Abstract
Here we focused on the co-occurrence pattern on regional and local scales, and on the niche differences of two species of congeneric beetles (Ochthebius quadricollis and O. lejolisii, Hydraenidae) exclusive of supratidal rockpools. Abundances of adults and larval stages from both species and environmental variables were obtained in 10 pools from 12 localities along the Iberian Mediterranean coast. To determine the local co-existence pattern, we monthly sampled two localities in an annual cycle. On regional and local scales, we found negative correlations between both species’ pool abundances, which suggest spatio-temporal segregation based on their different environmental responses. The OMI analysis detected interspecific niche differences, larger in larvae than adults. The best regression models obtained for O. quadricollis larvae included depth, conductivity, and fine sediments as the main explanatory variables with a positive effect, and distance to sea and CPOM with a negative effect. For O. lejolisii larvae, the best models included CPOM and periphyton with positive effects, while pool area, depth and conductivity negatively affected. Our results suggest that subtle interspecific differences in ecological niches, mainly those related to pool hydroperiod and salinity, could determine spatio-temporal storage effects as the principal mechanisms of co-existence on local and regional scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The co-existence of species in natural communities has historically fascinated scientists (e.g., Hutchinson, 1959; Amarasekare, 2003; Hart et al., 2017; Luo et al., 2022). Despite major theoretical developments in past decades, there is still very few little empirical research (Amarasekare, 2003; Hawlena, 2022), which can even be more complex and challenging when referring to congeneric species.
The classic theory assumes that closely related taxa inhabiting a given space have a series of common traits that allow them to pass the filter imposed by environmental conditions, as well as some ecological differences that enable stable co-existence (Gause, 1934; Hutchinson, 1959; MacArthur & Levins, 1967; Den Boer, 1979; Chase, 2003). These differences are usually considered to be species’ niches (Hutchinson, 1957). Niche differences are important for species co-existence because they enable each species to limit its own growth rates more effectively than that of competitors (Peterson et al., 2011). This can be achieved by several mechanisms, of which spatio-temporal storage effects are key co-existence mechanisms if competing species are different in terms of their environmental responses (Tilman, 1982; Chesson, 2000). Thus, when species differ in their responses to abiotic environment variation, they should reach high abundances in the most favorable habitat patches by experiencing fierce intraspecific competition. In contrast, they are rare in unfavorable patches and mostly endure interspecific competition (Pironon et al., 2018). Spatio-temporal heterogeneity may also facilitate species co-occurrence on fine scales (Burgazzi, 2018), but environmental data are rarely compiled at an adequate high resolution to empirically evaluate their influence (Letten et al., 2015; Williams et al., 2022). In observational studies, on the local scale the environment is usually treated as spatially homogeneous and co-existence is attributed to independent processes, such as resource partitioning or the temporal storage effect (Letten et al., 2015).
In this context, the ecological niche analysis is key for understanding the co-existence of related species, and even conspecific populations (Husson et al., 2017; Johnston et al., 2022). Niche displacement may explain how closely related species with co-occurrence on a regional or local scale can also co-exist on the microhabitat scale. These often subtle niche differences are more difficult to discern in extreme environments, where identifying possible niche displacements is complicated by the wide range of species’ tolerance to environmental variables, such as temperature, desiccation or salinity (Arribas et al., 2019; Marrone et al., 2023).
Coastal habitats are among the most dynamic on Earth given their simultaneous exposure to terrestrial, oceanic and atmospheric processes (Parvizi et al., 2022). Of them, the intertidal zone has a long history of ecological study, particularly the vertical zonation phenomenon and how it is modified by exposure to tidal and wave actions (see the review in Hawkins et al., 2020). A vast variety of field and laboratory experimental studies has been conducted to explore the role of physical and biological factors, including competition, predation and behavior, in setting distribution patterns, as well as causes, including how organisms respond to the environmental stressors associated with emersion periods, such as desiccation and temperature (e.g., Southward, 1958; Foster, 1971). In these habitats, the co-existence of closely related species can be common (e.g., Boaventura et al., 2002; Conde-Padín et al., 2007; Stickle et al., 2017; Casal et al., 2018; Blakeslee et al., 2021; Vecchioni et al., 2021), where the upper end of a species vertical distribution is principally determined by not only its abiotic stress tolerance to emersion temperature and desiccation (Connell, 1970, 1972; Wolcott, 1973; Menge & Sutherland, 1987), but also by biotic interactions at the lower end of the species range (Paine, 1966, 1969; Wethey, 1984; Somero, 2002).
Compared to intertidal zones, the co-existence of related species in supratidal zones has been studied much less (Villastrigo et al., 2022). Supratidal rockpools are recognized as one of the most challenging living environments worldwide (Fig. 1C), characterized by harsh rapidly changing environmental conditions (Brandes et al., 2015; Sabatelli et al., 2016, 2021; Parvizi et al., 2022), especially on the Mediterranean coast (Izquierdo & Mikolajewicz, 2019). These pools are subjected to intense salinity fluctuations due to the combined effect of marine (splash, waves) and terrestrial water inputs (Telesh et al., 2013; Vinagre et al., 2015), which determine an extremely variable hydroperiod. During raining seasons, rockpools can be flooded from days to several months, sometimes by severe storms, and they also undergo continuous evaporation and desiccation, especially in summer. This leads to not only the formation of extremely hypersaline pools (Brandes et al., 2015), with concentrations exceeding 200 g l−1 (Mirón-Gatón et al., 2022b), but also to drastic changes in habitat availability. Besides, marine rockpools undergo marked temperature fluctuations on seasonal and daily scales (Mirón-Gatón et al., 2022a) due to intense solar radiation in areas that lack emergent or rooted vegetation. In pools, a biofilm can develop on the rock substrate and filamentous algae as primary producers, and large deposits of Posidonia oceanica (L.) Delile leaf remains and fine sediment can accumulate. Thus, supratidal rocky coasts constitute a fragmented and highly heterogeneous area with conditions varying along the gradient of distance to sea; from more constant conditions in pools near the coastline, but strongly exposed to waves and changes in sea level, to more distant temporary pools with vast environmental variation. These fluctuating extreme conditions constrain species from being able to live in such habitats, which results in communities with poor species richness that consist mainly of Mollusca and Crustacea of marine origin, and Diptera and Coleoptera of continental origin (Margalef, 1949). Coleopterans of the genus Ochthebius Leach, 1815 (Family Hydraenidae) are the predominant macroinvertebrate to inhabit coastal supratidal rockpools in many parts of the world, and congener species of Ochthebius from two evolutionary lineages (Cobalius and Ochthebius subgenera) in the same localities, and even in the same pools, are frequently found (Villastrigo et al., 2022).
Our study focused on two representative species of these true rockpool Ochthebius lineages: O. (Ochthebius) quadricollis Mulsant, 1844 and O. (Cobalius) lejolisii Mulsant & Rey, 1861 (see habitus in Fig. 1A, B). Both species show partial sympatry on the Mediterranean coast of the Iberian Peninsula (Fig. 2A) and constitute an excellent model system to investigate co-existence mechanisms in closely related species on spatio-temporal scales. Previous studies have found different, but overlapping, responses to temperature (Mirón-Gatón et al., 2022a) and salinity between the adult and young life stages of these Ochthebius species (Mirón-Gatón et al., 2022b). Additionally, differences in their abundance and life cycle duration throughout the year (Velasco et al., 2022) point out that spatio-temporal storage effects are two of the principal co-existence mechanisms in these species. Yet despite recent advances, information on their habitat specificity is still scarce. Overall, this study aims to: (i) analyze the spatio-temporal occurrence patterns of the two above-mentioned congeneric Ochthebius species; (ii) estimate and compare the environmental niche of these species’ adult and larval stages by considering that the larval niche describes the optimum conditions under which reproduction and population growth are maximized; (iii) determine the environmental variables that drive their distribution and abundance patterns. We hypothesized niches differentiation between the studied Ochthebius species that, albeit subtle, allows their spatio-temporal segregation to result in stable co-existence on local and regional scales.
Methods
Study species
Ochthebius quadricollis and O. lejolisii have an eastern Atlantic and western/central Mediterranean distribution. O. quadricollis has a wider distribution in the western Mediterranean, while O. lejolisii is more commonly found on the Atlantic coast, but extends to the south Iberian Mediterranean coast where both species frequently co-occur, and even occasionally appear with another third Ochthebius species (O. subinteger Mulsant & Rey, 1861 or O. evae Villastrigo, Hernando, Millan & Ribera, 2020) (Villastrigo et al., 2022). Adults of both species are small organisms (about 2 mm), macropterous with weak flight ability that enables them to cover short distances between nearby pools (Hase, 1926; Jacquin, 1956). O. quadricollis adults are generally more active than those of O. lejolisii, and the latter are often found buried under the sediment of drying pools (Mirón-Gatón et al., 2022a, b). Both species’ breeding period lasts for most of the year and presents a temporal overlap, mainly in spring and autumn. Both species are fast-growing organisms with multivoltine cycles and overlapped cohorts. They complete at least three (O. lejolisii) or four generations per year (O. quadricollis) on the Spanish SE Mediterranean coast (Velasco et al., 2022).
Field sampling and life stage identifications
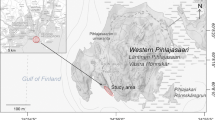
In order to analyze both species’ spatial co-occurrence patterns in the study area (regional scale), 12 Iberian Mediterranean coast localities were selected (Fig. 2B) that covered localities and pools with a wide range of environmental conditions (Table S1). In each locality, 10 pools were randomly sampled by following a distance gradient to the sea level during the more favorable reproductive seasons for both species (autumn, November 2018 or spring, May 2019). To determine their abundance patterns, 5 min were spent per pool to collect adult and larval stages with soft entomological forceps and brushes. We previously measured several physico-chemical variables per pool (distance to sea, area, length, width, depth, temperature, conductivity, fine sediment cover) and biological variables (periphyton and coarse particulate organic matter -CPOM- cover) as potential food resources (Table S1). To analyze the temporal co-existence patterns on the local scale, the same sampling protocol was monthly repeated from October 2018 to September 2019 in two sampling localities: “Cala Reona” (Cartagena, Murcia) and “Cala de las Pulgas” (Águilas, Murcia) (yellow spots, Fig. 2B). Both species co-occur in these two localities, but differ in terms of their abundance, with O. quadricollis being dominant at Cala Reona and O. lejolisii at Cala de las Pulgas.
A total of 1275 specimens of Ochthebius quadricollis and 782 of O. lejolisii were collected in the field (see more details in Table S1). They were stored in 96% EtOH and transported to the laboratory in small hermetically sealed boats. In the laboratory, species were morphologically identified under a Leica M165 C stereo microscope. Species’ larval stages were identified by the shape of the head oviruptor teeth in the first instar and by chaetotaxy for the remaining instars (see Delgado & Soler, 1995, 1997 for details).
Data analysis
We applied three approaches to investigate the spatio-temporal co-existence and storage mechanisms between the two Ochthebius species, and to characterize their environmental niches. We firstly applied Spearman’s correlation test to explore the spatial co-occurrence of the total abundance for both species, as well as adult and larval abundances separately, by considering both species’ abundance data in each sampled locality and their abundance in each pool. Subsequently on the local scale, we compared the time changes of the abundance data in the two intensively sampled localities (Cala Reona and Cala de las Pulgas) and tested their temporal correlation by considering locality and the pool monthly abundance data.
Considering all the pools sampled in the studied localities (see Fig. 2B), we applied an outlying mean index (OMI) analysis (Dolédec et al., 2000) to characterize the environmental niche of adult and larval stages separately by its niche position (NP), niche marginality (NM) and niche breadth (NB). The OMI analysis is a well-suited ordination technique to calculate niche metrics and to identify the most influential environmental factors for community structure and organization. NP was estimated as the species score of the first two ordination axis (Table S2). NM refers to the distance between the mean habitat conditions used by species and the mean habitat conditions of the study area. NB, also named tolerance in OMI, measures the amplitude of each species’ distribution along the sampled environmental gradients (Dolédec et al., 2000). The OMI analysis also identifies the variables that best differentiate the environmental niches of the studied species. A permutation test (Monte-Carlo tests with 1000 permutations) was applied to check the significance of the OMI analysis by indicating if species marginality was significantly higher than expected by chance. The OMI analysis was performed using the ‘ade4’ package (Dray & Dufour, 2007) with the R software, version 4.2.0 (R Development Core Team, 2022).
Finally, to determine the environmental variables driving the abundance patterns of adults and larval stages in both species, zero-inflated Poisson (ZIP) regression models were applied because our data included a high proportion of zeros (Lambert, 1992; Agarwal et al., 2002) (preliminary models using Poisson and negative binomial distributions performed poorly). Modeling was done by the R package ‘glmmTMB’ (Brooks et al., 2017). All the models included the fixed effects of environmental variables and a single random effect to account for dependence among samplings within the same locality. Before modeling, we performed single non parametric correlation tests (Spearman rank) to explore the relations among variables using the ‘cor’ function of R and ‘pairs.panels’ of the ‘psych’ package (Revelle, 2022) to select non redundant variables (Fig. S1). The explanatory variables were transformed (logarithmic or square root transformation in each case) and standardized to adequately compare and estimate their effect sizes. Finally, we followed a multimodel approach (Burnham & Anderson, 2002) with the ‘MuMIn’ R package (Bartoń, 2022) to identify the best fit models and to assess the relative importance of predictors. Model selection was conducted by fitting all the possible models and using Akaike’s Information Criterion adjusted for small sample sizes (AICc). We identified the best model based on AICc and calculated the relative importance of each predictor variable by summing the AICc weights across all the models in the set of the most likely models (ΔAICc < 2).
Results
Abundance correlations
Both species co-occurred in 9 of the 12 sampled localities, with O. quadricollis dominating in 6 localities and O. lejolisii in 3 (Table S1). Positive correlations were generally observed between the adult and larval stage abundances of each species (Fig. 3). On the regional scale, by considering the abundance in each locality as the sum of the species abundances in the set of sampled pools, there were no significant correlations between both species (Fig. 3A). However, the pool larval abundance of O. quadricollis correlated negatively with the total and adult abundances of O. lejolisii (Spearman's ρ − 0.23 and ρ − 0.24, both P-value < 0.05, respectively, Fig. 3B), but there was no significant correlation between both species’ total abundance.
Spearman’s correlation coefficients among the abundances of adults, larvae and the total of Ochthebius quadricollis and O. lejolisii on the regional scale when considering total abundance at the locality (A) and pool (B) levels in all the sampled locations. Correlation coefficients on the local scale obtained between species/stages when considering monthly abundances at the local and pool levels for Cala Reona (C and D, respectively) and Cala de las Pulgas (E and F, respectively). Legend: Q Total abundance of O. quadricollis; QA Abundance of O. quadricollis adults; QL Abundance of O. quadricollis larvae; L Total abundance of O. lejolisii; LA Abundance of O. lejolisii adults; LL Abundance of O. lejolisii larvae. Levels of significance: *(P-value < 0.05), **(P-value < 0.01), ***(P-value < 0.001). Spearman's correlation coefficients range from − 1 to + 1
On the local scale, by taking both the whole locality and the individual pools data, at Cala Reona the monthly total abundance of O. quadricollis correlated negatively with the larvae abundance of O. lejolisii (Spearman's ρ − 0.63 P-value < 0.05, Fig. 3C and ρ − 0.25 P-value < 0.01, Fig. 3D, respectively). The total abundance of O. quadricollis tended to increase during the studied annual period, while the abundance of O. lejolisii decreased, especially in summer (Fig. 4A). However, at Cala de las Pulgas, where O. lejolisii was the dominant species, its monthly total abundance in the set of pools, and the abundance of the larval and adult stages separately, did not correlate with those of O. quadricollis (Figs. 3E, 4B). When considering pool abundances, significant positive correlations appeared between species, and for both the total and adult stages (Spearman's ρ 0.3 and ρ 0.4, both P-value < 0.001, respectively, Fig. 3F).
Environmental niches
The OMI analysis revealed that the first two axes accounted for 92.2% of species' environmental variation, with 70.6% explained by the first axis and 21.5% by the second. Although there was a significant overlap between the O. quadricollis larval and adult niches (Fig. 5A), larvae were less tolerant (narrower NB) and showed more marginality (NM) than adults (Fig. 5B). Their environmental niches were positively related to pool area, depth and CPOM (Fig. 5A). Both O. lejolisii larvae and adults had wider niches than O. quadricollis, and the larval stage presented greater marginality (Fig. 5A, B). Moreover, the niches of O. lejolisii larvae and adults were closely related to the cover of fine sediment and periphyton and distance to sea (Fig. 5A).
A Two-dimensional plot of the OMI analysis representing the position of the environmental niches (elipses) of Ochthebius quadricollis and O. lejolisii according to their life stages. Black points represent sampled pools from all the localities. B The NM of the Ochthebius species/stages vs. the NB (tolerance) of both species/stages
Environmental variables driving abundance patterns
The best models, obtained with AICc < 2 (Table S3), for the abundance of O. quadricollis adults and larvae included conductivity, CPOM, depth and distance to sea as the principal explanatory variables. For O. lejolisii, only two models were selected for larvae and adults. Both models also included temperature and CPOM, but with a different relative importance for larvae and adults. The deviances explained by the best models (Tables 1, 2) were generally low, except for the O. lejolisii larvae (54.2%).
According to the results of the best model for O. quadricollis (Table 1) and the relative importance of the selected variables obtained from the subset of models (Table S3), adults’ abundance increased with distance to sea of the pool and CPOM cover, while these variables had the inverse effect on larvae abundance. Conductivity also had an important and different effect on the abundance of both larvae and adults, and was positive in larvae and negative in adults. Pool depth was another important factor to affect larvae abundance, and had a higher value in the deepest pool. However, for O. lejolisii (Table 2), pool depth had a negative effect on both the larval and adult stages, as did pool area on larvae. Conductivity only negatively affected larvae abundance, while temperature and fine sediment cover positively affected adults abundance. Both O. lejolisii larvae and adults were positively related to CPOM cover, whereas periphyton was negatively related to adults abundance and positively with larvae.
Discussion
Our field study reports, for the first time, quantitative pieces of evidence for spatio-temporal storage co-existence mechanisms in O. quadricollis and O. lejolisii, two water beetle inhabitants from western Mediterranean supratidal rockpools. Our results support the hypothesis that there were differences in the environmental niche of the two congeneric species that allowed their co-existence in highly dynamic and heterogenous systems like supratidal rockpools. For each species, it also found some differences in the environmental niche between the larval and adult stages with larval niches describing the optimum conditions under which reproduction and population growth is maximized and by showing larger interspecific differences than adult niches. O. lejolisii larvae had the widest NB (i.e., tolerant organisms that can maintain fitness over a broader range of abiotic conditions), but the highest NM, which also occurred in less common habitats in the sampling area, such as the less saline and ephemeral pools. The O. quadricollis larvae, with low marginality and NB values, occurred in common habitats in the sampling area.
Species co-existence in most animal communities is likely to result from multiple mechanisms (Amarasekare, 2003). With the Ochthebius species from supratidal rockpools, spatio-temporal storages appeared to be particularly important, similarly to what has been observed in continental zooplanktons like copepods, cladocerans and rotifers (Brendonck & De Meester, 2003; Montero-Pau et al., 2011) and intertidal species (Connell, 1983; Olabarria et al., 2001; Ysebaert & Herman, 2002; Benedetti-Cecchi, 2003). The negative abundance correlations found between species (Fig. 3B–D) point out spatio-temporal segregation on the local scale based on different environmental responses and habitat suitability, which varied spatially along the gradient of distance to sea and throughout the year. However, different species co-existence patterns were observed between localities, where one species dominated in abundance over another, which can be related to the availability of suitable habitats for each species. At Cala Reona, where O. quadricollis dominates, pools with its high abundances displayed lower O. lejolisii abundances (Fig. 3C, D). At Cala de las Pulgas, where O. lejolisii dominates, both species abundances were positively correlated (Fig. 3F). In the former, O. quadricollis was favored by very high habitat availability on the rocky coastal platform, and can respond with a high population growth rate throughout the year and obtain higher abundances. However, due to the narrower supratidal zone at Cala de las Pulgas, the presence of smaller shallower temporary pools is common, which provide a more favorable habitat for O. lejolisii than for O. quadricollis. Nonetheless the overall lower availability of suitable habitats for both species may limit their population sizes.
For the successful colonization of organisms in supratidal zones, two types of physiological adaptation are required under harsh selective pressure (Zhang et al., 2016): (1) strong desiccation resistance, which is facilitated by improved water retention; (2) efficient osmoregulation ability when exposed to fluctuating salinity. The differential susceptibility of organisms and populations to environmental stress influences the outcome of biological interactions and the structure of communities (Arnér, 1997). In our case, the most limiting factor for both studied species was water availability, determined principally by pool surface and depth, its proximity to the sea and the precipitation that fluctuates over time, which alter species’ responses. O. quadricollis preferentially occupies larger deeper pools with a long hydroperiod near the sea, while O. lejolisii prefers smaller shallower pools located further away from the coastline that often dry out, which is consistent with their different physiological tolerance to desiccation (Mirón-Gatón et al., 2022b). Salinity is another crucial environmental factor that determines habitat suitability and the distribution of organisms in supratidal rockpools (McAllen & Taylor, 2001; Zhang et al., 2016). In our study, conductivity had a significant, but heterogeneous, effect on the abundance of both species’ adult and larval stages. Although both species are euryhalines and may withstand extreme salinity fluctuations, the abundance of the O. quadricollis larvae increased with conductivity, while the abundance of O. lejolisii larvae seemed to be disadvantaged. However, recent laboratory experiments have shown that O. lejolisii has greater physiological tolerance to salinity than O. quadricollis, and both species’ larvae and eggs are more tolerant than adults (Mirón-Gatón et al., 2022b). The discordance between the observed saline niches and their fundamental niches was probably due to the combined effect of salinity and other environmental stressors, such as water availability and temperature. The two co-existing species also differed in terms of their temperature tolerance (Mirón-Gatón et al., 2022a): O. quadricollis was more tolerant to heat, while O. lejolisii better withstood cold temperatures. While oviposition and larval development in O. quadricollis were limited to winter, they did not seem to be limited by low temperatures in O. lejolisii (Velasco et al., 2022). However in summer, high temperatures (up to 38.5°C) and the drying out of shallow pools, mainly those located far from the coastline, diminished the abundance of both O. lejolisii larvae and adults in the study area. This species can resist extreme salinity and temperature conditions in dry pools by hiding as larvae or adults under sediment or in rock crevices (Villastrigo et al., 2022). This observational information was concordant with the significant relation found between the abundance of O. lejolisii adults and the fine sediment cover. This behavior response allows adults and larvae to resist until pools are refilled with autumn rains, and it activates development and reproduction. Furthermore, the production of resistant desiccation eggs in O. lejolisii (Mirón-Gatón et al., 2022b) could ensure stable co-existence on large temporal scales, like those that occur in zooplankton (e.g., Daphnia) by allowing species to remain inactive under harsh environmental conditions and a buffered population growth, which increases when the abiotic environment is favorable (Amarasekare, 2003). In contrast O. quadricollis can maintain as an active population and can successfully reproduce in summer in pools near the sea that remain flooded (Velasco et al., 2022). Therefore, the stable co-existence of both congeneric species via temporal storage is achieved with buffered population growth (Chesson, 2000, 2003, 2018). Populations of both species showed inverse fluctuations throughout the year due to their differential response to spatio-temporal habitat availability variation. As a result, each species was more active and abundant at different times of the year (O. lejolisii in winter and O. quadricollis in summer), which led to a narrower temporal overlap and, consequently, decreased the intensity of the interspecific competition between them. Diminishing population growth when the abiotic environment is unfavorable is offset by increasing population growth when the abiotic environment is favorable. In addition, mechanisms that are not entirely based on niche differentiation, such as differences in life cycle, likely interact with niches in the observed co-existence patterns. Under laboratory conditions, and at constant optimal temperature (20ºC) and salinity (35 g.l−1), O. quadricollis showed a shorter overall life cycle and more successful egg hatching than O. lejolisii (Velasco et al., 2022), which conferred O. quadricollis more demographic success.
On the regional scale, both species could co-exist via a spatial storage effect combined with dispersal (see Amarasekare, 2003 for more examples) because interspecific niche differences ensure that species have favorable locations in the landscape, where they experience relatively little interspecific competition. Regional co-existence is facilitated by the number of pools in a patchy environment (Ranta, 1982). Classic studies of species co-existence of Daphnia and corixids (Hemiptera) in Baltic coastal rockpools suggest that frequent local extinctions and random colonizations of empty pools maintain the regional co-existence pattern, even when the local co-existence in the same pool would be unlikely due to resource competition (Vepsäläinen, 1978; Ranta, 1979, 1982; Pajunen, 1979a, b, 1982; Hanski & Ranta, 1983; Bengtsson, 1988). Our results do not provide clear evidence for resource partitioning between species, but this co-existence mechanism may not be important because they are similar sizes and probably exploit common resources. Although the diets of both species have not yet been studied, Hydraenidae species generally feed on periphyton, filamentous algae and vegetal detritus mixed with mineral particles (Perkins, 1980; Jäch et al., 2016; Valladares et al., 2018). The models obtained in our study showed positive relations between the abundance of O. lejolisii larvae with periphyton and CPOM, and the abundance of the adults of both species with CPOM. Interestingly, plants/macroalgal waste, such as Posidonia leaf remains, are colonized by microorganisms and can serve as an important food resource because they can also be used by these species as substrate on which to lay eggs.
Conclusions
Our field study reports, for the first time, quantitative pieces of evidence for spatio-temporal storage co-existence mechanisms in O. quadricollis and O. lejolisii, two water beetles that inhabit western Mediterranean supratidal rockpools. Our results suggest that subtle interspecific differences in ecological niches, principally based on their differential responses to hydroperiod and salinity related to pool size and distance to sea, play an important role in both species’ co-existence on local and regional scales. Further studies into biotic interactions, dispersal ability and metapopulation dynamics will help to complete and gain a better understanding of the complex dynamics of these interesting ecosystems and their fauna.
Data availability
All relevant data are within the paper and its Supplementary Material.
References
Agarwal, D. K., A. E. Gelfand & S. Citron-Pousty, 2002. Zero-inflated models with application to spatial count data. Environmental and Ecological Statistics 9: 341–355. https://doi.org/10.1023/A:1020910605990.
Amarasekare, P., 2003. Competitive coexistence in spatially structured environments: a synthesis. Ecology Letters 6: 1109–1122. https://doi.org/10.1046/j.1461-0248.2003.00530.x.
Arnér, M., 1997. Organisms and food webs in rock pools: responses to environmental stress and trophic manipulation. Doctoral Thesis, University of Stockholm.
Arribas, P., C. Gutiérrez-Cánovas, M. Botella-Cruz, M. Cañedo-Argüelles, J. Carbonell, A. Millán, S. Pallarés, J. Velasco & D. Sánchez-Fernández, 2019. Insect communities in saline waters consist of realized but not fundamental niche specialists. Philosophical Transactions of the Royal Society B: Biological Sciences 374(20180008): 20180008. https://doi.org/10.1098/rstb.2018.0008.
Bartoń, K., 2022. MuMIn: multi-model inference. R package version 1.46.0. [available on internet at https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf].
Benedetti-Cecchi, L., 2003. The importance of the variance around the mean effect size of ecological processes. Ecology 84: 2335–2346. https://doi.org/10.1890/02-8011.
Bengtsson, J., 1988. Life histories, interspecific competition and regional distribution of three rockpool Daphnia species. Doctoral Thesis, University of Uppsala.
Blakeslee, A. M., A. W. Miller, G. M. Ruiz, K. Johannesson, C. André & M. Panova, 2021. Population structure and phylogeography of two North Atlantic Littorina species with contrasting larval development. Marine Biology 168: 1–16. https://doi.org/10.1007/s00227-021-03918-8.
Boaventura, D., P. Ré, L. Cancela da Fonseca & S. J. Hawkins, 2002. Intertidal rocky shore communities of the continental Portuguese coast: analysis of distribution patterns. Marine Ecology 23: 69–90. https://doi.org/10.1046/j.1439-0485.2002.02758.x.
Brandes, M., D. C. Albach, J. C. Vogt, E. Mayland-Quellhorst, G. Mendieta-Leiva, S. Golubic & K. A. Palinska, 2015. Supratidal extremophiles: cyanobacterial diversity in the rock pools of the Croatian Adria. Microbial Ecology 70: 876–888. https://doi.org/10.1007/s00248-015-0637-0.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84. https://doi.org/10.1023/A:1024454905119.
Brooks, E. M., K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Maechler & B. M. Bolker, 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9: 378–400. https://doi.org/10.32614/RJ-2017-066.
Burgazzi, G., S. Guareschi & A. Laini, 2018. The role of small-scale spatial location on macroinvertebrate community in an intermittent stream. Limnetica 37: 319–340. https://doi.org/10.23818/limn.37.26.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. Springer-Verlag, New York. https://doi.org/10.1007/b97636
Casal, G., S. Aceña-Matarranz, D. Fernández-Márquez & N. Fernández, 2018. Distribution and abundance patterns of three coexisting species of Patella (Mollusca Gastropoda) in the intertidal areas of the NW Iberian Peninsula: implications for management. Fisheries Research 198: 86–98. https://doi.org/10.1016/j.fishres.2017.10.011.
Chase, J. M., 2003. Community assembly: when should history matter? Oecologia 136: 489–498. https://doi.org/10.1007/s00442-003-1311-7.
Chesson, P., 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31: 343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343.
Chesson, P., 2003. Quantifying and testing coexistence mechanisms arising from recruitment fluctuations. Theoretical Population Biology 64: 345–357. https://doi.org/10.1016/S0040-5809(03)00095-9.
Chesson, P., 2018. Updates on mechanisms of maintenance of species diversity. Journal of Ecology 106: 1773–1794. https://doi.org/10.1111/1365-2745.13035.
Conde-Padín, P., J. W. Grahame & E. Rolán-Alvarez, 2007. Detecting shape differences in species of the Littorina saxatilis complex by morphometric analysis. Journal of Molluscan Studies 73: 147–154. https://doi.org/10.1093/mollus/eym009.
Connell, J. H., 1970. A predator-prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecological Monographs 40: 49–78. https://doi.org/10.2307/1942441.
Connell, J. H., 1972. Community interactions on marine rocky intertidal shores. Annual Review of Ecology and Systematics 3: 169–192. https://doi.org/10.1146/annurev.es.03.110172.001125.
Connell, J. H., 1983. On the prevalence and relative importance of interspecific competition: evidence from field experiments. The American Naturalist 122: 661–696. https://doi.org/10.1086/284165.
Delgado, J. A. & A. G. Soler, 1995. Description of the larva of Ochthebius (Cobalius) subinteger Mulsant & Rey, 1861 (Coleoptera, Hydraenidae). Graellsia 51: 121–128. https://doi.org/10.3989/graellsia.1995.v51.i0.402.
Delgado, J. A. & A. G. Soler, 1997. Morphology and Chaetotaxy of larval Hydraenidae (Coleoptera) III. The genus Calobius Wollaston, 1854. Aquatic inSects 19: 165–175. https://doi.org/10.1080/01650429709361651.
Den Boer, P. J., 1979. Exclusion or coexistence and the taxonomic or ecological relationship between species. Netherlands Journal of Zoology 30: 278–306. https://doi.org/10.1163/002829679X00421.
Dolédec, S., D. Chessel & C. Gimaret-Carpentier, 2000. Niche separation in community analysis: a new method. Ecology 81: 2914–2927. https://doi.org/10.1890/0012-9658(2000)081[2914:NSICAA]2.0.CO;2.
Dray, S. & A. B. Dufour, 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20. https://doi.org/10.18637/jss.v022.i04.
Foster, B. A., 1971. Desiccation as a factor in the intertidal zonation of barnacles. Marine Biology 8: 12–29. https://doi.org/10.1007/BF00349341.
Gause, G. F., 1934. The Struggle for Existence: A Classic of Mathematical Biology and Ecology. The Williams & Wilkins company, Baltimore. https://doi.org/10.5962/bhl.title.4489
Hanski, I. & E. Ranta, 1983. Coexistence in a patchy environment: three species of Daphnia in rock pools. Journal of Animal Ecology 52: 263–279. https://doi.org/10.2307/4599.
Hart, S. P., J. Usinowicz & J. M. Levine, 2017. The spatial scales of species coexistence. Nature Ecology & Evolution 1: 1066–1073. https://doi.org/10.1038/s41559-017-0230-7.
Hase, A., 1926. Zur Kenntnis der Lebensgewohnheiten und der Umwelt des marinen Käfers Ochthebius quadricollis Mulsant (Hydrophilidae). Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 16: 141–179. https://doi.org/10.1002/iroh.19260160303.
Hawkins, S. J., K. E. Pack, K. Hyder, L. Benedetti-Cecchi & S. R. Jenkins, 2020. Rocky shores as tractable test systems for experimental ecology. Journal of the Marine Biological Association of the United Kingdom 100: 1017–1041. https://doi.org/10.1017/S0025315420001046.
Hawlena, H., 2022. Coexistence research requires more interdisciplinary communication. Ecology and Evolution 12: e8914. https://doi.org/10.1002/ece3.8914.
Husson, B., P. M. Sarradin, D. Zeppilli & J. Sarrazin, 2017. Picturing thermal niches and biomass of hydrothermal vent species. Deep Sea Research Part II: Topical Studies in Oceanography. 137: 6–25. https://doi.org/10.1016/j.dsr2.2016.05.028.
Hutchinson, G. E., 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427. https://doi.org/10.1101/SQB.1957.022.01.039.
Hutchinson, G. E., 1959. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist 93: 145–159. https://doi.org/10.1086/282070.
Izquierdo, A. & U. Mikolajewicz, 2019. The role of tides in the spreading of Mediterranean Outflow waters along the southwestern Iberian margin. Ocean Modelling 133: 27–43. https://doi.org/10.1016/j.ocemod.2018.08.003.
Jäch, M. A., R. G. Beutel, J. A. Delgado & J. A. Díaz, 2016. Hydraenidae Mulsant, 1844. In Beutel, R. G. & R. A. B. Leschen (eds), Handbook of Zoology, vol. IV Arthropoda: Insecta. Part 38. Coleoptera, Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga (partim), vol. 1. 2nd ed. Walter De Gruyter, Berlin & New York: 316–345.
Jacquin, A., 1956. Recherches biologiques sur Ochthebius quadricollis Mulsant (Coléoptère Hydrophilide). Bulletin De La Société D’histoire Naturelle De L’afrique Du Nord 47: 270–290.
Johnston, E. C., A. S. Wyatt, J. J. Leichter & S. C. Burgess, 2022. Niche differences in co-occurring cryptic coral species (Pocillopora spp.). Coral Reefs 41: 767–778. https://doi.org/10.1007/s00338-021-02107-9.
Lambert, D., 1992. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 34: 1–14. https://doi.org/10.2307/1269547.
Letten, A. D., D. A. Keith, M. G. Tozer & F. Hui, 2015. Fine-scale hydrological niche differentiation through the lens of multi-species co-occurrence models. Journal of Ecology 103: 1264–1275. https://doi.org/10.1111/1365-2745.12428.
Luo, M., S. Wang, S. Saavedra, D. Ebert & F. Altermatt, 2022. Multispecies coexistence in fragmented landscapes. Proceedings of the National Academy of Sciences 119: e2201503119. https://doi.org/10.1073/pnas.2201503119.
MacArthur, R. H. & R. Levins, 1967. The limiting similarity, convergence and divergence of coexisting species. The American Naturalist 101: 377–385. https://doi.org/10.1086/282505.
Margalef, R., 1949. Sobre la ecología de las larvas del mosquito Aëdes mariae. Publicaciones Del Instituto De Biología Aplicada 6: 83–101.
Marrone, F., D. Fontaneto & L. Naselli-Flores, 2023. Cryptic diversity, niche displacement and our poor understanding of taxonomy and ecology of aquatic microorganisms. Hydrobiologia 850: 1221–1236. https://doi.org/10.1007/s10750-022-04904-x.
McAllen, R. & A. Taylor, 2001. The effect of salinity change on the oxygen consumption and swimming activity of the high-shore rockpool copepod Tigriopus brevicornis. Journal of Experimental Marine Biology and Ecology 263: 227–240. https://doi.org/10.1016/S0022-0981(01)00308-2.
Menge, B. A. & J. P. Sutherland, 1987. Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. The American Naturalist 130: 730–757. https://doi.org/10.1086/284741.
Mirón-Gatón, J. M., M. Botella-Cruz, A. J. García-Meseguer, A. Millán & J. Velasco, 2022a. Thermal tolerance differs between co-occurring congeneric beetle species in marine supratidal rockpools. Marine Ecology Progress Series 681: 185–196. https://doi.org/10.3354/meps13916.
Mirón-Gatón, J. M., M. Botella-Cruz, A. J. García-Meseguer, A. Millán & J. Velasco, 2022b. Discordant pattern between realised and fundamental saline niches in two supralittoral Ochthebius species (Coleoptera: Hydraenidae). Ecological Entomology. https://doi.org/10.1111/een.13220.
Montero-Pau, J., E. Ramos-Rodriguez, M. Serra & A. Gomez, 2011. Long-term coexistence of rotifer cryptic species. PLoS ONE 6: e21530. https://doi.org/10.1371/journal.pone.0021530.
Olabarria, C., J. L. Carballo & C. Vega, 2001. Spatio-temporal changes in the trophic structure of rocky intertidal mollusc assemblages on a tropical shore. Ciencias Marinas 27: 235–254. https://doi.org/10.7773/cm.v27i2.461.
Paine, R. T., 1966. Food web complexity and species diversity. The American Naturalist 100: 65–75. https://doi.org/10.1086/282400.
Paine, R. T., 1969. The Pisaster-Tegula interaction: prey patches, predator food preference, and intertidal community structure. Ecology 50: 950–961. https://doi.org/10.2307/1936888.
Pajunen, V. I., 1979a. Competition between rock pool corixids. Annales Zoologici Fennici 16: 138–143.
Pajunen, V. I., 1979b. Quantitative analysis of competition between Arctocorisa carinata (Sahlb.) and Callicorixa producta (Reut.) (Hemiptera, Corixidae). Annales Zoologici Fennici 16: 195–200.
Pajunen, V. I., 1982. Replacement analysis of non-equilibrium competition between rock pool corixids (Hemiptera, Corixidae). Oecologia 52: 153–155. https://doi.org/10.1007/BF00363828.
Parvizi, E., C. I. Fraser & J. M. Waters, 2022. Genetic impacts of physical disturbance processes in coastal marine ecosystems. Journal of Biogeography 49: 1877–1890. https://doi.org/10.1111/jbi.14464.
Perkins, P. D., 1980. Aquatic beetles of the family Hydraenidae in the Western Hemisphere: classification, biogeography and inferred phylogeny (Insecta: Coleoptera). Quaestationes Entomologicae 16: 5–554.
Peterson, A. T., J. Soberón, R. G. Pearson, R. P. Anderson, E. Martínez-Meyer, M. Nakamura & M. B. Araújo, 2011. Ecological Niches and Geographic Distributions (Monographs in Population Biology 49). Princeton University Press, Princeton. https://doi.org/10.1515/9781400840670
Pironon, S., J. Villellas, W. Thuiller, V. M. Eckhart, M. A. Geber, D. A. Moeller & M. B. García, 2018. The ‘Hutchinsonian niche’as an assemblage of demographic niches: implications for species geographic ranges. Ecography 41: 1103–1113. https://doi.org/10.1111/ecog.03414.
R Development Core Team, 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [available on internet at https://www.R-project.org].
Ranta, E., 1979. Niche of Daphnia species in rock pools. Archivfiir Hydrobiologie 87: 205–223.
Ranta, E., 1982. Animal communities in rock pools. Annales Zoologici Fennici 19: 337–347.
Revelle, W., 2022. psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University, Evanston, Illinois. R package version 2.2.5. [available on internet at https://CRAN.R-project.org/package=psych].
Sabatelli, S., P. Audisio, G. Antonini, E. Solano, A. Martinoli & M. Trizzino, 2016. Molecular ecology and phylogenetics of the water beetle genus Ochthebius revealed multiple independent shifts to marine rockpools lifestyle. Zoologica Scripta 45: 175–186. https://doi.org/10.1111/zsc.12141.
Sabatelli, S., P. Ruspantini, P. Cardoli & P. Audisio, 2021. Underestimated diversity: cryptic species and phylogenetic relationships in the subgenus Cobalius (Coleoptera: Hydraenidae) from marine rockpools. Molecular Phylogenetics and Evolution 163: 107243. https://doi.org/10.1016/j.ympev.2021.107243.
Somero, G. N., 2002. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integrative and Comparative Biology 42: 780–789. https://doi.org/10.1093/icb/42.4.780.
Southward, A. J., 1958. Note on the temperature tolerances of some intertidal animals in relation to environmental temperatures and geographical distribution. Journal of the Marine Biological Association of the United Kingdom 37: 49–66. https://doi.org/10.1017/S0025315400014818.
Stickle, W. B., E. Carrington & H. Hayford, 2017. Seasonal changes in the thermal regime and gastropod tolerance to temperature and desiccation stress in the rocky intertidal zone. Journal of Experimental Marine Biology and Ecology 488: 83–91. https://doi.org/10.1016/j.jembe.2016.12.006.
Telesh, I., H. Schubert & S. Skarlato, 2013. Life in the salinity gradient: discovering mechanisms behind a new biodiversity pattern. Estuarine, Coastal and Shelf Science 135: 317–327. https://doi.org/10.1016/j.ecss.2013.10.013.
Tilman, D., 1982. Resource Competition and Community Structure (Monographs in Population Biology 17). Princeton University Press, Princeton. https://doi.org/10.2307/j.ctvx5wb72
Valladares, L. F., J. A. Díaz, J. Garrido, C. E. Sáinz-Cantero & J. A. Delgado, 2018. Coleoptera Hydraenidae. In Ramos, M. A. et al. (eds), Fauna Ibérica, vol. 44. Museo Nacional de Ciencias Naturales. CSIC, Madrid: 520.
Vecchioni, L., M. Arculeo, V. Cottarelli & F. Marrone, 2021. Range-wide phylogeography and taxonomy of the marine rock pools dweller Tigriopus fulvus (Fischer, 1860) (Copepoda, Harpacticoida). Journal of Zoological Systematics and Evolutionary Research 59: 839–857. https://doi.org/10.1111/jzs.12457.
Velasco, J., J. M. Mirón-Gatón, A. J. García-Meseguer & M. Botella-Cruz, 2022. Life cycle differences between two coexisting species of supratidal rockpools: Ochthebius quadricollis Mulsant, 1844 and Ochthebius lejolisii Mulsant & Rey, 1861 (Coleoptera, Hydraenidae). Suplementos Del Boletín De La Asociación Española De Entomología 4: 131–136.
Vepsäläinen, K., 1978. Coexistence of two competing corixid species (Heteroptera) in an archipelago of temporary rock pools. Oecologia 37: 177–182. https://doi.org/10.1007/BF00344989.
Villastrigo, A., C. Hernando & A. Millán, 2022. The Ochthebius (Coleoptera, Hydraenidae) from western Palaearctic supratidal rockpools. Suplementos Del Boletín De La Asociación Española De Entomología 4: 100–108.
Vinagre, C., I. Leal, V. Mendonça & A. A. V. Flores, 2015. Effect of warming rate on the critical thermal maxima of crabs, shrimp and fish. Journal of Thermal Biology 47: 19–25. https://doi.org/10.1016/j.jtherbio.2014.10.012.
Wethey, D. S., 1984. Sun and shade mediate competition in the barnacles Chthamalus and Semibalanus: a field experiment. The Biological Bulletin 167: 176–185. https://doi.org/10.2307/1541346.
Williams, P. J., A. K. Moeller, A. Granados, H. Bernard, R. C. Ong & J. F. Brodie, 2022. Food availability alters community co-occurrence patterns at fine spatiotemporal scales in a tropical masting system. Oecologia 200: 169–181. https://doi.org/10.1007/s00442-022-05252-2.
Wolcott, T. G., 1973. Physiological ecology and intertidal zonation in limpets (Acmaea): a critical look at" limiting factors". The Biological Bulletin 145: 389–422. https://doi.org/10.2307/1540048.
Ysebaert, T. & P. M. Herman, 2002. Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Marine Ecology Progress Series 244: 105–124. https://doi.org/10.3354/meps244105.
Zhang, P., J. Sun, S. Wang, D. He & L. Zhao, 2016. Influences of desiccation, submergence, and salinity change on survival of Ligia cinerascens (Crustacea, Isopoda): high potential implication for inland migration and colonization. Hydrobiologia 772: 277–285. https://doi.org/10.1007/s10750-016-2673-2.
Acknowledgements
We thank Susana Pallarés, Vanesa Céspedes and Adrián Villastrigo for field assistance, Cayetano Gutiérrez for his assistance in advising about the statistical analysis and David Sánchez-Fernández for his useful methodological advice and discussion of the results.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by Excellence Research Project CGL2017-84157P (J.V.) (Spanish Ministry of Science and Innovation) co-funded by FEDER funds. A.J.G.-M. (FPI Program), J.M.M.-G. (FPU program) and M.B.-C. (University of Murcia) are supported by predoctoral grants.
Author information
Authors and Affiliations
Contributions
JV designed and coordinated the study; AJG-M and PA carried out the data analyses and AJG-M drafted the manuscript. All the authors participated in the field collection, discussed the results and the manuscript revision, and gave their final approval for it to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Meseguer, A.J., Abellán, P., Mirón-Gatón, J.M. et al. Fine-scale niche differences allow the co-existence of congeneric aquatic beetles in supratidal rockpools. Hydrobiologia 851, 471–485 (2024). https://doi.org/10.1007/s10750-023-05333-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05333-0