the same behavior as in Lago di Caldonazzo and Mondsee.’ Depth profiles down to 40 m in June 1901 from Attersee, a nearby but comparable lake, indicated the diatom species Asterionella gracillima (Hanztsch) Heiberg 1863 and Fragilaria crotonensis Kitton 1869 as very abundant (Brunnthaler, 1901) like findings in Mondsee (Brehm & Zederbauer, 1906). No records about phytoplankton can be found for more than the next five decades. The status of the lake can be deduced, however, from Haempel (1929) who typified the lake as ‘turbid, meso-oligotroph at the initial state of eutrophication’ (Secchi depth about 4 m). Liepolt (1931, 1935) attempted a first monograph of Mondsee without the plankton component but with a description of algal zonation in the littoral. Patches of Aphanothece piscinalis Rabenhorst 1865 (currently Aphanothece stagnina (Sprengel) A. Braun 1863) near the inflow at the western end, were regarded as due to ‘the advanced eutrophication in this section of the lake’. The extensive quantitative plankton study in autumn 1932 and summer 1933 (Ruttner, 1937) unfortunately excluded Mondsee, but reported O. rubescens as an abundant, deep living species at about 20 m from Wolfgangsee close to Mondsee, verified again by Findenegg (1959a). Since this cyanobacterium was not present in Mondsee at that time, the author concluded ‘Mondsee seems to be a different lake type’. The presence of P. rubescens before 1968 can be circumstantial substantiated from 14C profiles with deep production maxima, likely from this taxon (Steemann Nielsen, 1959; Findenegg, 1959b).
Diatom stratigraphy from a sediment core (Klee & Schmidt, 1987) provided evidence for a transitional state in the 1930s. Centric diatoms shifted from Stephanodiscus alpinus Hustedt 1942 and Cyclotella bodanica, Eulenstein ex Grunow 1878 to Aulacoseira subarctica (O. Müller) E.Y. Haworth 1990 and S. neoastrea Håkansson & Hickel 1986. The rising level of trophy was substantiated by an abrupt appearance of Fragilaria crotonensis (flared type) and Tabellaria flocculosa var. asterionelloides (Grunow) Knudson 1952 during the 1950s (Klee & Schmidt, 1987; Dokulil & Kofler, 1995).
Period before and during eutrophication—1957 to 1978
Quantitative assessment of phytoplankton from Mondsee resumed in 1957 (Findenegg, 1959a, 1959b) and has been carried out almost continuously until present. Among the four large lakes of the region studied by Findenegg, Mondsee had the highest mean biomass in the top 12 m (250 to 1000 mg m−3) in 1957 to 1959 depending on season. Diatoms dominated winter, spring, and autumn while summer samples were characterized by Peridinium and Ceratium species. Findenegg (1969) reported algal biomasses in the water column between 3.5 and 16 g m−2 for the years 1958 to 1961 (Fig. 1). Values decreased somewhat in 1962/63 most likely because of the impact by the road construction on the northern hillside producing large in-lake turbidity (Einsele, 1963). Analysis of turbidity layers dated by radionuclides (Irlweck, 1985; Swierczynski et al., 2009), diatoms, and palaeo-pigments from sediment cores provides additional evidence (Schmidt 1985, 1991; Schultze, 1985).
Phytoplankton biovolume (BV) in Mondsee 1957 to 2020: Gray area—all individual data as mm3 l−1. Symbols on green line—annual average BV as mm3 l−1. Values of pink symbols are reconstructed BV. The thin red line indicates changes in annual average total phosphorus (TP) as µg l−1. Time periods: I—pre-eutrophication1957 to 1967 (gray), II—eutrophication 1968 to 1979 (redish), III—oligotrophication 1980 to 1993 (greenish), IV—variable 1994 to 2020 (white). The insert shows the regression of TP versus BV. All data are integrated values 0 to 20 m. Red arrows mark hot summer years, brown arrows indicate years with Dinobryon blooms, and arrows in cyan mark those with Microcystis blooms. More information in the text
Biomass recovered in the following years, but diatoms remained rare. Species composition in spring 1968 was not different from years before. In May, biovolume drastically increased and structure switched to larger forms such as Ceratium and Tabellaria. Blooms of Anabaena and Ceratium finally led to the outbreak of P. rubescens in fall 1968 throughout the water column and hence switched into eutrophic state (Danecker, 1969; Findenegg, 1969).
In the years following the Oscillatoria-invasion (Findenegg, 1973), total phytoplankton as well as P. rubescens biomass increased (Fig. 1) and bloomed in summer 1971 at the lake surface as Bruschek (1971) and Schultz (1971) annotated. The reconstructed biomass for 1973 to 1976 decreased in the first two years and then increased again. The highest total biomass was reached in 1978 (Schwarz, 1979a, b) corresponding with a peak in O. rubescens (Fig. 2). The eutrophication period thereafter ended largely when the wastewater treatment plant built in 1973 went into full operation including P-flocculation.
Biovolume of Planktothrix rubescens in Mondsee 1969 to 2020. Cyan area—all individual sampling dates as mm3 l−1. Symbols on blue line are annual average BV as mm3 l−1 (blue scale at right side). Red line is TP as in Fig. 1 (red scale to the right). Insert shows the regression of TP versus P. rubescens BV. Variance and regression parameters added
Oligotrophication period—1979 to 1993
From an annual average maximum of 3.6 mm3 l−1 in 1979, total phytoplankton BV decreased zigzagging during the following years down to 1.5 mm3 l−1 in 1993 (Fig. 1). During these years P. rubescens rapidly declined from an annual average of 2.1 to 0.08 mm3 l−1 in 1985 and remained thereafter around that value until 1993 (Fig. 2). The decline of P. rubescens at that time was mainly associated with an increase of diatoms (Fig. 3). The increase and fluctuation of phytoplankton largely reflected the changes in TP which determined 42% of the variability in total BV (insert in Fig. 1) and 77% (r2 = 0.77, P < 0.001) of P. rubescens BV (insert in Fig. 2). Similarly, annual changes in chlorophyll-a significantly depended on TP (r2 = 0.76, P < 0.001). During the recovery period, occasional algal blooms developed at dates were marked with arrows (Fig. 1). Outbursts of Dinobryon spp., dominated by Dinobryon sociale Ehrenberg 1834 in June 1984 and a larger one in August 1988 alternated with blooms of Microcystis flos-quae (Wittrock) Kirchner 1898 in September1985 and October 1986 (Figs. 1, 6).
Phytoplankton development for more than four decades, from 1978 to 2020. A The six major taxonomic phytoplankton groups as percentages of the total phytoplankton biovolume. B and C Re-adjusted cumulative partial sums (RAPS): B for total BV, Cyano Cyanobacteria, Bacill Bacillariophyceae (diatoms) and C for Crypto Cryptophyceae, Chryso Chrysophyceae, Dino Dinophyceae (Dinoflagellates), Green all green algae s.l., including Euglenophyceae and Desmidiales. Long dashed lines mark zero level, short, colored dashed lines mark the major shifts. For more explanation refer to the text
The decrease in biovolume and the structural changes depicted in Figs. 1 and 2 were the result of adjustments in environmental parameters. The longest data records over five decades are shown for SWT, TP, and euphotic depth in Fig. 4, which cover well the point in time in year 1980 for opposed development of environmental conditions. The TP concentrations rapidly declined from 17 µg l−1 1979 to 5 µg l−1 1982 while SWT started to increase around 1980 at a rate of 0.6 °C per decade (Fig. 4A), concurrent with an air temperature rise (0.5 °C per decade). Absolute annual maximum air temperature was greater than 30 °C in all years and exceeded 35 °C in the years 2003, 2009, 2013 (36.4 °C), 2015 and 2019. Total summer precipitation (June–August) was much below 400 mm in 2003, 2015 and 2019 (308 mm) and tends to decrease with increasing maximum temperature. In accordance with the phytoplankton decrease, Zeu increased from 10 m in 1979 to 20 m depth in 1985 and became more variable as indicated by the range in Fig. 4b. TP then remained around a mean of 10 ± 1.2 µg l−1 until 2004, slightly decreased again 2005 reaching 7 ± 0.8 µg l−1 thereafter. The difference of 3 µg in the means was statistically significant (t-test, P < 0.001).
Time series records of environmental constrains over five decades. A Lake surface water temperature (°C) from 1957 to 2020 with two regression lines before and after 1980. Variance and regression parameters for post-1980 data are inserted. In addition, TP is added to mark main period of eutrophication as red line from Figs. 1 and 2. B Euphotic depth in meters at 1% ambient light level (Zeu) measured or converted from Secchi depth in meter. Blue line indicates the annual averages, the blueish area the range between maximum to minimum of depth Zeu for each year
Climate warming period at low TP 1993 to 2020
The period after 1993 was characterized by highly variable phytoplankton biovolumes with annual averages ranging from 0.33 to 1.42 mm3 l−1 (average 0.77 mm3 l−1) peaking in the years 1996, 2003, 2008 and 2020 (Fig. 1). Nine years from the interval 2000 to 2020 were associated with hot summers marked with red arrows in Figs. 1 and 8, partly affecting the amount and structure of the assemblages. The years 1996 and 2003, a year with the hottest summer on record were associated with the presence of P. rubescens. Annual averages reached 1.16 and 3.16 mm3 l−1, respectively. The taxon tended to increase in volume after 2012 (Fig. 2). The variability in both total and cyanobacterial biovolume occurred at a mean concentration of 7.3 ± 0.6 µg l−1 TP (min 6.5, max 8.9 in 2003). At the same time, SWT has increased from an annual mean of 10.9 °C in 1993 to 13.5 °C in 2019 while average Zeu declined from 20 to 13.3 m (Fig. 4a, b).
Long-term change in assemblage structure
The composition of the phytoplankton assemblage was dominated by diatoms and cyanobacteria over four decades from 1978 to 2020 (Fig. 3a) Cyanobacterial biovolume consisted of virtually only two species, P.rubescens and Microcystis flos-aquae. Contribution of P. rubescens decline from 1978 onwards with occasional maxima at depth, replaced by sporadic blooms from Microcystis in fall 1985, 1986, and particularly 1988 and 1990 when blooms extended from August till October (Fig. 3a, cyano%). Alternation of the two subgroups, centrics and penates, dominated the internal structure of diatom populations. Larger diatoms such as Tabellaria and Aulacoseira dominated the years 1978 to 1998 but declined from 98% in 1986 to 35% 1998 when solitary centric diatoms began to gain more and more importance reaching 88% 2007, dropped thereafter again reaching 92% 2020. Occasional outbreaks of Dinobryon species, main component D. sociale, occurred among the chrysophytes 1984 to 1990 and again between 2003 and 2009 (Fig. 3a).
Relative shifts in assemblage structure
The simple detection methods for different periods are cumulative sums, here visualized as RAPS. The domination of cyanobacteria and diatoms during the long observation interval was indicated by the largely similar trends in their RAPS with total BV in Fig. 3b. All algal groups abruptly shifted slightly upwards in 1982 but main trend changes occurred at the end of 1986 and October 1990 for diatoms and cyanobacteria, respectively, mirroring the general decline in total BV (Fig. 3b). The course of the RAPS curve of the diatoms identifies three periods. When values changed from positive to negative in 1993, oligotrophication ended. The variable period thereafter can be separated into two sub-periods from 1994 to the end of 2010 and 2011 to 2020 when values varied around the mean. Average diatom BV of 0.36 ± 0.24 mm3 l−1 during oligotrophication increased to 0.39 ± 0.21 mm3 l−1 in the first interval and to 0.41 ± 0.21 mm3 l−1 in the last decade. Although these data were not significantly different, they reflect an increase in contribution to total BV from 27% via 47% to 56%. The trend in RAPS of the chrysophytes shifted in October 1990 from increasing to declining. The shift corresponded with total BV and cyanobacteria because of stabilized TP concentrations and declined Planktothrix biovolumes (Fig. 3c). Cryptophytes, dinophytes, and green algae were present throughout the four decades but became more important from 2003 onwards when all three classes had biovolumes above average (Fig. 3c).
Seasonal pattern of phytoplankton
The average seasonal pattern of the environmental traits, such as, nutrient concentration, Secchi depth, SWT, as well as the biovolume of phytoplankton entities are shown for the period 1982–2002 in Fig. 5a. Thermal summer stratification lasts from the mid May to the end of October. Seasonally elevated values for nutrient concentrations and Secchi depth are observed from late winter to spring and from autumn to the end of the year. Lowest values occur during summer. Concentrations, of TP are highest in spring, have a second lower peak in late summer to early autumn and increase toward winter. The seasonal dynamic of phytoplankton varies according to taxonomic groups. Solitary and filamentous centric diatoms develop a pronounced spring peak where filamentous forms reach biovolumes more than twice that of the centrics (Fig. 5b). Chroococcal cyanobacteria develop pronounced biovolumes during late summer stratification, i.e., in early autumn, when annual SWT maximum has been passed until autumn overturn. The oscillatorian cyanobacteria, essentially P. rubescens, display two maxima, one in summer before annual peak of SWT during thermal summer stratification and a second one during autumn overturn (Fig. 5c). The cryptophytes and pennate diatoms have a bimodal development with peaks in spring and fall. Minima are reached during thermal summer stratification, in June and September, respectively (Fig. 5).
Seasonal pattern of biweekly observation covering lifetime acclimation of phytoplankton organisms and environmental constrains: A Seasonal cycle for nutrients (SRSi, N-NO3, TP), SWT and Secchi depth and time-span of thermal stratification, B and C the same as in A but for phytoplankton groups with one main peak development throughout seasons (B) and with pronounced bimodal seasonal development (C). sol c diat solitary centric diatoms, fil c diat filamentous centric diatoms (Aulacoseira spp.), cro cyan chroococcales cyanobacteria, crypte cryptophytes, osc cyan oscillatorian cyanobacteria, penn diat pennate diatoms. Thermal summer stratification is from Julian Day (JD) 132 to 303. All data represent long-term averages over 21 years (1982–2002)
Short-term structural persistence vs. net change of phytoplankton
To differentiate the compositional shifts among biovolume fractions, Bray–Curtis similarity of biweekly pairs of phytoplankton composition was plotted against the biweekly net change rates of Chl-a (Fig. 6a). The data points follow a unimodal dome-shaped distribution. Extreme low or extreme high net change rates are associated with low values of Bray–Curtis similarity and thus represent strong shifts of phytoplankton fractions within two successive weeks during short-term break down or rapid build-up of phytoplankton yield. The unimodal dome-shaped distribution was approximated by a nonlinear fit (r = 0.39, F = 46,85, P < 0.0001, Fig. 6a). A net change rate of Chl-a around zero indicates no change in standing crop of phytoplankton between two successive sampling dates. The highest Bray–Curtis similarity of phytoplankton composition of a biweekly sampling pair is achieved with 96% resemblance. The necessary but not sufficient condition for keeping such high resemblance between successive phytoplankton development over two weeks, is a net change rate of Chl-a of zero or almost zero. This means that such extreme high resemblance among successive phytoplankton is only found when no change in standing crop occurs along biweekly sampling. In turn, however, a net change rate close to zero, marks not necessarily a period of no compositional change. A net change rate of chl-a close to zero, e.g., ranging from − 0.02 to 0.02 d−1, is associated with similarity values between 40 and 96% (Fig. 6a). Plotting frequency distribution histogram for Bray–Curtis similarity values, negative skewness (Rsk = − 1.013) becomes obviously (Fig. 6b), which indicates a prevalence of persistence against flexibility of phytoplankton structure when comparing successive biweekly development. Most frequently are Bray–Curtis similarity values ranging between 80 and 85% similarity for phytoplankton structure of successive sampling pairs. In contrast to Bray–Curtis similarity, the data of net change rates of Chl-a follow a normal distribution (Kolmogorov–Smirnov distance = 0.035, P = 0.122, Fig. 6c).
Net change-dependent structural persistence of phytoplankton assemblages covering the lifetime response of phytoplankton organisms. A Dome-shaped curve of the relationship between net change rate of Chl-a (d-1) and persistence of phytoplankton composition expressed as Bray–Curtis similarity (%), both calculated for sampling pairs of successive intervals. The gray line in A indicates a nonlinear regression (see results), the blue dashed line outlines the dome-shaped distribution curve of all points. Data from A are separately displayed in histograms in B and C. B Bray–Curtis Similarity data follow an asymmetric distribution pattern of negative skewness, C Data of net change rates passed the test for normal distribution. For A–C, all at biweekly intervals, n = 528
Long-term persistence of phytoplankton structure beyond the generation time
The 21-year time series for compositional change of phytoplankton expressed by Bray–Curtis similarity is shown in Fig. 7. Diatoms form a dominant fraction in the phytoplankton assemblages, contributing on average with 31% to total biovolume (maximum about 80%, see also Fig. 3a). Selecting the most diatom rich years for half of the 527 samples, their average contribution is 48% to total phytoplankton BV. The compositional changes of phytoplankton fluctuated considerably within seasonal cycles and interannually over the two decades. The annual median values of Bray–Curtis similarity, followed by polynomial trend, coincide with the long-term annual trend of SRSi.
Long-term compositional shifts within phytoplankton assemblages over generations (expressed by Bray Curtis similarity, see method, polynomial trend with n = 528, blue points indicate annual median values) and long-term data and year-by-year polynomial trend of the environmental constrain SRSi (= Soluble Reactive Silica). The time series cover 21 years of observation, from 1982 to 2002
Diversity and disturbance
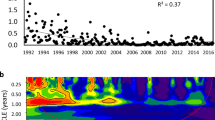
Diversity of phytoplankton assemblages based on the Shannon index H′ was highly variable between observations varying between 0.37 in 1983 and 3.24 in 2014 (Fig. 8) indicating lifetime acclimation of assemblages. Diversities less than 1 were associated with blooms or mass developments of a single taxon (Fig. 8b). Besides the blooms of D. sociale and M. flos-aquae in the 1980s, mass occurrences of Aulacoseira islandica (O. Müller) Simonsen 1979 dominated April 1983 and March 1998. Microcystis dominated again in July 1990 and December 1992. In January 2003 P. rubescens prevailed (Figs. 2, 4, 8) while February 2020 saw a massive presence of A. subarctica plus Stephanodiscus neoastrea (Fig. 8b).
Phytoplankton diversity calculated from biovolumes. A Evenness (left scale) and number of taxa (right scale) as annual averages. B Shannon diversity index (H′) as black line for monthly averages and as red line with symbols for annual averages (left scale). H values for individual species-specific data points in blue (right scale). Arrows: Brown—Dinobryon bloom, Cyan—Microcystis bloom; Red—hot summers with explanation on the right side (from HISTALP). See text for more explanations
Annual average H’ has increased from 1.94 in 1982 to 2.79 in 2001 at a rate of 0.4 per decade. After a drop to a minimum of 1.68 in 2003, diversity reached a maximum of 3.24 in 2005. The minimum in 2003 was due to a reduction in taxa number from 50 to 32 (Fig. 8a) possibly because of the hottest summer on record in Austria. Similarly, the decay in diversity from 2.74 in 2012 to 1.71 in 2020 was essentially triggered by a sequence of 8 hot summers in 9 years, of which four ranked 2 to 5 in temperature records. Number of taxa declined, from 2012 to 2014, from 68 to 43, was 51 in 2015, and reached a maximum of 72 species in 2016 declining afterward to 57 in 2019. Evenness declined from 0.6 in 2014 to 0.4 in 2020, indicating shifts in population structure.
Environmental constraints
Using the three environmental variables SWT, Zeu, and TP described in Fig. 4 as proxies for climatic, light and nutrient conditions relevant for phytoplankton development, the total biovolume (BV) of phytoplankton can be predicted from a multiple linear regression using SWT, Zeu and TP as independent variables:
The correlation matrix contains details of specific relationships and their significance (Table 4). TP related significantly to total biovolume, cyanobacteria—specifically P. rubescens—and total diatoms plus the pennate subgroup. The centric diatoms and all other classes depended more on SWT. In addition, chrysophytes, cryptophytes, and dinophytes correlated positively with Zeu, but negatively to TP and P. rubescens. Chrysophytes and cryptophytes correlated also negatively to TP.
Applying SWT, Zeu and TP as relevant constrains of phytoplankton development in multivariate statistics, PCA clearly separated algal biovolumes into periods related to trophic level (Fig. 9). The pre-eutrophication years 1962 to 1965 appear detached and un-influenced. The years 1966 to 1981 related to the eutrophic period were largely affected by TP. All other years covering the oligotrophication and the variable period were associated with Zeu and SWT. Hot summers affected annual average biovolumes as indicated in Fig. 9.
Principal component analysis (PCA). Biplot of annual average biovolumes (BV) for the years 1962 to 2020 versus total phosphorus (TP) concentrations, surface water temperature (SWT) and mean euphotic depth (Zeu). Symbols: Gray circles—data prior to eutrophication, red triangles—data during eutrophication period, green diamonds—variable post-eutrophication period with hot summer years marked by pink triangles. Statistics: Variance for PC1 is 60.7% and for PC2 21.3%. Loadings for PC: SWT = 0.56, for Zeu = 0.59 and for TP = 0.58; for PC2: SWT = 0.81, for Zeu = − 0.25 and for TP = 0.53
The PCA biplot of second versus first principal component captured most of the variance (PC1 61%, PC2 21%) of environmental variables SWT, Zeu and TP. The opposed vectors TP and Zeu indicate their inverse relationship, while SWT is independent from both (Fig. 9). According to the biplot display, the years 1962–1965 cannot be linked to any state along the inverse TP: Zeu relationship. The data points are displayed at the opposite of the SWT vector. It indicates that the early lake period in this study, is linked to low SWT (“cold water period”) but not to any trophic alteration. Data for the 1969 to 1981 period close to the TP vector and distant from the Zeu-vector indicate the main characteristics during that time, i.e., enhanced TP concentrations and shallow Zeu. The cluster of the sampling years from 1982 comprise to situations, namely the declining period (oligotrophication) until about 1993 and the phase thereafter of low TP, high Zeu, and rising temperatures.
Phytoplankton families linked to environmental variables indicated relation of TP to total cyanobacterial and P. rubescens BV. Pennate diatoms were less influenced by TP. Chrysophytes, dinophytes, and chlorophytes centered around Zeu while biovolumes of cryptophytes, conjugates, and centric diatoms clustered with SWT (Fig. 10).
Discussion
Long-term approaches have merits in ecology in general and in phytoplankton particularly because of their short generation time. A generation time of about 100 years in higher plants such as trees reduces to days up to weeks in plankton assemblages (Padisák et al. 1988; Halstvedt et al., 2007). As phytoplankton is a diverse cluster of polyphyletic entities (Falkowski et al., 2004), their members of distinctive physiological traits can act as sensitive sentinels for short and long-term structural changes, acclimation processes as well as trends and shifts in environmental traits. In this context, the presence or absence of plankton key species in early observations can have consequences for the present situation in a lake. Recent advances in methodology would now open the possibility to reconstruct the history of taxa of harmful blooms (Savichtcheva et al., 2011, 2015) such as Planktothrix in this study or other filamentous cyanobacteria such as Cylindrospermopsis (Fuentes et al., 2008) in an urban oxbow lake (Teubner et al., 2018).
Planktothrix rubescens - in situ observations over 100 years.
The unexpected appearance and rise of P. rubescens in late 1968 was a common phenomenon of eutrophication in the mid-20thcentury. Similar signals in several lakes in Austria, Europe, and worldwide attracted attention and triggered a broad debate on causes, consequences, and possible correctives (Liepolt, 1956; Vollenweider, 1968; Schindler, 1977).
According to earlier studies about P. rubescens in two alpine lakes, Mondsee and Ammersee, it was shown that this cyanobacterium lives well adjusted to ambient low light conditions at the lowest edge of the euphotic zone and can benefit from enhanced phosphorus exposure due to eutrophication and global warming (Teubner, 2006; Teubner & Greisberger, 2007; Dokulil & Teubner, 2012). The main picture of long-term development of P. rubescens over more than five decades, however, as clearly shown in this study from 1969 to 2022, follows the history of eutrophication in Mondsee, and shifted to global warming in recent decades. It thus confirms findings of former studies by others that environmental stressors shifted from nutrient enrichment to global warming (Achleitner et al., 2007; Luger et al., 2021), although the eutrophication had a vaster dimension of biomass development than global warming. A reason for the particular strong development of phytoplankton yield during TP enrichment must be seen in the linear response of phytoplankton biomass increase at low trophic level, most relevant for Mondsee of oligotrophic to mesotrophic lake reference background. The yield response to further enhancement of nutrient supply slows down at high trophic levels, such as hypertrophic lakes (e.g., Forsberg & Ryding, 1980), due to saturating nutrient level usually not relevant for pristine alpine lakes. In turn, initiated by restoration measures, TP concentrations and Planktothrix biovolumes declined during oligotrophication as observed in many lakes (Jeppesen et al., 2005), even if the decrease in biovolume of phytoplankton started with a delay for years when compared with the decrease in TP in Mondsee (Dokulil & Teubner, 2005). With sustained low TP in Mondsee, biovolumes of P. rubescens remained low in the 1990s and early 2000s, sometimes even below detection limit.
An argument for a less strong yield response by P. rubescens to climate change against eutrophication, can be seen in the counterbalance or side effects of global warming. According to an earlier study, P. rubescens benefits only partly from global warming which shifted lake phenology (Dokulil & Teubner, 2012). While P. rubescens benefits from an earlier onset of thermal stratification due to global warming, the prolongation of thermal stratification and a delay of autumn overturn seem to be rather exhausting for growth. Thus, the global warming response by increased yield of P. rubescens is rather modest in lake Mondsee. Nevertheless, rising water temperatures rather than small changes in TP at sustained low concentration level caused a return of moderate biovolume development (< 1 mm3 l−1) in the years 2002–2005 and from 2011 onwards. Furthermore, flooding events in 2002 and 2013 yielded in short-term TP-peaks and associated growth of P. rubescens (Luger et al., 2021; Kurmayer et al., 2022). In this view, recurring exposure to TP which stimulates again the growth of P. rubescens, is forced by extreme events linked to climate change. It thus shows the tight coupling of direct (TP surplus by eutrophication) and indirect (TP surplus by climate change effects) nutrient addition which finally addresses a stimulated growth of P. rubescens (Bergkemper & Weisse, 2018). Similar phenomena occurred in several European lakes (Teubner et al., 2003; Anneville et al., 2004, 2015; Salmaso, 2010; Posch et al., 2012; Pomati et al., 2015).
In Mondsee, the wax and wane of P. rubescens, has to be discussed in few of further concurrent phytoplankton species. While under nutrient-rich conditions, alternate shifts occurred between P. rubescens and other cyanobacteria and diatoms, during the recovery phase alternate blooms of cyanobacterial Chroococcales and Chrysophyceae are worth to notify. The Microcystis bloom in autumn 1986 was likely a result of the nutrient input by a landslide documented in the lake sediment (Swierczynski et al., 2009). A bloom of Dinobryon sociale in August 1988 rendered the water brownish. This outburst was a result of hatching from resting stages as traps placed above the sediment indicated (Dokulil & Skolaut, 1991). Iron made available through chelation by organic substances released from diatoms was presumably the activator. Feeding of this mixotrophic genus affected the structure and number of planktonic bacteria (Sommaruga & Psenner, 1989). The irregular appearance and the occasional blooms are characteristics of Dinobryon (Reynolds et al., 1993; Padisák, 1995).
Long-term shifts and increasing impact of climate change
Because of a regime shift in the mid-1980s, climate warming affected phytoplankton assemblages in lakes worldwide (Woolway et al., 2017; Kraemer et al., 2021). Livingstone & Dokulil (2001) and Dokulil et al. (2010) summarized impacts of global warming on lake water temperature for Mondsee and other lakes in Europe. Upward trends in maximum SWT (Dokulil et al., 2021) became progressively more pronounced affecting biovolume in Mondsee. Extended periods of warmer water temperatures in summer strongly affect phytoplankton assemblages. The absolute maximum temperature as well as the duration and exceedance of critical temperatures became increasingly important for the metabolism of the plankton organisms. Such intervals expended over the last few decades and surface temperatures admixed deeper into the epilimnion affecting species growth rates (Dokulil et al., 2021).
Periods of extremely hot summer weather are a recurrent phenomenon discussed by Kyselý et al. (2000). Based on the heat waves 1995 and 2003, Meehl and Tebaldi (2004) forecasted more intense and frequent heat waves for the twenty-first century. Off the 10 hottest summers, eight occurred since 2000 in Austria (https://www.zamg.ac.at/cms/de/klima/news/2021-unter-den-waermsten-jahren-der-messgeschichte).
A few of these hot summers have been documented in lake observations. The European heat wave of 2003 produced the highest ever recorded surface temperature and strong thermal stability (Jankowski et al., 2006; Dokulil et al., 2010) causing high BV of Planktothrix and low biodiversity in Mondsee. Blooms or no blooms of cyanobacteria during a heat wave seem to depend on the species involved suggesting differential benefits (Huber et al., 2012).
The response of phytoplankton in Mondsee to the July heat wave 2015 was studied by Bergkemper and Weisse (2017). The increase in water temperature fostered chrysophytes and particularly cyanobacteria which increased in biovolume relative to previous years. The peak of P. rubescens shifted from 11 to 16 m depth corresponding to the preferences of this specific cyanobacterium (Dokulil & Teubner, 2012). The main driver for vertical distribution is light quality and quantity (Greisberger & Teubner, 2007; Oberhaus et al., 2007). In addition to the heat wave, two heavy rain events in March 2015 and February 2016 were analyzed (Bergkemper & Weisse, 2018). At both events, cryptophytes declined, followed by rapid recovery. The spring peak of the centric diatoms and all other families, however, were unaffected by the precipitation.
Shifts in phytoplankton biomass and diversity under climate warming are still controversial. The fluctuations in phytoplankton diversity, abundance, and community structure observed in Mondsee correspond largely to those reported by Benedetti et al. (2019). Long-term trends in diversity were evident in Danish lakes under re-oligotrophication (Özkan et al., 2016). Phytoplankton diversity increased in periods of strongly decreasing biomass. In contrast to the observations in the present study, Özkan et al. (2016) could not verify a substantial climatic control. A report on future climate influences on phytoplankton diversity indicated increasingly alteration and unstable response leading to reduced resilience (Henson et al., 2021). Additionally, winter conditions with or without ice-cover influence plankton diversity (Lenard & Wojciechowska, 2013) as substantiated by the lack of winter ice-cover in recent years in Mondsee and other Austrian alpine lakes (Ficker et al., 2017; Niedrist et al., 2018).
Persistence of functional taxonomic traits of phytoplankton and seasonal pattern
Structural persistence within short time intervals relies on phytoplankton growth, dormancy, or losses among taxonomic groups. Growth relates to the potential of phytoplankton cell division, which largely depends on resource availability of nutrients and light, but also on other conditions like allelopathic substances which may hamper cell development (e.g., Śliwińska-Wilczewska et al., 2021). While growth conditions in the research laboratory are often set at one cell division per day (balanced exponential growth maintaining cultures prior to experiments, growth rate of about 0.69; e.g., Ragni et al., 2008; Boatman et al., 2018), such development is the exception rather than the rule for phytoplankton development 365 days a year, except for short periods of exponential growth, e.g., in spring. This way, the life span of phytoplankton organisms can be extended for weeks (Feuillade & Krupka, 1986; Halstvedt et al., 2007), which aside of growth conditions also depends on taxonomic traits. In this view, structural persistence of phytoplankton assessed stepwise at biweekly to monthly intervals is close to the timescale of the lifespan of phytoplankton organisms of various phylogenetic traits.
Using Chl-a as rough proxy of phytoplankton yield, net change rates of Chl-a different from zero stand for an increase or decrease of phytoplankton yield, i.e., the periods of predominant growth or losses of phytoplankton. In turn, zero net change rates of Chl-a indicate periods of dormancy or perfect balance between increases and losses. The structural change, expressed by Bray–Curtis similarity, along net change in this study, revealed a dome-shaped curve for Mondsee. Such a dome-shaped relationship of net change-dependent structural persistence is also shown for phytoplankton of alpine Ammersee (Teubner et al., 2003, 2006) and ciliate assemblages of alpine Traunsee (Sonntag et al., 2006). Dome-shaped functional curves in ecology are reported by others, e.g., commonly for population growth dependence from population density (e.g., Bacher et al., 1997; Hutchings & Kuparinen, 2014) or species response curves along environmental gradients (e.g., Rydgren et al., 2003; Bal et al., 2011). In case of the net change-dependent structural persistence of phytoplankton in Mondsee, extreme yield growth or yield loss is associated with extreme structural shifts in phytoplankton assemblages. As shown in an earlier food web study by Qu et al. (2021), the protozoan species development was in particular tightly connected with phytoplankton in Mondsee, and thus might force structural shifts in phytoplankton assemblages by feeding pressure. Furthermore, in Lake Mondsee the clear water phase is described by lake phenology (Dokulil et al., 1990; Dokulil & Teubner, 2012) as typical for other lakes. The clear water phase is not necessarily only associated with a clear species shift of phytoplankton community, but also seen, e.g., by shifts within protozoan assemblages in Mondsee. The rough picture of seasonal development for phytoplankton and also for small-sized grazers exemplified for Mondsee, however, are not four, but only two seasonal distinct assemblages (Teubner, 2000; Forster et al., 2021). Seasonality in temperate lakes is thus reduced to two main seasons only, namely the cold season, lasting from winter to spring, and the warm season, from summer to autumn. It implies that a pronounced species overturn occurs only twice a year, from spring to summer and from autumn to winter (Teubner et al., 2000). In turn, a persistence of phytoplankton structure is only achieved if net change rates are close to zero; but a net change rate of zero does not necessarily mean that no structural change is possible. It could be shown that in particular metalimnetic phytoplankton assemblages, dominated by P. rubescens in alpine Ammersee, are more resistant against structural changes than the epilimnetic phytoplankton (Teubner et al., 2003, 2006). Another aspect concerning short-term development of phytoplankton traits becomes obvious when looking at the frequency distribution for values of both, the net changes and the structural similarity of phytoplankton assemblages. While values of net change rates satisfy a normal distribution, the Bray–Curtis values are expressed by a negative skewed distribution. The latter implies that structural persistence in phytoplankton assemblages is more common than structural fluctuation and thus supports the ‘plankton paradox’ by Hutchinson (1961). Tracking the structural persistence of phytoplankton assemblages, the capability of organisms to adjust during their lifetime comes to the fore. Phytoplankton organisms need to cope with or react to their environment during their lifetime to succeed. Such a lifetime response concerns the rapid adjustment for phosphate acquisition (i.e., Aubriot et al, 2011) or for photosynthetic activity and acclimation utilizing light across daytime (e.g., Masojídek et al, 2001; Ragni et al., 2008) within a few minutes to hours. The acclimation to ambient light by photosynthetic efficiency and pigment pattern has been shown for phytoplankton in Mondsee and other alpine lakes (Teubner et al., 2001; Greisberger & Teubner, 2007). Seasonal lifetime acclimation is consistent and repetitive over prolonged periods. It thus contributes to stability of the phytoplankton community, which is reflected by seasonal pattern of phytoplankton groups of diverse taxonomic traits (Anneville et al., 2018; Fu et al., 2021). Diatoms develop in particular well, as long as there is enough Silica available for growth during mixing in Mondsee. With summer stratification in deep alpine lakes, epilimnetic phytoplankton growth becomes in particular exhausted by Si (Teubner, 2003) and diatom biomass rapidly declines (Dokulil & Skolaut, 1991). Cyanobacteria can benefit from thermal stratification or phases of high-water transparency living in deep metalimnetic layers (Greisberger & Teubner, 2007; Dokulil & Teubner, 2012). Seasonal unimodal or bimodal distribution pattern thus finally rely on the adjustment of species to cope successfully with their environment at least over a certain period of time. The phytoplankton assemblage as a whole, as a bulk of the many phytoplankton groups of different taxonomic traits, follows a bimodal seasonal distribution pattern in Mondsee as earlier shown in Dokulil and Teubner (2012). Such bimodal phytoplankton development is shaped by thermal stratification during the growing season and is typically described for deep mountain lakes, as shown by a synoptic study phytoplankton seasonality of lakes in Italy (Morabito et al., 2018).
Conclusions
Long-term ecological research for phytoplankton focuses on tracking environmental change which may have an impact on the lake ecology in a broader sense. According to the phytoplankton assessment of alpine Lake Mondsee for more than six decades (1958 to 2020), we conclude the following concerns for long-term ecological research of phytoplankton in lakes:
-
Phytoplankton groups of similar taxonomic traits are suitable entities to track structural shifts of phytoplankton assemblages in plankton research and this way avoid the taxonomic rigidity of the species concepts in ecology.
-
Response of taxonomic traits of phytoplankton to environmental constrains can be studied at two main timescales relevant for primary producers: covering the adjustment of organism’s during lifetime (1) and over generations (2).
-
Biweekly to monthly observations cover well the temporal resolution of lifetime adjustment which is in a longer perspective reflected by the seasonal pattern.
-
The persistence of taxonomic traits along weeks is predominant and thus an essential element for stabilizing phytoplankton structure rather than structural fluctuations.
-
Studying the response to environmental constrains at timescales over generations might focus not only on the average extent of stressors but also cover their range of values, as biodiversity is in particular vulnerable against extreme environmental situations.
-
Single key taxa, as is exemplified here for Planktothrix rubescens, which are omnipotent players in phytoplankton assemblages and are occurring during different environmental scenarios in the long-term, are planktonic taxa of suitable functional traits to follow the impact of various environmental stressors over large time-spans, along many generations of these organisms. Their detailed study is thus of particular importance for the contribution to long-term ecological research (LTER).
References
Achleitner, D., H. Gassner & A. Jagsch, 2007. Longterm Development of the Trophic Situation of Lake Mondsee and Lake Irrsee, Schriftenreihe BAW, Wien: (in German).
Anneville, O., S. Souissi, S. Gammeter & D. Straile, 2004. Seasonal and inter-annual scales of variability in phytoplankton assemblages: comparison of phytoplankton dynamics in three peri-alpine lakes over a period of 28 years. Freshwater Biology 49: 98–115. https://doi.org/10.1046/j.1365-2426.2003.01167.x.
Anneville, O., I. Domaizon, O. Kerimoglu, F. Rimet & S. Jaquet, 2015. Blue-green algae in a ‘“greenhouse century”’? New insights from field data on climate change impacts on cyanobacteria abundance. Ecosystems 18: 441–458. https://doi.org/10.1007/s10021-014-9837-6.
Anneville, O., G. Dur, F. Rimet & S. Souissi, 2018. Plasticity in phytoplankton annual periodicity: an adaptation to long-term environmental changes. Hydrobiologia 824: 121–141. https://doi.org/10.1007/s10750-017-3412-z.
Anneville, O., C.-W. Chang, G. Dur, S. Souissi, F. Rimet & C.-H. Hsieh, 2019. The paradox of re-oligotrophication: the role of bottom–up versus top–down controls on the phytoplankton community. Oikos 128: 1666–1677. https://doi.org/10.1111/oik.06399.
Aubriot, L., S. Bonilla & G. Falkner, 2011. Adaptive phosphate uptake behaviour of phytoplankton to environmental phosphate fluctuations. FEMS Microbiology Ecology 77(1): 1–16. https://doi.org/10.1111/j.1574-6941.2011.01078.x.
Bacher, C., P. Duarte, J. G. Ferreira, M. Héral & O. Raillard, 1997. Assessment and comparison of the Marennes-Oléron Bay (France) and Carlingford Lough (Ireland) carrying capacity with ecosystem models. Aquatic Ecology 31(4): 379–394. https://doi.org/10.1023/A:1009925228308.
Bal, G., E. Rivot, E. Prévost, C. Piou & J. L. Baglinière, 2011. Effect of water temperature and density of juvenile salmonids on growth of young-of-the-year Atlantic salmon Salmo salar. Journal of Fish Biology 78(4): 1002–1022. https://doi.org/10.1111/j.1095-8649.2011.02902.x.
BAW, H.G., 2010. Natürliche und künstliche Seen Österreichs größer als 50 ha. Stand 2009. Schriftenreihe des Bundesamtes für Wasserwirtschaft 33: 425 S. [available on internet at https://info.bml.gv.at/service/publikationen/wasser/naturlichekunstl.html].
Benedetti, F., L. Jalabert, M. Sourisseau, B. Becker, C. Cailliau, C. Desnos, A. Elineau, J.-O. Irisson, F. Lombard, M. Picheral, L. Stemmann & P. Pouline, 2019. The seasonal and inter-annual fluctuations of plankton abundance and community structure in a north Atlantic marine protected area. Frontiers Marine. Science 6: 214. https://doi.org/10.3389/fmars.2019.00214.
Bergkemper, V. & W. Weisse, 2017. Phytoplankton response to the summer 2015 heat wave: a case study from prealpine Lake Mondsee, Austria. Inland Waters 7: 88–99. https://doi.org/10.1080/20442041.2017.1294352.
Bergkemper, V. & W. Weisse, 2018. Do current European lake monitoring programs reliably estimate phytoplankton community changes? Hydrobiologia 824: 143–162. https://doi.org/10.1007/s10750-017-3426-6.
Boatman, T. G., K. Oxborough, M. Gledhill, T. Lawson & R. J. Geider, 2018. An integrated response of Trichodesmium erythraeum IMS101 growth and photo-physiology to iron, CO2, and light intensity. Frontiers in Microbiology 9: 624. https://doi.org/10.3389/fmicb.2018.00624.
Brehm, V. & E. Zederbauer, 1906. Beiträge zur Planktonuntersuchung alpiner Seen. Acta ZooBot Austria 56: 19–32.
Brunnthaler, J., 1901. II. Zusammensetzung des Phytoplanktons. Österreichische Botanische Zeitschrift 51: 78–82.
Bruschek, E., 1971. Burgunderblutalge behindert die Setzlingsaufzucht in der Fischzuchtanstalt Kreuzstein. Österreichs Fischerei 24: 124–126.
Danecker, E., 1969. Bedenklicher Zustand des Mondsees im Herbst 1968. Österreichs Fischerei 22: 25–31.
Dokulil, M.T., 1993. Long-term response of phytoplankton population dynamics to oligotrophication in Mondsee, Austria. Verhandlungen Internationale Vereinigung Limnologie 25: 657–661.
Dokulil, M. T., 2017. Peri-alpine Lakes in the Anthropocene: deterioration and rehabilitation: an Austrian success story. Acta ZooBot Austria 154: 1–53.
Dokulil, M. T., 2020. High mountain and lowland lakes in the Anthropocene: an Austrian long-term perspective (IBP to LTER). Acta ZooBot Austria 157: 161–213.
Dokulil, M. T. & C. Skolaut, 1991. Aspects of phytoplankton seasonal succession in Mondsee, Austria, with particular reference to the ecology of Dinobryon Ehrenb. Verhandlungen Internationale Vereinigung Limnologie 24: 968–973. https://doi.org/10.1080/03680770.1989.11898892.
Dokulil, M. T. & S. Kofler, 1995. Ecology and autecology of Tabellaria flocculosa var. asterionelloides Grunow (Bacillariophytes) with remarks on the validation of the species name. In: Marino, D. & M. Montresor (eds), Proceedings of the 13th International Diatom Symposium 1994: 23–38, Biopress Limited, Bristol.
Dokulil, M. T. & K. Teubner, 2005. Do phytoplankton communities correctly track trophic changes? An assessment using directly measured and palaeolimnological data. Freshwater Biology 50: 1594–1604. https://doi.org/10.1111/j.1365-2427.2005.01431.x.
Dokulil, M. T. & K. Teubner, 2012. Deep living Planktothrix rubescens modulated by environmental constraints and climate forcing. Hydrobiologia 698: 29–46. https://doi.org/10.1007/s10750-012-1020-5.
Dokulil, M. T., A. Herzig & A. Jagsch, 1990. Trophic relationships in the pelagic zone of Mondsee, Austria. Hydrobiologia 191: 199–212. https://doi.org/10.1007/BF00026053.
Dokulil, M. T., K. Teubner, A. Jagsch, U. Nickus, R. Adrian, D. Straile, T. Jankowski, A. Herzig & J. Padisák, 2010. The impact of climate change on lakes in Central Europe. In George, G. (ed), The Impact of Climate Change on European Lakes Springer, Doordrecht: 387–410.
Dokulil, M. T., E. de Eyto, S. E. Maberly, L. May, G. A. Weyhenmeyer & R. I. Woolway, 2021. Increasing maximum lake surface temperature under climate change. Climatic Change 165: 56. https://doi.org/10.1007/s10584-021-03085-1.
Einsele, W., 1963. II. Schwere Schädigungen der Fischerei und der biologischen Verhältnisse im Mondsee durch Einbringung von lehmig-tonigem Berg-Abraum. Der spezielle Fall und seine allgemeinen Lehren. Österreichs Fischerei 16: 1–9.
Falkowski, P. G., M. E. Katz, A. H. Knoll, A. Quigg, J. A. Raven, O. Schofield & F. J. R. Taylor, 2004. The evolution of modern eukaryotic phytoplankton. Science 305(5682): 354–360. https://doi.org/10.1126/science.1095964.
Feuillade, M. & H. Krupka, 1986. Assimilation des acides aminés par Oscillatoria rubescens DC (Cyanophycée). Archiv Für Hydrobiologie 107: 441–463.
Ficker, H., M. Luger & H. Gassner, 2017. From dimictic to monomictic: empirical evidence of thermal regime transitions in three deep alpine lakes in Austria induced by climate change. Freshwater Biology 62: 1335–1345. https://doi.org/10.1111/fwb.12946.
Findenegg, I., 1959a. Das pflanzliche Plankton der Salzkammergutseen. Österreichs Fischerei 5–6: 32–35.
Findenegg, I., 1959b. Die Gewässer Österreichs. Ein limnologischer Überblick. Hg. Biol. Stat. Lunz aus Anlaß des 14. Intern. Limnologiekongresses 1959b, Österreich. 68 S.
Findenegg, I., 1965. Limnologische Unterschiede zwischen den österreichischen und ostschweizerischen Alpenseen und ihre Auswirkung auf das Phytoplankton. Vierteljahrschrift Der Naturforschenden Gesellschaft Zürich 110: 289–300.
Findenegg, I., 1969. Die Eutrophierung des Mondsees im Salzkammergut. Wasser- Und Abwasser-Forschung 4: 139–144.
Findenegg, I., 1973. Vorkommen und biologisches Verhalten der Blaualge Oscillatoria rubescens DC. In den österreichischen Alpenseen. Carinthia II 163: 317–330.
Forsberg, C. & S. O. Ryding, 1980. Eutrophication variablesand trophic state indices in 30 waste receiving Swedish lakes. Archiv Fur Hydrobiologie 89: 189–207.
Forster, D., Z. Qu, G. Pitsch, E. P. Bruni, B. Kammerlander, T. Pröschold, B. Sonntag, T. Posch & T. Stoeck, 2021. Lake ecosystem robustness and resilience inferred from a climate-stressed protistan plankton network. Microorganisms 9(3): 549. https://doi.org/10.3390/microorganisms9030549.
Fu, H., D. Yuan, K. Özkan, L. S. Johansson, M. Søndergaard, T. B. Lauridsen & E. Jeppesen, 2021. Patterns of seasonal stability of lake phytoplankton mediated by resource and grazer control during two decades of re-oligotrophication. Ecosystems 24: 911–925. https://doi.org/10.1007/s10021-020-00557-w.
Fuentes, S., J. Rick, P. Scherp, A. Chistoserdov & J. Noel, 2008. Development of Real-Time PCR assays for the detection of Cylindrospermopsis raciborskii. In: Proceedings of the 12th International Conference on Harmful Algae 397: 353–355. ISSHA and IOC of UNESCO, 2008, Copenhagen.
Garbrecht, J. & G. P. Fernandez, 1994. Visualization of trends and fluctuations in climatic records. Water Resource Bulletin 30: 287–306. https://doi.org/10.1111/j.1752-1688.1994.tb03292.x.
Greisberger, S. & K. Teubner, 2007. Does pigment composition reflect phytoplankton community structure in differing temperature and light conditions in a deep alpine lake? An approach using HPLC and delayed fluorescence (DF) techniques. Journal of Phycology 43: 1108–1119. https://doi.org/10.1111/j.1529-8817.2007.00404.x.
Haempel, O., 1929. Vergleichende Untersuchungen an drei oester. Alpenseen und die Begründung einer fisch.-biolog. Station in Oesterreich, Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 4: 333–339. https://doi.org/10.1080/03680770.1929.11898412.
Hajnal, É. & J. Padisák, 2008. Analysis of long-term ecological status of Lake Balaton based on the ALMOBAL phytoplankton database. Hydrobiologia 599: 227–237. https://doi.org/10.1007/s10750-007-9207-x.
Halstvedt, C. B., T. Rohrlack, T. Andersen, O. Skulberg & B. Edvardsen, 2007. Seasonal dynamics and depth distribution of Planktothrix spp. in Lake Steinsfjorden (Norway) related to environmental factors. Journal of Plankton Research 29(5): 471–482. https://doi.org/10.1093/plankt/fbm036.
Hamilton, P. B., 1990. The revised edition of a computeruzed plankton counter for plankton, periphyton and sediment diatom analyses. Hydrobiologia 194: 23–30.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: Paleontological Statistics software package for education and data analysis. Palaeontologica Electronica 4: 1–9.
Henson, S. A., B. B. Cael, S. R. Allen & S. Dutliewicz, 2021. Future phytoplankton diversity in a changing climate. Nature Communications 12: 5372. https://doi.org/10.1038/s41467-021-25699-w.
Huber, V., C. Wagner, D. Gerten & R. Adrian, 2012. To bloom or not to bloom: contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 169: 245–256. https://doi.org/10.1007/s00442-011-2186-7.
Hutchings, J. A. & A. Kuparinen, 2014. A generic target for species recovery. Canadian Journal of Zoology 92: 371–376. https://doi.org/10.1139/cjz-2013-0276.
Hutchinson, G. E., 1961. The paradox of the plankton. The American Naturalist 95: 135–143. https://doi.org/10.1086/282171.
Ibanez, F., J. Fromentin & J. Castel, 1993. Application de la méthode des sommes cumulées á l´analyse des séries chronologiques en océanographie. C.R.Acad.Sci.Paris, Sciences de la vie/Life Sciences 316: 754–748.
Imhof, O.E., 1885. Faunistische Studien in achtzehn kleineren und größeren österreichischen Süßwasserbecken. Sitzb. Akad. Wiss. Mat.-nath. Kl. 91: 203–226. [available on internet at: https://www.zobodat.at/publikation_series.php?id=7341].
Irlweck, K., 1985. Radionuclide studies on sediments from Mondsee. In Danielopol, D., R. Schmidt & E. Schultze (eds), Contributions to the Paleolimnology of the Trumer Lakes (Salzburg) and the Lakes Mondsee, Attersee and Traunsee (Upper Austria) Limnologisches Institut Mondsee, ÖAW: 89–98.
ISO, 7888, 1985. Water Quality—Determination of Electrical Conductivity, International Organization for Standardization, Geneva:, 1–6.
ISO, 11905-1, 1997. Water Quality—Determination of Nitrogen, International Organization for Standardization, Geneva:, 1–13.
ISO, 6878, 2004. Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method, International Organization for Standardization, Geneva:, 1–20.
Jankowski, T., D. M. Livingstone, H. Bührer, R. Forster & P. Niederhauser, 2006. Consequences of the 2003 European heat wave for lake temperature profiles, thermal stability, and hypolimnetic oxygen depletion: Implications for a warmer world. Limnology Oceanography 51: 815–819. https://doi.org/10.4319/lo.2006.51.2.0815.
Jantsch, A., 1977. Untersuchungen an der Mondseeache als Verbindung eines eutrophen Sees mit einem oligotrophen See und Sedimentationsprozesse. Arbeiten Aus Dem Labor Weyregg 2: 52–62.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. F. Hampton, K. Kangur, J. Köhler, S. Körner, E. Lammens, T. L. Lauridsen, M. Manea, R. Miracle, B. Moss, P. Nöges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lake responses to reduced nutrient loading: an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771. https://doi.org/10.1111/j.1365-2427.2005.01415.x.
Jochimsen, M. C., R. Kümmerlin & D. Straile, 2012. Compensatory dynamics and the stability of phytoplankton biomass during four decades of eutrophication and oligotrophication. Ecology Letters 16(81): 89. https://doi.org/10.1111/ele.1201.
Kamenir, Y., Z. Dubinsky & T. Zohary, 2006. The long-term patterns of phytoplankton taxonomic size-structure and their sensitivity to perturbation: a Lake Kinneret case study. Aquatic Sciences 68: 490–591. https://doi.org/10.1007/s00027-006-0864-z.
Klee, R. & R. Schmidt, 1987. Eutrophication of Mondsee (Upper Austria) as indicated by the diatom stratigraphy of a sediment core. Diatom Research 2: 55–76. https://doi.org/10.1080/0269249X.1987.9704985.
Kraemer, B. M., R. M. Pilla, R. I. Woolway, O. Anneville, S. Ban, W. Colom-Montero, S. P. Devlin, M. T. Dokulil, E. E. Gaiser, et al., 2021. Climate change drives widespread shifts in lake thermal habitat. Nature Climate Change 11: 521–529. https://doi.org/10.1038/s41558-021-01060-3.
Kurmayer, R., M. Luger & H. Blatterer, 2022. Auftreten von roten Cyanobakterien-Blüten in den Alpenseen am Beispiel des Mondsees gestern und heute. Österreichs Fischerei 75: 139–153.
Kyselý, J., J. Kalvova & V. Květoň, 2000. Heat Waves in the South Moravian Region during the period 1961–1995. Studia Geophysica Et Geodaetica 44: 57–72. https://doi.org/10.1023/A:1022009924435.
Lenard, T. & W. Wojciechowska, 2013. Phytoplankton diversity and biomass during winter with and without ice cover in the context of climate change. Polish Journal Ecology 61: 739–748.
Liepolt, R., 1931. Zur Kenntnis einiger Alpenseen. Der Mondsee. Wien, Boku Diss.,108 S. + Karte. [available on internet at: https://permalink.obvsg.at/bok/AC00546873].
Liepolt, R., 1935. Limnologische Untersuchungen der Ufer- und Tiefenfauna des Mondsees und dessen Stellung zur Seentypenfrage. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 32: 164–236.
Liepolt, R., 1956. Die Verunreinigung von Gewässern durch Siedlungsabwässer. Wasser Und Abwasser 1956: 9–19.
Liepolt, R., 1957. Die Verunreinigung des Zellersees. Wasser Und Abwasser 1957: 9–38.
Livingstone, D. M. & M. T. Dokulil, 2001. Eighty years of spatially coherent Austrian lake surface temperatures and their relationship to regional air temperature and the North Atlantic Oscillation. Limnology Oceanography 46: 1220–1227. https://doi.org/10.4319/lo.2001.46.5.1220.
Luger, M., B. Kammerlander, H. Blatterer & H. Gassner, 2021. Von der Eutrophierung in die Klimaerwärmung – 45 Jahre limnologisches Monitoring Mondsee. Österreichische Wasser- Und Abfallwirtschaft 73: 418–425. https://doi.org/10.1007/s00506-021-00786-w.
Masojídek, J., J. U. Grobbelaar, L. Pechar & M. Koblížek, 2001. Photosystem II electron transport rates and oxygen production in natural waterblooms of freshwater cyanobacteria during a diel cycle. Journal of Plankton Research 23: 57–66. https://doi.org/10.1093/plankt/23.1.57.
Meehl, G. A. & C. Tebaldi, 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997. https://doi.org/10.1126/science.1098704.
Morabito, G., M. G. Mazzocchi, N. Salmaso, A. Zingone, C. Bergami, G. Flaim & A. Pugnetti, 2018. Plankton dynamics across the freshwater, transitional and marine research sites of the LTER-Italy Network. Patterns, fluctuations, drivers. Science of the Total Environment 627: 373–387. https://doi.org/10.1016/j.scitotenv.2018.01.153.
Müller-Jantsch, A., 1979. Untersuchungen an der Mondseeache und Sedimentationsmessungen im Attersee. Arbeiten Aus Dem Labor Weyregg 3: 107–120.
Niedrist, G. H., R. Psenner & R. Sommaruga, 2018. Climate warming increases vertical and seasonal water temperature differences and inter-annual variability in a mountain lake. Climatic Change 151: 473–490. https://doi.org/10.1007/s10584-018-2328-6.
Oberhaus, L., J. F. Briand, C. Leboulanger, S. Jaquet & J. F. Humbert, 2007. Comparative effects of the quality and quantity of light and temperature on the growth of Planktothrix agardhii and P. rubescens. Journal of Phycology 43: 1191–1199. https://doi.org/10.1111/j.1529-8817.2007.00414.x.
Oberrosler, I. E., 1979. Tiefenprofile des Phytoplanktons im Mondsee 1977/78. Arbeiten Aus Dem Labor Weyregg 3: 93–94.
GZÜV OÖ, 2005. 2007–2020: not separately referenced. [available on internet at: https://www.land-oberoesterreich.gv.at/211482.htm].
Ostrovsky, I., A. Rimmer, Y. Z. Yacobi, A. Nishri, A. Sukenik, O. Hadas & T. Zohary, 2013. Long-term changes in the lake kinneret ecosystem: the effects of climate change and anthropogenic factors. In Goldman, C. R., M. Kumagai & D. Robarts (eds), Climatic Change and Global Warming of Inland Waters Wiley-Blackwell, Oxford: 271–293. https://doi.org/10.1002/9781118470596.
Özkan, K., E. Jeppesen, T. A. Davidson, R. Bjerring, L. S. Johansson, M. Søndergaard, T. L. Lauridsen & J.-C. Svenning, 2016. Long-term trends and temporal synchrony in plankton richness, diversity and biomass driven by re-oligotrophication and climate across 17 Danish lakes. Water 8: 427. https://doi.org/10.3390/w8100427.
Padisák, J., 1995. Long-term changes of phytoplankton in the northeastern Siófok Basin of Lake Balaton, Hungary, between 1933 and 1994. In: Proceedings of the 6th International Conference on the Conservation and Management of lakes Kasumigaura '95: 813–817. [available on internet at: http://real.mtak.hu/id/eprint/3217].
Padisák, J., L. G. Tóth & M. Rajczy, 1988. The role of storms in the summer succession of the phytoplankton community in a shallow lake (Lake Balaton, Hungary). Journal of Plankton Research 10: 249–265. https://doi.org/10.1093/plankt/10.2.249.
Pomati, F., C. Tellenbach, B. Matthews, P. Venail, B. W. Ibelings & R. Ptacnik, 2015. Challenges and prospects for interpreting long-term phytoplankton diversity changes in Lake Zurich (Switzerland). Freshwater Biology 60: 1052–1059. https://doi.org/10.1111/fwb.12416.
Posch, T., O. Köster, M. M. Salcher & J. Pernthaler, 2012. Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nature Climate Change 2: 809–813. https://doi.org/10.1038/nclimate1581.
Psenner, R. & R. Sommaruga, 1989. Are rapid changes in bacterial biomass caused by shifts from top-down to bottom-up control? Limnology Oceanography 37: 1092–1100. https://doi.org/10.4319/lo.1992.37.5.1092.
Qu, Z., D. Forster, E. P. Bruni, D. Frantal, B. Kammerlander, L. Nachbaur, G. Pitsch, T. Posch, T. Pröschold, K. Teubner, B. Sonntag & T. Stoeck, 2021. Aquatic food webs in deep temperate lakes: key species establish through their autecological versatility. Molecular Ecology 30: 1053–1071. https://doi.org/10.1111/mec.15776.
Ragni, M., R. L. Airs, N. Leonardos & R. J. Geider, 2008. Photoinhibition of PSII in Emiliania huxleyi (Haptophyta) under light stress series: the roles of photoacclimation, photoprotection, and photorepair. Journal of Phycology 44: 670–683. https://doi.org/10.1111/j.1529-8817.2008.00524.x.
Raven, J. A. & R. J. Geider, 2003. Adaptation, acclimation and regulation in algal photosynthesis. In Larkum, A. W. D., S. E. Douglas & J. A. Raven (eds), Photosynthesis in Algae. Advances in Photosynthesis and Respiration, Vol. 14. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1038-2_17.
Reynolds, C. S., J. Padisák & I. Kóbor, 1993. A localized bloom of Dinobryon sociale in Lake Balaton: some implications for the perception of patchiness and the maintenance of species richness. Abstracta Botanica 17: 251–260.
Rott, E., 1981. Some results from phytoplankton counting intercalibrations. Schweizerische Zeitschrift Hydrologie 43: 35–62.
Ruggiu, D., G. Morabito, P. Panzani & A. Pugnetti, 1998. Trends and relations among basic phytoplankton characteristics in the course of the longterm oligotrophication of Lake Maggiore (Italy). Hydrobiologia 369(370): 243–257. https://doi.org/10.1023/A:1017058112298.
Ruttner, F., 1937. Limnologische Studien an einigen Seen der Ostalpen. Archiv für Hydrobiologie 32: 167–319.
Rydgren, K., R. H. Økland & T. Økland, 2003. Species response curves along environmental gradients. A case study from SE Norwegian swamp forests. Journal of Vegetation Science 14: 869–880. https://doi.org/10.1111/j.1654-1103.2003.tb02220.x.
Salmaso, N., 2010. Long-term phytoplankton community changes in a deep subalpine lake: responses to nutrient availability and climatic fluctuations. Freshwater Biology 55: 825–846. https://doi.org/10.1111/j.1365-2427.2009.02325.x.
Savichtcheva, O., D. Debroas, R. Kurmayer, C. Villar, J. P. Jenny, F. Arnaud, M. E. Perga & I. Domaizon, 2011. Quantitative PCR enumeration of total/toxic Planktothrix rubescens and total cyanobacteria in preserved DNA isolated from lake sediments. Applied and Environmental Microbiology 77: 8744–8753. https://doi.org/10.1128/AEM.06106-11.
Savichtcheva, O., D. Debroas, M. E. Perga, F. Arnaud, C. Villar, E. Lyautey, A. Kirkham, C. Chardon, B. Alric & I. Domaizon, 2015. Effects of nutrients and warming on Planktothrix dynamics and diversity: a palaeolimnological view based on sedimentary DNA and RNA. Freshwater Biology 60: 31–49. https://doi.org/10.1111/fwb.12465.
Schagerl, M., R. Siedler, E. Konopáčova & S. S. Ali, 2022. Estimating biomass and vitality of microalgae for monitoring cultures: a roadmap for reliable measurements. Cells 11: 2455. https://doi.org/10.3390/cells11152455.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes: natural mechanisms compensate for deficiencies of nitrogen and carbon in eutrophied lakes. Science 195: 260–262.
Schmidt, R., 1991. Diatomeenanalytische Auswertung laminierter Sedimente für die Beurteilung trophischer Langzeittrends am Beispiel des Mondsees (Oberösterreich). Wasser Und Abwasser 35: 109–123.
Schmidt, R., Jens Müller & J. Froh, 1985. Laminated sediments as a record of increasing eutrophication of the Mondsee. In Danielopol, D., R. Schmidt & E. Schultze (eds), Contributions to the Paleolimnology of the Trumer Lakes (Salzburg) snd the Lakes Mondsee, Attersee and Traunsee (Upper Austria) Limnologisches Institut Mondsee, ÖAW: 122–131.
Schröder, R., 1969. Ein summierender Wasserschöpfer. A summarizing water sampler. Archiv Für Hydrobiologie 66: 141–143.
Schultz, G., 1971. Erste Ergebnisse von 3 Jahren Seenuntersuchungen (1968 1969, 1970) an einigen Salzkammergutseen und Seen des Salzburger Flachgaues. Österreichs Fischerei 24: 149–158.
Schultze, E., 1985. Carotenoids from selected cores of the Trumer Lakes and the Lake Mondsee (trophic development and human impact). In Danielopol, D., R. Schmidt & E. Schultze (eds), Contributions to the Paleolimnology of the Trumer Lakes (Salzburg) snd the Lakes Mondsee, Attersee and Traunsee (Upper Austria) Limnologisches Institut Mondsee, ÖAW: 52–65.
Schwarz, K., 1979a. Entwicklung des Phytoplanktons im Mondsee von 1968–1978. Österreichs Fischerei 32: 213–216.
Schwarz, K., 1979b. Das Phytoplankton des Mondsees 1978. Arbeiten Aus Dem Labor Weyregg 3: 83–92.
Schwarz, K., 1981. Das Phytoplankton im Mondsee 1980. Arbeiten Aus Dem Labor Weyregg 5: 110–118.
Śliwińska-Wilczewska, S., K. A. Wiśniewska, G. Budzałek & Z. Konarzewska, 2021. Phenomenon of allelopathy in cyanobacteria. In Rastogi, R. P. (ed), Ecophysiology and Biochemistry of Cyanobacteria. Springer, Singapore. https://doi.org/10.1007/978-981-16-4873-1_11.
Sonntag, B., T. Posch, S. Klammer, K. Teubner & R. Psenner, 2006. Ciliates and flagellates in an oligotrophic deep alpine lake: contrasting variability with seasons and depths. Aquatic Microbial Ecology 43: 193–207. https://doi.org/10.3354/ame043193.
Steemann, Nielsen E., 1959. Untersuchungen über die Primärproduktion des Planktons in einigen Alpenseen Österreichs. Oikos 10: 24–37. https://doi.org/10.2307/3564904.
Swierczynski, T., S. Lauterbach, P. Dulski & A. Brauer, 2009. Die Sedimentablagerungen des Mondsees (Oberösterreich) als ein Archiv extremer Abflussereignisse der letzten 100 Jahre. In Schmidt, R., C. Matulla & R. Psenner (eds), Klimawandel in Österreich, alpine space - man & environment, Vol. 6. Innsbruck University Press, Innsberg: 115–126.
Teubner, K., 2000. Synchronized changes of planktonic cyanobacterial and diatom assemblages in North German waters reduce seasonality to two principal periods. Archiv Hydrobiologie, Special Issues Advances Limnology 55: 565–580.
Teubner, K., 2003. Phytoplankton, pelagic community and nutrients in a deep oligotrophic alpine lake: ratios as sensitive indicators of the use of P-resources (DRP:DOP:PP and TN:TP:SRSi). Water Research 37(7): 1583–1592. https://doi.org/10.1016/S0043-1354(02)00535-3.
Teubner, K., A. Sarobe, M. R. Vadrucci & M. T. Dokulil, 2001. 14C photosynthesis and pigment pattern of phytoplankton as size related adaptation strategies in alpine lakes. Aquatic Sciences 63: 310–325. https://doi.org/10.1007/PL00001357.
Teubner, K., M. Tolotti, S. Greisberger, H. Morscheid, M. T. Dokulil & H. Morscheid, 2003. Steady state phytoplankton in a deep pre-alpine lake: species and pigments of epilimnetic versus metalimnetic assemblages. Hydrobiologia 502: 49–64. https://doi.org/10.1007/978-94-017-2666-5_5.
Teubner, K., M. Tolotti, S. Greisberger, H. Morscheid, M. T. Dokulil & V. Kucklentz, 2006. Steady state of phytoplankton and implications for climatic changes in a deep pre-alpine lake: epilimnetic versus metalimnetic assemblages. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 29: 1688–1692. https://doi.org/10.1080/03680770.2005.11902973.
Teubner, K., W. Kabas & I. E. Teubner, 2018. Phytoplankton in Alte Donau: response to trophic change from hypertrophic to mesotrophic over 22 years. In Dokulil, M. T., K. Donabaum & K. Teubner (eds), The Alte Donau: Successful Restoration and Sustainable Management. An Ecosystem Case Study of a Shallow Urban Lake. Aquatic Ecology Series, Vol: 10 Springer, Cham: 107–148.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitteilungen Der Internationalen Vereinigung Limnologie 9: 1–38.
Vollenweider, R. A., 1968. The scientific basis of lake and stream eutrophication, with particular reference to phosphorus and nitrogen as eutrophication factors. Technical Report OECD Paris. DAS/CSI/68 27: 1-182.
Winkler, L. W., 1888. Die Bestimmung des im Wasser gelösten Sauerstoffes. Berichte Der Deutschen Chemischen Gesellschaft 21: 2843–2854.
Woolway, R. I., M. T. Dokulil, W. Marszelewski, M. Schmid, D. Bouffard & C. J. Merchant, 2017. Warming of Central European lakes and their response to the 1980s climate regime shift. Climatic Change 142: 505–520. https://doi.org/10.1007/s10584-017-1966-4.
Znachor, P., J. Nedoma, J. Hejzlar, J. Sedá, J. Komárková, V. Kolář, T. Mrkvička & D. Boukal, 2020. Changing environmental conditions underpin long-term patterns of phytoplankton in a freshwater reservoir. Science of the Total Environment 710: 135626. https://doi.org/10.1016/j.scitotenv.2019.135626.
Zotina, T., O. Köster & F. Jüttner, 2003. Photoheterotrophy and light-dependent uptake of organic and organic nitrogenous compounds by Planktothrix rubescens under low irradiance. Freshwater Biology 48: 1859–1872. https://doi.org/10.1046/j.1365-2427.2003.01134.x.
Acknowledgments
Many thanks to Claudia Skolaut, Christina Bachmann-Kaiblinger, Sonja Berl-Greisberger, Liselotte Eisl and Albert Jagsch. Rainer Kurmayer provided additional data on Planktothrix rubescens. Data from GZÜV (https://www.land-oberoesterreich.gv.at/211482.htm), Gov. Upper-Austria, eHyd (https://ehyd.gv.a) and H2O Fachdatenbank (https://wasser.umweltbundesamt.at/h2odb/), both Federal Ministry, Republic of Austria, Agriculture, Forestry, Regions and Water Management is kindly acknowledged.
Funding
Open access funding provided by University of Vienna.
Author information
Authors and Affiliations
Contributions
Paper concept, data acquisition and long-term analysis MTD, functional taxonomic traits and long-term persistence beyond generation times KT, both KT and MTD assembled text and graphics.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests and no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Viktória B-Béres, Luigi Naselli-Flores, Judit Padisák & Gábor Borics / Trait- Based Approaches in Micro-Algal Ecology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dokulil, M.T., Teubner, K. Long-term adjustment of phytoplankton structure to environmental traits at timescales during lifetime development and over generations. Hydrobiologia 851, 823–847 (2024). https://doi.org/10.1007/s10750-023-05365-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05365-6