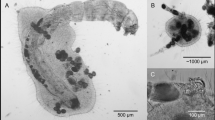
Abstract
We quantified the population growth of the predatory heliozoan Actinosphaerium eichhornii fed separately four rotifer prey (Anuraeopsis fissa, Brachionus calyciflorus, Brachionus havanaensis and Plationus patulus) at three densities (0.5, 1 and 2 ind. ml−1 per day). All the four prey rotifer species were consumed by A. eichhornii. Regardless of the prey species, increasing rotifer prey density resulted in an increased population growth of the heliozoans. Higher cyst production in A. eichhornii occurred at higher prey densities on all prey species. A. fissa offered at the lower densities of 0.5–1.0 ind. ml−1 day−1 did not lead to cyst production by A. eichhornii. The predator did not survive beyond 2 days on Chlorella vulgaris alone. When cultured together with heliozoans, A. fissa, B. calyciflorus and B. havanaensis were eliminated within 4 days. However, the prey rotifer Plationus patulus continued to survive for about 10 days with heliozoans. The highest rate of population increase of heliozoans (0.91 day−1) was recorded when fed B. calyciflorus at a density of 2 ind. ml−1 day−1, while of those of prey varied from 0.30 to 0.41 day−1 when fed Chlorella vulgaris at 1 × 106 cells ml−1 day−1. We have analyzed the impact of heliozoan predation on rotifers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predator–prey interactions in aquatic ecosystems are fundamental in understanding trophic dynamics (Bell et al., 1991). Among the main freshwater zooplankton communities, ciliates, rotifers, cladocerans and copepods are numerically abundant (Ejsmont-Karabin et al., 2019). Within the crustacean communities, there are only few predatory cladocerans while, cyclopoids, especially in adult stages, are predominantly predatory (Dodson & Frey, 2001; Williamson & Reid, 2001). Amongst rotifers many species are herbivores, and many others are omnivores or carnivores (Koste, 1978). Many members of Notommatidae and Asplanchnidae are predatory (Arndt, 1993a). Among protists, heliozoans are primarily freshwater, and are often predatory (Arndt, 1993b). Although heliozoans are mainly found on or near the benthos, they are also frequently planktonic, especially in shallow lakes (Mikrjukov & Milyutina, 2001).
Heliozoans feed on a great variety of food items ranging from picoplankton to mesozooplankton (Bell et al., 2006). However, in shallow waterbodies, heliozoans often capture planktonic rotifers, and other smaller invertebrates such as gastrotrichs (Weithoff & Bell, 2022). Heliozoans do not actively pursue their prey items. They possess extrusomes of various types, which facilitate prey adhesion and capture (Mikrjukov & Patterson, 2001). Therefore, large, and motile prey are most vulnerable and when they come in contact with axopodia, they are entangled and engulfed (Fenchel, 1982).
Actinosphaerium is a freshwater heliozoan genus which is capable of engulfing large prey items, such as rotifers, cladocerans, copepods and gastrotrichs (Mikrjukov & Milyutina, 2001) and complete ingestion of a prey item may require about a few hours. Actinosphaerium eichhornii (Ehrenberg, 1840) forms syncytium to capture larger prey, such as insect larvae (Kumler et al., 2020), which rarely escape (Taylor & Sanders, 2001).
Prey defence strategies against invertebrate predators include morphological adaptations such as elongation of body size or spine length or the presence of minute spicules (Garza-Mouriño et al., 2005). Behavioural strategies include fast swimming rates as in the case of Hexarthra and Polyarthra (Kak & Rao, 1998; Diéguez & Gilbert, 2002) and vertical migration (Gilbert, 2014). Energetic strategies include reduced body size so as to yield least energy when captured by a predator (Sarma & Nandini, 2007). Physiological features include presence of toxins as in the case of warts of Sinantherina (Wallace et al., 2023). High population growth rates of a rapidly reproducing prey might offset the loss incurred from predatory rotifers (Gilbert, 2017).
As in the case of other invertebrate predators (Nandini & Sarma, 1999), heliozoans show both numerical responses, whereby an increase in prey availability leads to increased food consumption and thus increased predator abundance (Kumler et al., 2020). Species of Actinosphaerium reproduce by binary fission which gives them greater advantage of rapid population build-up in a shorter duration (Mikrjukov & Patterson, 2001). For example, when fed on a suitable prey, an individual of Actinosphaerium eichhornii is capable of reaching great abundances within a few hours (Mikrjukov & Milyutina, 2001). Since many species of rotifers do not have such high population growth rates, in the presence of A. eichhornii, these zooplankton prey may experience population extinction (Serra et al., 2018). However, heliozoans show density-dependent effects, where increased population abundance and diminishing food resources, actinophryid members produce cysts through autogamy which results in a population crash (Mikrjukov & Patterson, 2001). During the encystment, individuals of an Actinosphaerium species withdraw their exopodia, adhere to the substratum, called primary cyst and at this stage it cannot capture any prey. From this primary cyst varying numbers of secondary cysts are formed which when fuse produce zygotes (Taylor & Sanders, 2001).
Most of the previous works considered prey selection, prey handling time and population growth of heliozoans (Grebecki & Hausmann, 1993; Weithoff & Bell, 2022). However, studies have rarely simultaneously quantified the unconsumed prey, production of cysts, and population growth of both prey and the predator. We hypothesize that predatory heliozoans, due to their higher population growth rates, rapidly diminish the prey rotifer populations in mixed cultures and with increase in predator abundance, cyst production sets in. In addition, on the abundance of the predator will also depend on prey size and density.
We studied the population growth of Actinosphaerium eichhornii fed different prey species offered at different densities. We also aimed at quantifying the cyst numbers in relation to the population density of heliozoans. Further, we also aimed at quantifying the prey abundances when cultured in the presence of A. eichhornii.
Material and methods
Culture of prey rotifers
We cultured four prey rotifer species (Anuraeopsis fissa Gosse, 1851, Brachionus calyciflorus Pallas, 1766, Brachionus havanaensis Rousselet, 1911 and Plationus patulus (Müller, 1786)), all of which were isolated from a shallow waterbody (Lake Xochimilco, Mexico City). Starting from a single parthenogenetic individual, each rotifer species was separately cultured in moderately hardwater (EPA medium) (Weber, 1993). All the rotifer species are herbivorous and hence were fed on the green alga Chlorella vulgaris. The algae were mass-cultured using standard Bold basal medium (Borowitzka & Borowitzka, 1988). Log phase algae were concentrated by centrifugation and resuspended in a small volume of distilled water. Freshly harvested alga at a density of 1 × 106 cells ml−1 was added to each rotifer culture vessel. To keep the rotifer cultures healthy, the EPA medium was replaced daily, and fresh alga was added. The pH of the rotifer culture tanks was 7.2–7.4). All rotifer cultures were maintained at 24 ± 1 °C and under continuous fluorescent illumination.
Culture of predatory heliozoans
Several heliozoan individuals were collected using a plankton net of 50 µm from the same waterbody from which prey rotifers were originally collected. Using a finely drawn Pasteur pipette under a stereo microscope, one single healthy individual of Actinosphaerium eichhornii (Ehrenberg, 1840) was isolated from the plankton collection and placed in a small Petri dish (5 cm diameter × 1.5 cm depth) containing 10 ml of EPA medium. To this we added a few individuals (2–4 ind. ml−1) of mixed rotifer species as prey. To keep the rotifers in the heliozoan cultures active, a very low density of Chlorella vulgaris (0.01 × 106 cells ml−1), which was below the threshold for the growth of the brachionids (Nandini et al., 2007), was added. Daily, the medium of the heliozoan cultures was replaced. Uneaten rotifers and empty loricae were removed and fresh prey items were added. As the predator density increased, higher prey numbers were added. To prevent cyst formation and subsequent crash of predators, were periodically removed some individuals of A. eichhornii and maintained population at low density (1 ind. ml−1). Other culture conditions were similar to those of prey rotifers mentioned earlier.
Test design
Population growth of A. eichhornii offered fixed prey densities
Population growth experiments of the heliozoan were separately but simultaneously conducted using four prey species (A. fissa, B. calyciflorus, B. havanaensis and P. patulus). For each prey we used three densities (0.5, 1.0 and 2.0 ind. ml−1). The initial density of A. eichhornii was 0.1 ind. ml−1. In all we used 48 (four prey species × three densities × four replicates) Petri dishes containing 10 ml EPA medium. Thus, into each Petri dish we introduced one (pre-starved for 2 h) predator individual and one of the four rotifer prey species at the chosen density. To keep the rotifer prey active, a few drops of Chlorella were added to the test containers. Following the initiation of the predator’s population growth, daily we quantified number of individuals of A. eichhornii in each test container and uneaten prey and cysts, if any, were counted and removed. Following this, the test medium was completely replaced with fresh EPA medium containing corresponding prey species and density. The experiments were discontinued after 15 days, by which time the heliozoan populations began to decline in each replicate.
Population growth of rotifers and heliozoans in mixed cultures
To test the rotifer population growth in the presence of the heliozoans, we offered the predator each of the four rotifer species at a single prey density of 1 ind. ml−1. To ensure the population growth of prey in the presence (treatments) or absence (controls) of heliozoans, the herbivorous rotifers were offered Chlorella vulgaris at a density of 1.0 × 106 cells ml−1. We also used controls for predators (with only alga at 1.0 × 106 cells ml−1 but no prey). For each control and treatment, we used four replicates. Thus, in all we used 36 Petri dishes (four controls for rotifers × four replicates (= 16) + 4 treatments (with predators) × four replicates (= 16) + 4 controls for heliozoans at one algal density) containing 10 ml medium with Chlorella vulgaris at density of 1.0 × 106 cells ml−1. Into each Petri dish containing 10 ml EPA medium with alga at 1 × 106 cells ml−1), we introduced one individual A. eichhornii and/or 10 individuals of one of the four rotifer species.
Following initiation of the population growth experiments of prey rotifers and the predatory heliozoan, daily we counted the number of individuals from each replicate of controls and treatments. Cysts when found were also counted but removed. Later all individuals (prey and/or predator) from each replicate were transferred to fresh Petri dishes containing EPA medium and alga but no further prey individuals were added. For controls of rotifers, since the densities in each replicate were high, aliquots were used for quantification. The experiments were discontinued after 15 days by which time, population abundances of prey or predators were declined or reached an asymptotic phase.
Depending on the experiment, the following variables were obtained: Peak population density of rotifers and heliozoans, rate of population increase (r per day) for prey and predators, mean cyst production and total prey consumed by A. eichhornii. The population growth rate was derived using the exponential growth equation, r = (ln Pf-ln Pi)/t, where Pf: final population density, Pi initial population density, t is time in days (Krebs, 1985).
Data were statistically treated using one way ANOVA and for multiple comparison, post hoc (Tukey) tests were used following SigmaPlot ver. 11.
Results
Population growth of A. eichhornii offered fixed prey densities
The rotifer prey used in this study differed in body size (adult length, spines excluded, mean ± SE, n = 15, in µm): A. fissa 70 ± 5; B. calyciflorus: 160 ± 10; B. havanaensis 110 ± 5 and P. patulus 120 ± 5. The body size of the predator, A. eichhornii, varied from 50 to 250 µm, depending on the presence of prey items in the body.
All the four prey rotifer species were consumed by A. eichhornii. Regardless of prey species, increasing rotifer prey density resulted in an increased population growth of heliozoans. Data on the population abundances and cyst production by A. eichhornii fed four rotifer prey species at three densities are shown in Fig. 1. Higher abundances of heliozoans were obtained when fed B. calyciflorus but lowest on A. fissa. For all the tested prey species, regardless of the offered density, heliozoan showed exponential population growth almost immediately after 3–5 days. A. eichhornii fed A. fissa at the lowest density (0.5 ind. ml−1) did not survive beyond one week in all replicates. Higher cyst production in A. eichhornii occurred when fed higher prey densities of all the test prey species. A. fissa offered at densities of 0.5–1.0 ind. ml−1 day−1 did not lead to cyst production by A. eichhornii.
Population growth and cyst production (numbers 10 ml−1) in the heliozoan Actinosphaerium eichhornii fed daily on four rotifer species (Anuraeopsis fissa, Brachionus calyciflorus, Brachionus havanaensis and Plationus patulus) at three prey densities (5, 10 and 20 ind. 10 ml−1 day−1). Shown are mean ± SE based on four replicates
The peak population densities of A. eichhornii were significantly influenced by the prey density (p < 0.05, one-way ANOVA). However, the peak abundances of the predator fed at lower densities (0.5 and 1 ind. ml−1) were not significant for smaller rotifers (A. fissa and B. havanaensis). Regardless of prey species and density, the peak abundances of heliozoans varied from 0.1 to 7.1 ind. ml−1 (Fig. 2).
Peak population abundances (ind. 10 ml−1) of A. eichhornii fed on four rotifer species (Anuraeopsis fissa, Brachionus calyciflorus, Brachionus havanaensis and Plationus patulus) at three prey densities (5, 10 and 20 ind. 10 ml−1 day−1). Shown are mean ± SE based on four replicates. For a given prey species, data bars carrying similar alphabets are not significant (p > 0.05, Tukey test)
The number of uneaten (left) prey individuals during the first 3 days were about 30% but as the predator density increased, there were few prey individuals left after 24 h of feeding. During the experimental period, more than 80% of the prey individuals consumed were A. fissa, B. calyciflorus and B. havanaensis but only 46% of P. patulus was consumed (Fig. 3).
Since the number of cysts produced was quantified (and removed) daily, it was possible to obtain the mean number of cysts produced for each prey species during the entire experimental period (Fig. 4). The mean number of cysts produced was lowest (0.002 per ml) when fed A. fissa at a density of 0.5 ind. ml−1 while it was more than 36 ml−1 when fed B. calyciflorus at the highest prey density (2 ind. ml−1 day−1). Statistically, the cyst number was significantly (p < 0.05) affected by the prey density for all the tested rotifer species.
Mean cysts (numbers 10 ml−1) produced by the heliozoans in relation to the total prey numbers (Anuraeopsis fissa, Brachionus calyciflorus, Brachionus havanaensis and Plationus patulus) during the test period. Shown are mean ± SE based on four replicates. For a given prey species, data bars carrying similar alphabet are not significant (p > 0.05, Tukey test)
Population growth of rotifers and heliozoans in mixed cultures
The predator A. eichhornii did not survive beyond two days when Chlorella vulgaris only was added to the test jars. Therefore, these data are not presented in the results. However, each rotifer species in controls, when offered Chlorella vulgaris as food at a density of 1 × 106 cells ml−1, showed exponential growth. The smaller rotifer species, A. fissa reached higher abundances as compared to the larger species (B. calyciflorus). When cultured together with heliozoans, A. fissa, B. calyciflorus and B. havanaensis were eliminated within 4 days. However, Plationus patulus continued to survive for about 10 days in the presence of heliozoans. In mixed culture treatments, when the prey was eliminated, heliozoans also died within 2–3 days (Fig. 5). Under mixed culture conditions only a few cysts were produced by the heliozoans.
Population growth (ind. 10 ml−1) four rotifer species (closed symbol) (Anuraeopsis fissa, Brachionus calyciflorus, Brachionus havanaensis and Plationus patulus) cultured alone (controls) (left column) and in mixed cultures (right column, ind. 10 ml−1) with the predator Actinosphaerium eichhornii (open symbol). Shown are mean ± SE based on four replicates
The rate of population increase (r) of rotifers cultured alone and in the absence of predators, had r values in the range of 0.30 to 0.41 day−1. The r of A. eichhornii fed four prey species at different densities varied from nearly 0 to 0.91 day−1. The highest growth rates were obtained when fed B. calyciflorus at a density of 2 ind. ml−1 day−1. Statistically, the r was significantly (p < 0.05) influenced by the prey concentration (Fig. 6).
Rate of population increase per day of Actinosphaerium eichhornii fed four rotifer species (Anuraeopsis fissa, Brachionus calyciflorus, Brachionus havanaensis and Plationus patulus) at three prey densities (5, 10 and 20 ind. 10 ml−1 day−1). Shown are mean ± SE based on four replicates. For a given prey species, data bars carrying similar alphabet are not significant (p > 0.05, Tukey test)
Discussion
Most heliozoans of the genus Actinosphaerium are predatory often capturing other invertebrate prey such as gastrotrichs, rotifers, cladocerans and insect larvae (Gaponova et al., 2021). In this study, A. eichhornii was able to feed on rotifers, resulting in an increase in its population density. Lake Xochimilco is a system of shallow canals (depth < 2 m) and therefore many planktonic and benthic rotifers are caught while sampling with a plankton net (Sarma & Nandini, 2019). A. eichhornii, though a benthic species, also occurs in planktonic collections. Thus rotifers, both pelagic and littoral, are vulnerable to predation risk by actinosporid heliozoans.
Quantitative data on the population growth of heliozoans show that prey body size and density are important factors in determining the magnitude of abundances of the predators (Suzaki et al., 1980). This is also the case here with A. eichhornii. Of the four rotifer prey species, A. fissa is the smallest and B. calyciflorus is the largest. Thus, heliozoan abundances were higher on the larger on B. calyciflorus compared to those on Anuraeopsis. For blind and non-motile predators, prey encounter is dependent on the body size. Considering that most brachionid species have a swimming speed of about 1 mm per second (Snell et al. 1987), larger prey B. calyciflorus was more frequently captured than the smaller A. fissa. In addition, larger prey yields higher energy to the predator which is reflected in its population abundances (Moya-Laraño, 2011). This is evident in this work where B. calyciflorus-fed predators reached abundances more than thrice those fed on A. fissa at comparable prey densities. Also, the population growth rate of the predator was similar on the small sized B. havanaensis as on the less consumed P. patulus. These findings reinforce the hypothesis that small body size also offers defence against predators (Sarma & Nandini, 2007).
It is well-known that for any given prey size, the predator’s abundances are a function of available prey numbers (Krebs, 1985). This is also the case for A. eichhornii when fed rotifers at different densities, where higher prey densities resulted in higher predator numbers. Smaller prey in low densities offer protection against non-visual and non-motile predators (Cuthbert et al., 2021). Thus, both A. fissa and B. havanaensis at two densities (0.5 and 1.0 ind. ml−1) did not significantly result in higher predator abundances. Plationus patulus is typically tychoplanktic and is associated with macrophytes by way of attaching itself by thin transparent sticky threads secreted by the foot-glands (Koste, 1978). This offers some protection as they are less likely encountered by a heliozoan. Our microscopic observations of the test containers showed that many individuals of P. patulus occurred together with foot-gland secretions to which some heliozoans were caught. Such predator individuals were less successful to capture the prey items. Thus, the foot-gland secretions by P. patulus are probably not only useful to lessen the possibility of its encounters with heliozoans but are also helpful to trap or reduce the predator’s exopodia movements. We also observed that when the population abundances of the predators were higher than 2 ind. ml−1, nearly 30% of the individuals were detached from the Petri dish and reached the surface. While most cysts were attached to the bottom of the test containers, some cysts were also at the surface of the medium. The length width ratio is also important in determining prey preference. Here we observed that P. patulus is as wide as long and less preferred.
Cyst production in heliozoans is a function of population abundance and diminishing prey availability, which act as a cue to undergo encystment (Taylor & Sanders, 2001). This is possibly similar to rotifer resting egg production when the population densities are high and under limiting resources (Pourriot & Snell, 1983). In this work, the highest cyst production in A. eichhornii occurred when fed B. calyciflorus at 2 ind. ml−1 day−1. The lack of cyst production in lower prey densities of smaller prey probably indicates the unavailability of adequate energy for this process (Nelson & Garoian, 1982).
When both, the predators and prey, were cultured together, the prey rotifer did not survive beyond a few days, even though they had received adequate levels of alga (Nandini et al., 2007). This unstable predator–prey cycle happens due to many factors such as when (a): prey lacks adequate induced defences such as protective spines or is toxic (Wallace et al., 2023), (b) prey population growth rates are lower than those of the predators (Sarma et al., 2010) and (c) inducing density dependent population regulation in predators, such as cyst production leading to a crash in the predator density (Pourriot & Snell, 1983). Here, unlike other rotifer predators such as Asplanchna which result in spine elongation in the prey (Gilbert, 2022), we did not observe any remarkable changes in the morphology of prey species when cultured together with heliozoan predators. Though the foot-gland secretions in P. patulus reduced the intensity of prey capture by A. eichhornii, eventually the prey was eliminated. Population growth rates of A. eichhornii (ca. 0.91 day−1) were much higher than those of the prey rotifers (< 0.41 day−1) and this might be one of the reasons for the unstable state of predator–prey cycle (Mondal et al., 2021).
In predatory rotifers such as Asplanchna, higher population abundances induce sexual reproduction and thus resting egg production which leads to declining growth rates. In heliozoans, cyst production was observed when the densities of predators were close to 2 ind. ml−1. Cyst production continued even as the population continued to remain stable or increased further. Thus, it appears that A. eichhornii is able to maintain a high population density despite cyst production as long as sufficient energy resources are available.
Conclusions
Our study showed that Actinosphaerium eichhornii was a strong predator on rotifers. A. eichhornii showed typical numerical responses where increase in the prey availability resulted in increased predator abundance. Mixed cultures of heliozoans and rotifer showed that this predator–prey cycle was unstable even when the prey was offered adequate levels of algal food. Increased cyst production and population abundances of heliozoans continued if energy resources were not limiting. Our study did not consider prey selection by the heliozoans, although it is known that these predators show certain patterns of prey selection.
Data availability
Enquiries about data availability should be directed to the authors. Data will be made available on reasonable request.
References
Arndt, H., 1993a. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)—a review. Hydrobiologia 255: 231–246.
Arndt, H., 1993b. A critical review of the importance of rhizopods (naked and testate amoebae) and actinopods (heliozoa) in lake plankton. Marine Microbial Food Webs 7: 3–29.
Bell, S. S., E. D. McCoy & H. R. Mushinsky (eds), 1991. Habitat structure: population and community biology series,. Vol. 8. Springer, Dordrecht.
Bell, E. M., G. Weithoff & U. Gaedke, 2006. Temporal dynamics and growth of Actinophrys sol (Sarcodina: Heliozoa), the top predator in an extremely acidic lake. Freshwater Biology 51(6): 1149–1161.
Borowitzka, M. A. & L. J. Borowitzka, 1988. Micro-algal biotechnology, Cambridge University Press, London:
Cuthbert, R. N., T. Dalu, R. J. Wasserman, A. Sentis, O. L. F. Weyl, P. W. Froneman, A. Callaghan & J. T. A. Dick, 2021. Prey and predator density-dependent interactions under different water volumes. Ecology and Evolution 11(11): 6504–6512.
Diéguez, M. D. C. & J. J. Gilbert, 2002. Suppression of the rotifer Polyarthra remata by the omnivorous copepod Tropocyclops extensus: Predation or competition. Journal of Plankton Research 24(4): 359–369.
Dodson, S. I. & D. G. Frey, 2001. Chapter 21: Cladocera and other branchiopoda. In Thorp, J. H. & A. P. Covich (eds), Ecology and classification of North American freshwater invertebrates 2nd ed. Academic Press, London: 849–913.
Ejsmont-Karabin, J., K. Kalinowska & M. Karpowicz, 2019. Structure of ciliate, rotifer, and crustacean communities in lake systems of Northeastern Poland, Complexity in polish phonotactics: on features, weights, rankings and preferences (prosody, phonology and phonetics) Springer: 77–101.
Fenchel, T., 1982. Ecology of heterotrophic microflagellates–IV: Quantitative occurrence and importance as consumers of bacteria. Marine Ecology Progress Series 9: 35–42.
Gaponova, L., T. Suzaki, M. S. Islam & A. Kolosiuk, 2021. Interaction between centrohelid and actinophryid heliozoans: field and laboratory studies. Journal of Protistology 53: e004. https://doi.org/10.18980/jop.e004.
Garza-Mouriño, G., M. Silva-Briano, S. Nandini, S. S. S. Sarma & M. E. Castellanos-Páez, 2005. Morphological and morphometrical variations of selected rotifer species in response to predation: a seasonal study of selected brachionid species from Lake Xochimilco (Mexico). Hydrobiologia 546: 169–179.
Gilbert, J. J., 2014. Morphological and behavioral responses of a rotifer to the predator Asplanchna. Journal of Plankton Research 36: 1576–1584.
Gilbert, J. J., 2017. Non-genetic polymorphisms in rotifers: environmental and endogenous controls, development, and features for predictable or unpredictable environments. Biological Reviews 92: 964–992.
Gilbert, J. J., 2022. Food niches of planktonic rotifers: Diversification and implications. Limnology and Oceanography 67(10): 2218–2251.
Grebecki, A. & K. Hausmann, 1993. Motor behaviour of prey during first steps of food capture by Actinophrys sol. Acta Protozoologica 32: 157–164.
Kak, A. & T. R. Rao, 1998. Does the evasive behavior of Hexarthra influence its competition with cladocerans? Hydrobiologia 387: 409–419. https://doi.org/10.1023/A:1017055013639.
Koste, W., 1978. Rotatoria. Die Radertiere Mittel-europas, 2nd ed. Gebruder Borntraeger, Berlin and Stuttgart V. 1, text, 673 pp.; V. 2, plates, 476 pp.
Krebs, C. J., 1985. Ecology: the experimental analysis of distribution and abundance, Harper & Row, New York:
Kumler, W. E., J. Jorge, P. M. Kim, N. Iftekhar & M. A. R. Koehl, 2020. Does formation of multicellular colonies by choanoflagellates affect their susceptibility to capture by passive protozoan predators? Journal of Eukaryotic Microbiology 67(5): 555–565.
Mikrjukov, K. A. & I. Milyutina, 2001. Heliozoa as a component of marine microbenthos: a study of heliozoa of the White Sea. Ophelia 54: 51–73.
Mikrjukov, K. A. & D. J. Patterson, 2001. Taxonomy and phylogeny of Heliozoa–III: Actinophryids. Acta Protozoologica 40: 3–25.
Mondal, S., G. P. Samanta & J. J. Nieto, 2021. Dynamics of a predator-prey population in the presence of resource subsidy under the influence of nonlinear prey refuge and fear effect. Complexity 2021: 9963031.
Moya-Laraño, J., 2011. Genetic variation, predator-prey interactions and food web structure. Philosophical Transactions of the Royal Society B Biological Sciences 366(1569): 1425–1437. https://doi.org/10.1098/rstb.2010.0241.
Nandini, S. & S. S. S. Sarma, 1999. Effect of starvation time on the prey capture behaviour, functional response and population growth of Asplanchna sieboldi (Rotifera). Freshwater Biology 42: 121–130.
Nandini, S., S. S. S. Sarma, R. J. Amador-López & S. Bolaños-Muñoz, 2007. Population growth and body size in five rotifer species in response to variable food concentration. Journal of Freshwater Ecology 22: 1–10.
Nelson, L. G. & G. S. Garoian, 1982. The encysted stage of the heliozoan Echinosphaerium nucleofilum. Transactions of the American Microscopical Society 101(1): 59–65.
Pourriot, R. & T. W. Snell, 1983. Resting eggs in rotifers. Hydrobiologia 104: 213–224. https://doi.org/10.1007/BF00045970.
Sarma, S. S. S. & S. Nandini, 2007. Small prey size offers immunity to predation: a case study on two species of Asplanchna and three brachionid prey (Rotifera). Hydrobiologia 593: 67–76.
Sarma, S. S. S. & S. Nandini, 2019. Comparative population dynamics of six brachionid rotifers (Rotifera) fed seston from a hypertrophic, high altitude shallow waterbody from Mexico. Hydrobiologia 844(1): 55–65.
Sarma, S. S. S., M. H. Sagrario & S. Nandini, 2010. Allelopathic interactions between the predator (Asplanchna brightwellii) and prey (Brachionus calyciflorus) for coexistence. Allelopathy Journal 26(1): 131–138.
Serra, M., T. W. Snell & R. L. Wallace, 2018. Reproduction, overview by phylogeny: rotifera. In Skinner, M. K. (ed), Encyclopedia of reproduction, Vol. 6. Elsevier, Academic Press: 513–521.
Snell, T. W., M. J. Childress & E. M. Boyer, 1987. Assessing the status of rotifer cultures. Journal of the World Aquaculture Society 18: 270–277.
Suzaki, T., Y. Shigenaka, S. Watanabe & A. Toyohara, 1980. Food capture and ingestion in the large heliozoan, Echinosphaerium nucleofilum. Journal of Cell Science 42: 61–79. https://doi.org/10.1242/jcs.42.1.61.
Taylor, W. T. & R. W. Sanders, 2001. Protozoa. In Thorp, J. H. & A. P. Covich (eds), Ecology and classification of North American freshwater invertebrates 2nd ed. Academic Press, London: 43–96.
Wallace, R. L., K. M. Dash, T. Q. Araújo, E. J. Walsh, S. Das & R. Hochberg, 2023. Ultrastructural characterization of the putative defensive glands (warts) in the sessile, colonial rotifer Sinantherina socialis (Gnesiotrocha; Flosculariidae). Zoologischer Anzeiger 304: 10–20.
Weber, C.I., 1993. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 4th ed. United States Environmental Protection Agency, Cincinnati, OH, EPA/600/4–90/027F.
Weithoff, G. & E. M. Bell, 2022. Complex Trophic interactions in an acidophilic microbial community. Microorganisms 10: 1340.
Williamson, C. E. & J. W. Reid, 2001. Chapter 22: Copepoda. In Thorp, J. H. & A. P. Covich (eds), Ecology and classification of North American freshwater invertebrates 2nd ed. Academic Press, London: 915–954.
Acknowledgements
We thank Vasily Zlatogursky for identification of the heliozoans
Funding
This work was supported by a project PAPIIT UNAM IN208223.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no competing interests.
Ethical approval
Authors declare that the work presented here fully obeys the ethical guidelines established by our university.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Maria Špoljar, Diego Fontaneto, Elizabeth J. Walsh & Natalia Kuczyńska-Kippen / Diverse Rotifers in Diverse Ecosystems
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sarma, S.S.S., Nandini, S. Rotifer-heliozoan interactions: a population growth study. Hydrobiologia 851, 3125–3135 (2024). https://doi.org/10.1007/s10750-023-05315-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05315-2