Abstract
Wind is expected to be one of the main vectors of passive dispersal for small zooplankters between discrete, unconnected habitats. However, little is known about the differences in the dispersal capacity of species in relation to their propagule traits. Here we assessed the effect of volume and weight of diapausing eggs and substrate granulometry on the dispersal departure propensity of two differently body-sized rotifer species belonging to the Brachionus plicatilis complex using a wind tunnel experiment. Diapausing eggs of the larger species were also larger but, counterintuitively, were lifted by the wind to a greater extent than those of the smaller one. Further, diapausing eggs on the finer substrate were more exposed to the wind than those over the coarser one, and therefore higher departure rates were observed in the former. Overall, results show that wind is a relevant dispersal vector for the rotifers of the B. plicatilis species complex, with egg morphological traits and substrate granulometry being important factors modulating their dispersal. This study is a proof of concept for the departure phase of dispersal. Further studies on transfer and settlement phases are needed to get a complete picture of the dispersal potential of these organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal is an important life-history trait that allows survivorship of demes (Bonte & Dahirel, 2017). In a landscape of aquatic water bodies, especially when some of them are temporary, metapopulation extinction is avoided by the migration of individuals from nearby sources (Vanschoenwinkel et al., 2013) In zooplankters, the dominant dispersal propagule is generally the sexually produced diapausing egg. Passive spatial dispersal of propagules can occur through different biotic and abiotic agents (Havel & Shurin, 2004) such as water connections (hydrochory; e.g., Michels et al., 2001), animals (zoochory; e.g., Frisch et al., 2007), or wind (anemochory; e.g., Brendonck & Riddoch, 1999; Cáceres & Soluk, 2002; Cohen & Shurin, 2003; Havel & Shurin, 2004; Vanschoenwinkel et al., 2009). The latter is also recognized to influence the horizontal distribution of zooplankters over one water body (Thackeray et al., 2004), but it is a crucial vector for the effective transport of small metazoans among discrete, unconnected habitats (Sirianni, 2017; Vanschoenwinkel et al., 2008a).
Despite its critical importance, measurements of wind dispersal in zooplankton groups are rare compared with other organisms (e.g., plants), but increasingly growing in recent decades (Tesson et al., 2015). The net displacement between locations is determined by three stages of dispersal: departure, transfer and settlement (Bonte & Dahirel, 2017). Most studies on zooplankton dispersal rely on the third stage using gene flow measurements as direct evidence of population colonization and establishment (Louette et al., 2007; Ortells et al., 2012, 2014). Direct estimates of wind-borne transfer are challenging because they involve intercepting propagules by means of traps and windsocks (Vanschoenwinkel et al., 2008a, b; Vanschoenwinkel et al., 2009). To determine the capability of departure from the aquatic habitat by being airlifted, two nonexclusive approaches are applicable: installing propagule traps on the edges of the source water body and using wind-tunnel experiments under controlled conditions in the lab (Graham & Wirth, 2008; Pinceel et al., 2016; Rivas, et al., 2018). Wind-tunnel experiments further enable to search a relationship between dispersal capacity and specific propagule traits. It has been suggested that specific differences in egg size, shape or ornamentation may influence buoyancy, animal attachment or adhesion to the substrate (Cáceres et al., 2007; Pinceel et al., 2016; Vanschoenwinkel et al., 2009). Making use of a wind tunnel in a laboratory experiment, Pinceel et al. (2016) found differences in dispersal propensity among crustacean eggs of different size and shape on three distinct surface types. In their study, larger eggs were more easily airlifted and the rougher substrate offered spaces for wind protection. By contrast in the field, with the use of traps and windsocks, Vanschoenwinkel et al. (2009) found larger propagules over the substrate and smaller ones lifted above the rocky surface.
Recently, there have been important efforts to quantify wind dispersal (Sirianni, 2017; Ptatscheck et al., 2018; Rivas et al., 2019), but the overwhelming emphasis has been placed on large aquatic invertebrates (e.g., nematodes, water mites branchiopods, cladocerans, tardigrades), despite that small zooplankters—less than 300 µm—like rotifers are expected to be most readily dispersed by wind (Lopes et al., 2016; Moreno et al., 2016; Brendonck et al., 2017). Until now, direct measurements of rotifer wind dispersal are scarce (Jenkins & Underwood, 1998; Cáceres & Soluk, 2002; Lopes et al., 2016; Moreno et al., 2016; Ptatscheck et al., 2018; Rivas et al., 2019), and little is known on variation in dispersal propensity among rotifer species and in the features of their propagules.
The so-called resting or diapausing eggs of monogonont rotifers are encysted embryos, whose development is arrested, and encased in a three-layered shell that protects them from external stressors, like desiccation and temperature extremes (Wurdak et al., 1978; Clément & Wurdak, 1991; García-Roger, et al., 2019). The external characters of the diapausing egg—shape, size, color, and sculpturing (ornamentation)—appear to be species specific (Pourriot & Snell, 1983; Walsh et al., 2017; Guerrero-Jiménez et al., 2020). Eggs are either ovoid, as in Brachionus, or spherical, as in Asplanchna (Walsh et al., 2017; Guerrero-Jiménez et al., 2020). While there is little variation in the shape of the diapausing eggs, this is not the case for their size. In a metadata analysis, Walsh et al. (2017) estimated an average volume of rotifer diapausing eggs of 6.8 × 105 µm3 with a variation range from 0.11 × 105 to 97.4 × 105 µm3. The outer layer of the egg is a thick opaque shell which in some species may bear knobs or spines observable by scanning electron microscopy (Walsh et al., 2017; Guerrero-Jiménez et al., 2020).
Variation in the features of diapausing eggs appears to exist even between morphologically very similar species such as the cryptic species complex Epiphanes senta (Schröder & Walsh, 2007) and Brachionus plicatilis. For the latter, differences in size, shape and surface topography of their diapausing eggs have been reported for some species (Ciros-Pérez et al., 2001; Guerrero-Jiménez et al., 2020). However, it is unknown whether interspecific variation in these features might determine different wind-mediated dispersal capabilities of these cryptic species, which otherwise share most of their biology and ecology. Investigating these aspects will allow to characterize factors and processes in relation to dispersal and successful colonization in rotifers. Apart from the features of the propagules, substrate has also been recognized as an important factor in wind dispersal of zooplankton (Graham & Wirth, 2008; Vanschoenwinkel et al., 2010; Pinceel et al., 2016). The texture of the substrate influences the degree of exposure of the eggs and thus the wind speed necessary to lift them. Although there are some comparative studies among branchiopod groups (i.e., anostraca, notostraca, cladocera and spinicaudata) regarding the interaction between substrate grain and features of dormant stages (Pinceel et al., 2016), to date there are no data on the effect of type of substrate and its granulometry on the ability to disperse by wind in rotifers. The difference in the size of the diapausing eggs of the species of the B. plicatilis complex could interact with the grain size of the substrate, either favoring dispersal or generating shelters from the wind. This question has never been explored before.
In this study we focussed on wind dispersal of two related rotifer species belonging to the B. plicatilis complex. We performed a wind tunnel experiment with rotifer diapausing eggs to answer the following questions: Are there differences in the capacity of dispersal between rotifer species? And, to what extent variation in diapausing egg properties and substrate grain influences the propensity to be dispersed?
Methods
Production of experimental diapausing eggs
The diapausing eggs used in the experiments were produced under controlled laboratory conditions by monoclonal cultures of the species Brachionus plicatilis s.s. (six clones) and Brachionus rotundiformis (three clones). Differences in diapausing egg size between the two species can be observed in Fig. 1. The studied clones were established from the hatchlings of diapausing eggs isolated from the sediment egg bank of a temporary pond, Poza Sur, located in the East coast of Spain (Prat de Cabanes-Torreblanca Marsh Nature Reserve, Castellón, 40.089170 N, 0.101480 E). This is a small brackish pond (salinity range: 10–32 g l−1), roughly rectangular (10 × 7 m), and shallow (1 m, average depth), that typically dries out in summer (Gómez et al., 1995; García-Roger et al., 2008). The Cabanes-Torreblanca Marsh forms a coastal barrier-lagoon system with permanently flooded areas in an almost flat space located in the Oropesa Coastal Plain (Carmona et al., 2014; Segura-Beltran & Pardo-Pascual, 2019). This type of habitat is considered a highly dynamic environment where wind exposure, especially due to the sea breeze, is a very relevant factor (Blondel et al., 2010).
In Poza Sur, B. plicatilis and B. rotundiformis, which belong to the B. plicatilis cryptic species complex (Gómez et al., 2002; Suatoni et al., 2006), appear together in the water column during extended time periods (Gómez et al., 1995; Ortells et al., 2003; García-Roger et al., 2008; Gabaldón et al., 2017). Diapausing eggs morphologically identified as putatively belonging to the B. plicatilis complex were isolated from the sediment samples by the sugar flotation technique described in (García-Roger et al., 2005). Diapausing egg hatching was induced by individually transferring the eggs into 96-multiwell plates containing 150 µL of 6 g l−1 saline solution made with synthetic sea salts (Instant Ocean®; Aquarium Systems) and incubating the plates at 25 ºC under constant illumination (~ 160 µmol quanta m2 s−1) (García-Roger et al., 2006). The wells were checked for hatchlings every 24 h and 50 µL of rotifer culture medium were added to each well where a newborn female was observed. This medium consisted of a culture of the microalgae Tetraselmis suecica, used as food source to rotifers, and adjusted to a concentration of 250,000 cells ml−1. Microalgae were grown in 12 g l−1 modified F/2 medium (Guillard, 1975) prepared with synthetic sea salt (Instant Ocean®; Aquarium Systems) (hereafter, standard conditions). Microalgae density was estimated by using an automated cell counter based on image analysis (Celeromics Technologies). After a few days of clonal propagation from the diapausing egg hatchlings, the rotifer clones were transferred to test tubes with 15 ml of medium and maintained as stock cultures under standard conditions. B. plicatilis and B. rotundiformis clones were taxonomically identified according to their body size and shape, and dorsal spine morphology (Ciros-Pérez et al., 2001). From stock cultures—and in order to produce the diapausing eggs to be used in the experiments—mass cultures for each clone were started at low density by randomly transferring 50 females to flasks containing 500 ml of culture medium (initial density = 0.1 females ml−1). These cultures were raised under standard conditions and the microalgae concentration was monitored daily. If the microalgae concentration was lower than 250,000 cells ml−1, it was restored to this initial concentration by adding microalgae concentrated by centrifugation (1500×g, 10 min) to a low volume (< 1 ml). Pre-experimental cultures were also monitored daily for first males’ appearance (an indicator of the initiation of sexual reproduction). Once initiation of sexual reproduction was observed, the eggs produced within three days were harvested in B. plicatilis cultures, and within six days in the cultures of B. rotundiformis (where the production was slower). The aim was to gather an enough number of diapausing eggs for the experiments that were as similar in age as possible. The diapausing eggs were collected by filtering each culture through a 30-µm Nytal mesh sieve, which retained rotifers and eggs. Afterwards, the eggs were rinsed in 12 g l−1 saline solution and isolated under a stereomicroscope (Olympus SZX10) into Eppendorf tubes containing 40 g l−1 saline solution. Until their use in the experiments, these tubes were stored in the dark and 4 ºC to avoid the hatching of diapausing eggs. Before carrying the wind tunnel experiment and the measurements of weight and biometry, the diapausing eggs were soaked and rinsed sequentially in solutions of decreasing salinity up to distilled water.
Biometric traits of diapausing eggs
The length and width of 50 diapausing eggs from each clone and species were measured from microphotographs taken under an optical microscope Olympus BX51 at ×400 magnification, fitted with an Olympus ColorView IIIu 5 photocamera, and using cellˆP software (version 5.1, build 2600; Olympus) for image analysis. From these measurements, the volume was estimated using an ellipsoid approximation following the expression V = (π/12) (3 WL—W), where L is the length and W the width of the egg (Serrano et al., 1989).
The dry weight per egg was estimated for a minimum of three replicated batches of 40 eggs to a maximum of six replicated batches of 200 eggs, depending on the available eggs for each of the studied clones and species. Batches of eggs were isolated in pre-weighted aluminium foil containers made using the wells of 96-well tissue culture plates (Nunc™) as template. Then, they were dried in the oven at 55 ºC overnight and afterwards weighed. All measurements were carried out to the nearest 1.0 µg in a Thermo Cahn C-35 microbalance (Thermon Electron Cooperation) with the help of forceps. The average weight of a single egg was calculated within each batch. In all cases, we verified that coefficients of variation in weight estimate did not vary strongly among clones.
Wind-tunnel experiments
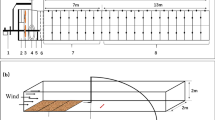
Aeolian dispersal of diapausing eggs was simulated in a wind tunnel built at the Laboratory of Aquatic Ecology, Evolution and Conservation (KU Leuven, Belgium). The tunnel is powered by a dual inlet centrifugal fan (D2E146-HT67-02, EBM-papst), mounted in a wooden frame and positioned at a 22° angle toward the presentation platform. The wind flow was directed through a Perspex tube with a diameter of 9 cm and a length of 23 cm until the platform, where the microscope slides with the diapausing eggs corresponding to each trial were placed. Realized wind speed was verified before each trial at the position of the presentation platform with an AN200 anemometer with a circular vane probe (AN200, EXTECH instruments). The wind tunnel device has the possibility to mount a fabric sock to collect the propagules lifted by the wind. However, the small size of Brachionus diapausing eggs does not allow them to be recovered afterwards. Therefore, instead of recording the number of eggs lifted from the slide by the wind and recovered in the sock, the number of eggs remaining on the slide was recorded following (Parekh et al., 2014). Consequently, our experiments focused on the dispersal departure of diapausing eggs (i.e., the first phase of the dispersal process). In a previous trial, the optimal wind speed for an observable outcome was found to be 6 km h−1. Consequently, wind velocity was set at this value using an in-line speed controller (REE 50, EBM-papst). This wind speed was in the same order of magnitude than the average wind speed in the wild for the natural populations from which experimental clones were isolated (data for 2021–2022; State Meteorological Agency—AEMET—Spanish Government).
To simulate the rough texture of the pond’s sandy sediment and study the effect of substrate particle size on wind dispersal, the lift-off of the eggs was tested on sandpaper of two different grain sizes, p80 (coarser grain, average diameter size = 200 µm) and p180 (finer grain, average diameter size = 80 µm) following Pinceel et al. (2016). These grain sizes correspond respectively to the fine and very-fine sand sediment granulometry (Wentworth, 1922). The granulometry of the pond sediment from which the eggs were isolated were 60–2000 µm. For each grain size, eight replicates were performed on each of the six clones of B. plicatilis, while in the three clones of B. rotundiformis, for which fewer eggs were available, the number of replicates ranged between two and eight.
For each wind tunnel trial (corresponding to the combination of 2 species × 3–6 clones × 2 grain substrate sizes × 2–8 replicates), 40 healthy-looking eggs were placed one by one, with the aid of a single-hair brush, in the center of a microscope slide lined with the sandpaper (Fig. 2). During each trial, the slide with the eggs was exposed to 6 km h−1 wind for a series of time intervals of 3, 5 and 10 min. After each exposure interval, the slide was checked under a stereomicroscope to count the remaining eggs. Then, the slide was placed again in the wind tunnel for the next wind exposure time period. The number of eggs remaining in the slide after each exposure corresponds to those eggs that were not airlifted. From these records, the number of dispersed eggs at each experimental time interval was estimated.
Microphotography of the two types of sandpaper, p80 (coarser grain, average diameter size = 200 µm) in the left panels and p180 (finer grain, average diameter size = 80 µm) in the right panels, showing the diapausing eggs of the two species (arrows): B. plicatilis in upper panels and B. rotundiformis in the bottom panels
Data analysis
We constructed a generalized linear mixed effect model (GLMM) to investigate whether there are differences in wind dispersal departure capacity of diapausing eggs between (1) species (B. plicatilis and B. rotundiformis), (2) the granulometry of substrate (p80 and p180 sandpaper), and (3) among different exposure times to wind (3, 5 and 10 min), all (1–3) taken as fixed-effect explanatory variables. Clone (nested within species) and egg (nested within clone) were considered as random-effect factors, the latter accounting for the fact that repeated observations (i.e., at the three-time intervals of wind exposure) were performed on each individual egg revealing its status (i.e., whether it dispersed or not). A binomial distribution of errors was assumed, and logit was used as link function in the GLMM. Significance of effects was tested after single term reductions of the full model (i.e., that including all the above-mentioned explanatory variables) followed by comparisons between reduced and the full models via likelihood ratio tests (LRTs). The significance level was set at P < 0.05.
In order to study whether the diapausing eggs within a given clone showed signs of differential dispersal departure capacity, we fitted linear regression models of the log-transformed counts of non-dispersing eggs (i.e., those remaining in the slide exposed to wind) against time in a clone-by-clone fashion. The logarithm of the initial number of diapausing eggs of a given clone exposed to the wind was set as a fixed intercept in each model. Then, the time distribution of the residuals of the data against each model (taken as a reference of constant dispersal rate) was used to estimate whether there are sets of eggs within a clone that dispersed from or remained in the experimental plates more than expected. Note that this approach is like the well-known survivorship curve analysis in ecology (Pearl, 1928) for which type II survivorship is taken as a reference (i.e., a constant proportion of individuals dying at each age interval).
The relationship between diapausing egg traits, weight and volume, and between these and their dispersal departure capacity (measured from the proportion of non-dispersing eggs) was explored by means of correlation analysis using the average values of each clone.
Analyses to investigate wind dispersal departure capacity of diapausing eggs of B. plicatilis and B. rotundiformis in relation to substrate grain and exposure time to wind were performed in R studio 3.5.3 (R Core Development Team) using the glmer and anova functions from package “lme4” (Bates et al., 2015), respectively for GLMM fitting and LRTs. Linear regression and analysis of residuals for the study of differential dispersal capacity at the within-clone level, as well as correlational analyses for diapausing egg traits, were carried out in Excel.
Results
Biometric traits of diapausing eggs
Diapausing egg linear dimensions (length and width), as well as volume, showed differences between the two species, with B. plicatilis consistently exhibiting greater values than B. rotundiformis (Table 1). Overall, the average values of B. plicatilis were 152.8 ± 1.7 µm for egg length and 113.6 ± 0.7 µm for egg width, while average values for B. rotundiformis were 112.0 ± 0.6 µm for egg length and 76.7 ± 0.6 µm for egg width. The average volume estimated from these measures was 13,613.5 ± 194.8 µm3 in B. plicatilis and 5,045.7 ± 145.2 µm3 in B. rotundiformis. The weight of B. plicatilis diapausing eggs averaged 0.41 ± 0.02 µg and those of B. rotundiformis averaged 0.18 ± 0.03 µg. In all cases, ranges were consistently wider among B. plicatilis clones. A positive and significant correlation was observed between the volume and the weight of diapausing eggs (Pearson’s r = 0.917, P < 0.001).
Wind tunnel experiment
The percentage of eggs dispersed at a moderate wind speed of 6 km h−1 during a period of ten minutes ranged from 19 to 89% for B. plicatilis, and from 6 to 74% for B. rotundiformis depending on substrate granulometry. Dispersal departure capacity of B. plicatilis and B. rotundiformis clones on substrates of different granulometry are depicted in Fig. 2. GLMM-based analyses revealed significant differences between the dispersal capacity of the diapausing eggs of both species (LRT’s χ2 = 243, P < 0.001); B. plicatilis dispersing more than B. rotundiformis, even with a light wind. Regarding substrate granulometry, the finest grain assayed (p180 sandpaper) resulted in higher dispersal rates than the coarsest (p80 sandpaper) for both species (RT’s χ2 = 1275.54, P < 0.001). Time exposure to wind had a significant positive effect on the dispersal capacity of the two species studied (LRT’s χ2 = 2272, P < 0.001). None of the interactions between fixed-effect explanatory variables were significant. Intraspecific variation in the dispersal departure capacity of diapausing eggs was observed (Fig. 3), with significant differences between the different clones of each species (LRT’s χ2 = 5550.2, P < 0.001), as well as between the eggs of a same clone (LRT’s χ2 = 345.14, P < 0.001).
Number of diapausing eggs remaining in the slides (i.e., not lifted) after each wind exposure time interval during the experiment for the clones studied of B. plicatilis (upper panel) and B. rotundiformis (lower panel). Note that this number of eggs is complementary to the number of eggs dispersed. Each line represents the dispersal dynamics of the diapausing eggs of a trial within a clone. Data are shown for two substrate granulometries, simulated with sandpaper of two different grain sizes, p80 (coarser grain, average diameter size = 200 µm) and p180 (finer grain, average diameter size = 80 µm)
At this within-clone level, the analysis of the deviations of the residuals of underlying linear models assuming constant dispersal rates with exposure time revealed differences between the diapausing eggs of a same clone with respect to their probability of being lifted by wind (Fig. 4). The differences were more pronounced for the clones of B. plicatilis—the species that dispersed the most—than for B. rotundiformis, and became more evident on p180 sandpaper. Diapausing eggs of B. rotundiformis dispersed at a constant rate on both substrate granulometries, whereas B. plicatilis diapausing eggs only dispersed at a constant rate on the coarser substrate (p80 sandpaper). When placed on the finer substrate (p180 sandpaper), the diapausing eggs of B. plicatilis dispersed more than expected under a constant rate during the first five minutes and less than expected after ten minutes of wind exposure.
Average of the deviation of the residuals for the diapausing egg dispersal rate at each wind exposure interval time in relation to a constant dispersion rate. Results are shown for each clone of the two species studied (B. plicatilis: Bp; B. rotundiformis: Br) in the two substrate granulometries assayed, p80 sandpaper (coarser grain, average diameter size = 200 µm) in blue and p180 sandpaper (finer grain, average diameter size = 80 µm) in red. The error bars represent the standard error
Results showed a negative correlation between the percentage of remaining diapausing eggs after ten minutes of wind exposure and egg volume (Pearson’s r = − 0.488, P = 0.040) and weight (Pearson’s r = − 0.407, P = 0.094).
Discussion
Our findings provide empirical evidence that wind is a relevant dispersal vector for small sized zooplankters such as the rotifers of the B. plicatilis species complex. In this manner, the diapausing eggs of the natural populations studied were effectively lifted in the established wind tunnel setup. Despite that small sized taxa are expected to disperse easily (Brooks & Dodson, 1965; Finlay, 2002) and that wind has been long recognized as a major dispersing vector for zooplankton dormant propagules over short distances (Cáceres & Soluk, 2002; Cohen & Shurin, 2003; Havel & Shurin, 2004; Vanschoenwinkel et al., 2008b; Lopes et al., 2016; Moreno et al., 2016), there are very few studies that have engaged in quantifying rotifer dispersal. Most of these studies have focused on population establishment based on manipulated mesocosms (Cáceres & Soluk, 2002; Cohen & Shurin, 2003), and some have adventured to catch rotifer propagules up in the air (Lopes et al., 2016; Moreno et al., 2016; Rivas et al., 2019). To date, there is only one previous study addressing wind dispersal of rotifer dormant propagules under controlled conditions (Rivas et al., 2018), and to our knowledge, none has quantified the differential dispersal output capacities of closely related species in relation to the biometric characteristics of their diapausing eggs.
Wind dispersal departures differed between the two species studied, which appears to be associated with differences in diapausing egg size and weight. The diapausing eggs of B. plicatilis, larger and heavier than those of B. rotundiformis (Munuswamy et al., 1996; Ciros-Pérez et al., 2001), were more prone to be airlifted than the eggs of B. rotundiformis even at low wind speed. The diapausing eggs of the two species studied differed in size. The mean diapausing egg volume of B. plicatilis was approximately 2.7 times that of B. rotundiformis, even though the volume of both species lies in the lower volume range of rotifer diapausing eggs according to data reported by Walsh et al. (2017). The percentage of eggs dispersed after 10 min of exposure to wind was consistently higher for B. plicatilis than for B. rotundiformis for both types of substrate granulometry. These results coincide with those of Pinceel et al. (2016) who found that larger propagules in several species of branchiopod crustaceans were more easily lifted by wind than smaller ones. Since the weight of diapausing eggs of both rotifer species showed a positive correlation with volume, this greater capacity of the heavier eggs to be lifted by the wind—like in Pinceel’s et al. (2016) study—is contrary to the rational expectation of a positive relationship between mass and airlifting. As these authors argue, a larger diameter (i.e., the two diameters in the case of the ellipsoidal diapausing eggs of Brachionus) provides a larger wind contact surface and thus can result in a stronger wind boost on the egg, which could compensate for the increased aerodynamic resistance and adhesion forces resulting from the larger size of the propagule. To our knowledge, except for the previous study by Pinceel et al. (2016), the relationship between zooplankton propagule mass and wind-dispersal departure has not been analyzed under controlled conditions. There are, however, previous reports stating that passively dispersed propagules tend to be smaller than active dispersers, and that their dispersed distances are not related to propagule mass (see Jenkins et al., 2007 for meta-analysis in several taxa including protists, Fungi, Plantae and Animalia). Because in this study we have emphasized on the departure phase of the dispersal process in rotifers, we do not have data on the distances reached based on the biometrical traits of the diapausing eggs of the species studied. Moreover, whether or not these wind uplift differences imply differences in the height attained and the dispersal distance traveled between the diapausing eggs of the two species studied cannot be ascertained from our study. It has been suggested that the higher ease for airlifting of bigger-sized eggs could result in frequent, short distance dispersal by tumbling near ground level, whereas smaller eggs could be airlifted longer and thus travel larger distances (Brendonck & Riddoch, 1999; Vanschoenwinkel et al., 2009). However, studies investigating the relationship between passive dispersal distance and propagule mass are not conclusive in this respect (Jenkins et al., 2007; Fontaneto, 2019; Alzate & Onstein, 2022). On the other hand, a previous wind-tunnel study by Rivas et al. (2018) reported that most diapausing eggs of B. plicatilis and the congeneric freshwater species Brachionus calyciflorus, whose diapausing eggs are of similar size and morphology, reached distances of several hundreds of meters, and that some could even travel up to 1,000 km, when exposed to a wind speed of ~ 40 km h−1.
Besides differences between species, significant differences in dispersal capacity were observed between clones of the same species. These differences may be related to variation in volume and weight of the diapausing eggs. Previous studies have reported variations of 30% in diapausing egg volume among clones within other species of the genus Brachionus (Liu & Niu, 2010). The coefficient of variation of diapausing egg volume of clones of the species studied was 9.1% for B. rotundiformis and 8.6% for B. plicatilis. Given that all eggs were produced under the same conditions, differences between clones must be genetically determined. Similarly, among clonal differences found in dispersal capacity can also have a genetic basis. On the contrary, within clonal differences are strictly due to environmental conditions, in this case, the egg being more or less exposed to the wind. It is precisely in this sense that the granulometry of the substrate emerges as a factor of influence on the dispersal capacity of rotifer diapausing eggs, as we show below.
Our findings showed that the grain size of the surface on which eggs were laid is relevant for dispersal. We used two different substrate granulometries, framed in the granulometry range of the sediment in the dried pond bed and areas of exposed soil from the margin of the pond during periods of low water in our study area, and from which diapausing eggs are expected to disperse away. Dispersal rate was higher in the finer grain sandpaper (p. 180) for both species than in the coarser grain one (p80), in a manner consistent with the findings by Pinceel et al. (2016) for several branchiopod crustacean propagules. The average grain-to-grain distance in the coarser grain sandpaper is 400 µm, whereas it is 130 µm in the finer grain sandpaper. Therefore, the diapausing eggs of both species can be interspersed between the grains of the coarser grain sandpaper. However, only the diapausing eggs of B. rotundiformis, smaller in size (112.0 µm ± 0.6), can be interspersed between the grains on the finer grain sandpaper, while the diapausing eggs of B. plicatilis lied down on the top of the grains of this substrate. As a result, the eggs of B. plicatilis were more exposed to wind when deposited on this substrate.
In the wind tunnel, diapausing eggs were dispersed at low wind speeds (6 km h−1). In the field, this wind speed is at the lower limit of the range reported for the region where the natural populations of B. plicatilis and B. rotundiformis studied are found, according to the records of the nearest meteorological station (39.95514157509477, − 0.07379782107939019; approx. 30 km from the study site). In the last two years, the maximum wind speed was 75.96 km h−1 and, having days without wind, the average wind speed was 8.1 km h−1. This indicates that natural dispersal of rotifer diapausing eggs would be likely even on days with light winds. For larger zooplankters, such as branchiopods and cladocerans, Graham & Wirth (2008) demonstrated with a portable wind tunnel, that wind velocities necessary for cyst movement in branchiopods were within the range of velocities experienced in the field, and minimum wind speed thresholds for dispersal have been estimated ranging from 5.28 to 12.4 km h−1 (Parekh et al., 2014). Parekh et al. (2014) estimated that a speed of 17.2 km h−1 for 10 s would be required to disperse 80% of the eggs of a branchiopod species. Regarding cladocerans, the range for minimum wind speed thresholds for dispersal have been estimated from 8.1 to 10.9 km h−1 (Pinceel et al., 2016). Furthermore, Pinceel et al. (2020) showed that when exposing ephippia for 210 min at a wind speed of 32.5 km h−1 the percentage of dispersed eggs ranged from 2 to 12%, and that this percentage increased from 5 to 30% when using a wind speed of 70 km h−1. Regarding our results, for a series of pre-experimental tests of wind speeds, values above 10 km h−1 provoked the loss of the vast majority of rotifer diapausing eggs (C. Arenas, pers. obs.), so we could not provide comparative data of wind speeds as those reported for the other groups of organisms. However, we have been able to provide data on the numbers of airlifted diapausing eggs at various time intervals over a total exposure period of 10 min. These data have shown how eggs have different probabilities of being lifted into the air, and that this probability varies over time depending on the availability of spaces sheltered from the wind and the effect of the wind speed itself to remove the eggs from the shelters.
Another methodological issue in our experiment has to do with the placement of the eggs on the substrate. This was carried out on dry substrates aiming to mimic conditions experienced by diapausing eggs during the dry season in temporary ponds, which is the period when eggs may be more exposed to the wind (Bilton et al., 2001; Vanschoenwinkel et al., 2008b; Tuytens et al., 2014; Moreno et al., 2016). It is worthy to mention that the ponds inhabited by B. plicatilis and B. rotundiformis are brackish, so the formation of a salt precipitate with the evaporation of water is a common circumstance in the dry period that would fix the diapausing eggs to the substrate and make it difficult to lift them. The effective wind speed for the dispersal of rotifer diapausing eggs in these systems should be one which lifts particles of dust and salt, even sediment fragments in which the eggs are embedded (Vanschoenwinkel et al., 2009), for which probably a wind speed higher than that tested here would be needed. Finally, the salinity of the water could also be a relevant aspect for a situation not initially considered here, namely the dispersal of floating or suspended eggs in the water column. In this sense, dispersal would not be restricted only to dry periods –although these could still be the most important– but also to the moment of the reflooding of the ponds. Note that diapausing eggs in contact with air could float more easily at that moment since their outer shell is porous, but denser or more viscous water may exert a greater impediment to lifting eggs through the air. Therefore, we call for the need to carry out experiments in a wind tunnel placing the eggs on water to test whether a higher speed is required for their effective dispersal.
This study represents a proof of principle on differences in dispersal capabilities among species and clones of rotifers due to diapausing egg features and substrate type. The controlled conditions in the laboratory do not mimic completely those occurring in the field, and our results represent only dispersal rates from the departure phase. Clearly, field quantification with wind traps and studies on colonization and community build up will enrich the picture with transfer and establishment processes like maintenance in the air or effective dispersal. However, it is a first step that we believe will encourage many more to come, bringing rotifer knowledge to the mainstream pool of dispersal studies.
Data availability
Data will be available upon reasonable request.
References
Alzate, A. & R. E. Onstein, 2022. Understanding the relationship between dispersal and range size. Ecology Letters 25: 2303.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32: 159–181.
Blondel, J., J. Aronson, J.-Y. Bodiou & G. Boeuf, 2010. The Mediterranean Region: Biological Diversity Through Time and Space, Oxford University Press, Oxford:
Bonte, D. & M. Dahirel, 2017. Dispersal: a central and independent trait in life history. Oikos 126: 472–479.
Brendonck, L. & B. J. Riddoch, 1999. Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda). Biological Journal of the Linnean Society 67: 87–95.
Brendonck, L., T. Pinceel & R. Ortells, 2017. Dormancy and dispersal as mediators of zooplankton population and community dynamics along a hydrological disturbance gradient in inland temporary pools. Hydrobiologia 796: 201–222.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Cáceres, C. E. & D. A. Soluk, 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408.
Cáceres, C. E., A. N. Christoff & W. J. Boeing, 2007. Variation in ephippial buoyancy in Daphnia pulicaria. Freshwater Biology 52: 313–318.
Carmona, P., J. M. Ruiz & M. I. Ibáñez, 2014. Coastal erosion and environmental change in the Cabanes-Torreblanca wetland (Castelló). Data for sustainable management. Boletín De La Asociación De Geógrafos Españoles 66: 477–483.
Ciros-Pérez, J., A. Gomez & M. Serra, 2001. On the taxonomy of three sympatric sibling species of the Brachionus plicatilis (Rotifera) complex from Spain, with the description of B. ibericus n. sp. Journal of Plankton Research 23: 1311–1328.
Clément, P. & E. Wurdak, 1991. Rotifera. In Harrison, F. W. & E. E. Ruppert (eds), Microscopic Anatomy of Invertebrates Wiley, New York: 219–297.
Cohen, G. M. & J. B. Shurin, 2003. Scale-dependence and mechanisms of dispersal in freshwater zooplankton. Oikos 103: 603–617.
Finlay, B. J., 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063.
Fontaneto, D., 2019. Long-distance passive dispersal in microscopic aquatic animals. Movement Ecology 7: 10.
Frisch, D., A. J. Green & J. Figuerola, 2007. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Sciences 69: 568–574.
Gabaldón, C., D. Fontaneto, M. J. Carmona, J. Montero-Pau & M. Serra, 2017. Ecological differentiation in cryptic rotifer species: what we can learn from the Brachionus plicatilis complex. Hydrobiologia 796: 7–18.
García-Roger, E. M., M. J. Carmona & M. Serra, 2005. Deterioration patterns in diapausing egg banks of Brachionus (Müller, 1786) rotifer species. Journal of Experimental Marine Biology and Ecology 314: 149–161.
García-Roger, E. M., M. J. Carmona & M. Serra, 2006. Hatching and viability of rotifer diapausing eggs collected from pond sediments. Freshwater Biology 51: 1351–1358.
García-Roger, E., X. Armengol, M. Carmona & M. Serra, 2008. Assessing rotifer diapausing egg bank diversity and abundance in brackish temporary environments: an ex situ sediment incubation approach. Fundamental and Applied Limnology / Archiv Für Hydrobiologie 173: 79–88.
García-Roger, E. M., E. Lubzens, D. Fontaneto & M. Serra, 2019. Facing adversity: dormant embryos in rotifers. The Biological Bulletin 237: 119–144.
Gómez, Á., M. Temprano & M. Serra, 1995. Ecological genetics of a cyclical parthenogen in temporary habitats. Journal of Evolutionary Biology 8: 601–622.
Gómez, A., M. Serra, G. R. Carvalho & D. H. Lunt, 2002. Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (Rotifera). Evolution 56: 1431–1444.
Graham, T. B. & D. Wirth, 2008. Dispersal of large branchiopod cysts: potential movement by wind from potholes on the Colorado Plateau. Hydrobiologia 600: 17–27.
Guerrero-Jiménez, G., E. Ramos-Rodríguez, M. Silva-Briano, A. Adabache-Ortiz & J. M. Conde-Porcuna, 2020. Analysis of the morphological structure of diapausing propagules as a potential tool for the identification of rotifer and cladoceran species. Hydrobiologia 847: 243–266.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In Smith, W. L. & M. H. Chanley (eds), Culture of Marine Invertebrate Animals. Springer, Boston.
Havel, J. E. & J. B. Shurin, 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49: 1229–1238.
Jenkins, D. G. & M. O. Underwood, 1998. Zooplankton may not disperse readily in wind, rain, or waterfowl. Hydrobiologia 387–388: 15–21.
Jenkins, D. G., C. R. Brescacin, C. V. Duxbury, J. A. Elliott, J. A. Evans, K. R. Grablow, M. Hillegass, B. N. Lyon, G. A. Metzger, M. L. Olandese, D. Pepe, G. A. Silvers, H. N. Suresch, T. N. Thompson, C. M. Trexler, G. E. Williams, N. C. Williams & S. E. Williams, 2007. Does size matter for dispersal distance? Global Ecology and Biogeography 16: 415–425.
Liu, W. & C. J. Niu, 2010. Polymorphism in resting egg size and hatching strategy in the rotifer Brachionus calyciflorus Pallas. Zoological Science 27: 330–337.
Lopes, P. M., R. Bozelli, L. M. Bini, J. M. Santangelo & S. A. J. Declerck, 2016. Contributions of airborne dispersal and dormant propagule recruitment to the assembly of rotifer and crustacean zooplankton communities in temporary ponds. Freshwater Biology 61: 658–669.
Louette, G., J. Vanoverbeke, R. Ortells & L. De Meester, 2007. The founding mothers: the genetic structure of newly established Daphnia populations. Oikos 116: 728–741.
Michels, E., K. Cottenie, L. Neys & L. De Meester, 2001. Zooplankton on the move: first results on the quantification of dispersal of zooplankton in a set of interconnected ponds. Hydrobiologia 442: 117–126.
Moreno, E., C. Pérez-Martínez & J. M. Conde-Porcuna, 2016. Dispersal of zooplankton dormant propagules by wind and rain in two aquatic systems. Limnetica 35: 323–336.
Munuswamy, N., A. Hagiwara, G. Murugan, K. Hirayama & H. J. Dumont, 1996. Structural differences between the resting eggs of Brachionus plicatilis and Brachionus rotundiformis (Rotifera, Brachionidae): an electron microscope study. Hydrobiologia 318: 219–223.
Ortells, R., A. Gómez & M. Serra, 2003. Coexistence of cryptic rotifer species: ecological and genetic characterisation of Brachionus plicatilis. Freshwater Biology 48: 2194–2202.
Ortells, R., C. Olmo & X. Armengol, 2012. Colonization in action: genetic characteristics of Daphnia magna Strauss (Crustacea, Anomopoda) in two recently restored ponds. Hydrobiologia 689: 37–49.
Ortells, R., J. Vanoverbeke, G. Louette & L. Meester, 2014. Colonization of Daphnia magna in a newly created pond: founder effects and secondary immigrants. Hydrobiologia 723: 167–179.
Parekh, P. A., M. J. Paetkau & L. A. Gosselin, 2014. Historical frequency of wind dispersal events and role of topography in the dispersal of anostracan cysts in a semi-arid environment. Hydrobiologia 740: 51–59.
Pearl, R., 1928. The rate of living, A. A. Knopf Inc., New York:
Pinceel, T., L. Brendonck & B. Vanschoenwinkel, 2016. Propagule size and shape may promote local wind dispersal in freshwater zooplankton-a wind tunnel experiment. Limnology and Oceanography 61: 122–131.
Pinceel, T., B. Vanschoenwinkel, M. Weckx & L. Brendonck, 2020. An empirical test of the impact of drying events and physical disturbance on wind erosion of zooplankton egg banks in temporary ponds. Aquatic Ecology 54: 137–144.
Pourriot, R. & T. W. Snell, 1983. Resting eggs in rotifers. Hydrobiologia 104: 213–224.
Ptatscheck, C., B. Gansfort & W. Traunspurger, 2018. The extent of wind-mediated dispersal of small metazoans, focusing nematodes. Scientific Reports 8: 6814.
Rivas, J. A., J. E. Mohl, R. S. Van Pelt, M.-Y. Leung, R. L. Wallace, T. E. Gill & E. J. Walsh, 2018. Evidence for regional Aeolian transport of freshwater micrometazoans in arid regions. Limnology and Oceanography Letters 3: 320.
Rivas, J. A., T. Schroder, T. E. Gill, R. L. Wallace & E. J. Walsh, 2019. Anemochory of diapausing stages of microinvertebrates in North American drylands. Freshwater Biology 64: 1303–1314.
Schröder, T. & E. J. Walsh, 2007. Cryptic speciation in the cosmopolitan Epiphanes senta complex (Monogononta, Rotifera) with the description of new species. Hydrobiologia 593: 129–140.
Segura-Beltran, F. & J. E. Pardo-Pascual, 2019. Fan deltas and floodplains in Valencian coastal plains. In Morales, J. A. (ed), The Spanish Coastal Systems. Dynamic Processes, Sediments and Management. Springer, Berlin.
Serrano, L., M. Serra & M. R. Miracle, 1989. Size variation in Brachionus plicatilis resting eggs. Hydrobiologia 186(187): 381–386.
Sirianni, K. M., 2017. Differential wind dispersal of cladoceran ephippia in a rock pool metacommunity. Aquatic Ecology 51: 203–218.
Suatoni, E., S. Vicario, S. Rice, T. Snell & A. Caccone, 2006. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: the rotifer-Brachionus plicatilis. Molecular Phylogenetics and Evolution 41: 86–98.
Tesson, S. V. M., B. Okamura, R. Y. Dudaniec, W. Vyverman, J. L. Ondahl, C. Rushing, A. Valentini & A. J. Green, 2015. Integrating microorganism and macroorganism dispersal: modes, techniques and challenges with particular focus on co-dispersal. Ecoscience 22: 109–124.
Thackeray, S. J., D. G. George, R. I. Jones & I. A. N. J. Winfield, 2004. Quantitative analysis of the importance of wind-induced circulation for the spatial structuring of planktonic populations. Freshwater Biology 49: 1091–1102.
Tuytens, K., B. Vanschoenwinkel, A. Waterkeyn & L. Brendonck, 2014. Predictions of climate change infer increased environmental harshness and altered connectivity in a cluster of temporary pools. Freshwater Biology 59: 955–968.
Vanschoenwinkel, B., S. Gielen, H. Vandewaerde, M. Seaman & L. Brendonck, 2008a. Relative importance of different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity. Ecography 31: 567–577.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2008b. Any way the wind blows—frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117: 125–134.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2009. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: differences in dispersal capacities and modes. Hydrobiologia 635: 363–372.
Vanschoenwinkel, B., A. Waterkeyn, M. Jocqué, L. Boven, M. Seaman & L. Brendonck, 2010. Species sorting in space and time—the impact of disturbance regime on community assembly in a temporary pool metacommunity. Journal of the North American Benthological Society 29: 1267–1278.
Vanschoenwinkel, B., F. Buschke & L. Brendonck, 2013. Disturbance regime alters the impact of dispersal on alpha and beta diversity in a natural metacommunity. Ecology 94: 2547–2557.
Walsh, E. J., L. May & R. L. Wallace, 2017. A metadata approach to documenting sex in phylum Rotifera: diapausing embryos, males, and hatchlings from sediments. Hydrobiologia 796: 265–276.
Wentworth, C. K., 1922. A scale of grade and class terms for clastic sediments. The Journal of Geology 30: 377–392.
Wurdak, E. S., J. J. Gilbert & R. Jagels, 1978. Fine structure of the resting eggs of the rotifers Brachionus calyciflorus and Asplanchna sieboldi. Transactions of the American Microscopical Society 97: 49–72.
Acknowledgements
We thank Daniel García-Sala for his assistance in image processing of figures.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by Grants AICO/2020/013 and CIGE2021-139 funded by GVA, Grant PID20201141536B-100 funded by MCINN and ERDF “A way of making Europe”, and fellowship FPU19/03779 funded by MCINN/AEI/10.13039/501100011033 and FSE “Investing in your future”. C. A-S. benefited from a travel Grant EST22/00601 to carry out the experiments at KU Leuven.
Author information
Authors and Affiliations
Contributions
Conceptualization: RO, LB, MJC and EMG-R. Conducting the research: CA-S and RO. Formal analysis: EMG-R and CA-S. Visualization: CA-S and EMG-R. First-draft elaboration: CA-S, RO, MJC and EMG-R. Writing, review and editing: CA-S, RO, MJC, EMG-R and LB. Funding: EMG-R and MJC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial relationship that could be interpreted as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Maria Špoljar, Diego Fontaneto, Elizabeth J. Walsh & Natalia Kuczyńska-Kippen / Diverse Rotifers in Diverse Ecosystems
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arenas-Sánchez, C., Brendonck, L., García-Roger, E.M. et al. Wind dispersal differences between rotifer cryptic species: a proof of principle from a wind tunnel experiment. Hydrobiologia 851, 2895–2907 (2024). https://doi.org/10.1007/s10750-023-05349-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05349-6