Abstract
Heavily modified water bodies (HMWB) have been seriously affected by human activities and natural processes promoting their imbalance, and impacting their functioning and biodiversity. This study explores a new approach of monitoring and assessing water quality in Mediterranean reservoirs using phytoplankton communities across a disturbance gradient, according to water framework directive. Phytoplankton and environmental data were sampled in 34 reservoirs over 8 years. Two types of reservoirs were analyzed: Type1 “run-of-river reservoirs” (located in the main rivers, with a low residence time); and Type2 “true reservoirs” (located in tributaries, with high residence time). The transition from deeper and colder reservoirs (reference sites) to shallow and warmer (impaired sites) was clear in Type2, correlated to organic pollution and mineral gradients. Impaired sites from both types showed a higher richness of tolerant taxa. Principal response curve (PRC) provided a concise summary of phytoplankton temporal dynamics and assessed ecosystem health for Mediterranean HMWBs. PRC will provide a powerful tool for environmental quality assessment and be incorporated into monitoring and assessment programs. This approach can help policymakers to manage natural capital to achieve multiple objectives, mainly increasing ecosystem services, and improve readability and interpretation of spatial patterns in temporal changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystems provide many benefits and services to human communities but are under severe pressure, reinforcing the need for monitoring (Gunkel et al., 2015). Therefore, there is a growing need for more effective integrated assessment and ecosystem-based management. Assessing the well-being of water resources and implementing mitigation measures has become a priority, reinforced by Water Framework Directive (WFD, Directive 2000/60/EC), which states the need for EU member states to maintain all water bodies free from pollution long after 2027. However, the progress of improving freshwater quality and reducing eutrophication is slow and remains behind targets. Indeed, a recent report (European Environment Agency, 2018) revealed that 60% of European water bodies fail to achieve good ecological and chemical status. Only by achieving its effective management, we will be able to address the new Green Deal EU strategy designed to combine existing policies (e.g., WFD, Nitrates Directive, and Biodiversity) with broader objectives related to climate change impacts, adaptation, and sustainable development goals in an integrated holistic framework (CNEGP, 2017; Bieroza et al., 2021). WFD is the first legislation establishing the innovative concept of the ecological status of surface waters based on Biological Quality Elements in addition to physical and chemical conditions (Boeuf & Fritsch, 2016). This is based on a reference system with the highest ecological status, including water bodies whose ecology, chemistry, hydrology, and morphology are in pristine conditions, with almost no impact by anthropogenic activities (reference approach). Setting a reference state is still a challenge for most water bodies. Heavily Modified Water Bodies (HMWB), such as reservoirs, must meet ‘good ecological potential’ (GEP) while maintaining their functions and ecosystem service delivery (i.e., hydroelectric powers, navigation, recreation, water storage/regulation, and flood protection) (MEA, 2005). Similar to Good Ecological Status, this objective may differ slightly from the best possible condition, Maximum Ecological Potential (MEP). The MEP represents the maximum ecological quality that could be achieved for these systems once all mitigation measures that do not have significant adverse effects on its specified use or in the broader environment have been applied (mitigation measures approach) (GIG, 2007; EU, 2019). Therefore, to measure the distance to the target, the relative deviation from the biological reference condition needs to be considered, mainly in terms of altered species composition and abundance or biomass of key biota (Denys et al., 2014; Dehini & Gomes, 2022).
In aquatic ecosystems, phytoplankton communities are key elements that regulate biogeochemical cycles (Litchman et al., 2015; Padedda et al., 2017; Liu et al., 2021). This community is one of the most important primary producers (Domis et al., 2013) and a crucial biological element considered within the WFD. Because of their particular sensitivity to water quality and anthropogenic pressures, phytoplankton communities are broadly used as trophic and ecological indicators to assess water quality in HMWB (Fetahi, et al., 2014; Lyche Solheim et al., 2014; Zhang et al., 2020). Consumers like zooplankton, fish, and invertebrate communities, are not included in the quality classification of this water bodies typology, accordingly to WDF (INAG, 2009; Almeida et al., 2020.
The implementation of the WFD has prompted the search for novel methodologies and biological indicators (Statzner et al., 2001; Simboura et al., 2005; Ekdahl et al., 2007) for the assessment of environmental quality because traditional approaches cannot entirely capture the natural variability of ecosystems. The development of indicator systems based on species-environment relationships has become a widely used approach for these tasks (Statzner et al., 2001; Dziock et al., 2006).
There is a growing need to describe the complexity of ecosystem dynamics, namely how communities change over space and time. However, spite the more holistic approach, many available tools provide ambiguous ordination diagrams, making unclear the detection of ecological impacts and the presentation of those impacts in an easily communicable way to policymakers, managers, and stakeholders who are usually not biologists (Pardal et al., 2004). The summary ordination plots and diagrams from traditional ordination methods (e.g., redundancy analysis: RDA; principal component analysis: PCA; or multi-dimensional scaling: MDS) can remain uninterpertable to non-specialists and consequently are difficult to interpret (Warwick & Clarke, 1991). This is especially true when a time factor is present within the data because temporal trajectories are often non-linear in such plots. One approach to deal with time dependency's complexity and overcome such difficulties is applying a novel application of principal response curves (PRCs) (Van den Brink & Ter Braak, 1999).
PRC analysis was initially conceived for aquatic ecotoxicology, assessing the effect of toxicants on freshwater communities (Van den Brink & ter Braak, 1999; Brock et al., 2009), but potentially it has much broader applications (Pardal et al., 2004; Auber et al., 2017; Vendrig et al., 2017). It has been widely applied in aquatic and terrestrial ecology and ecotoxicology (e.g., Verdonschot et al., 2015; Sittenthaler et al., 2019), microbiology (e.g., Fuentes et al., 2014; Paliy & ShanKar, 2016) and soil science (e.g., Cardoso et al. 2008; Van Paassen et al., 2020). In these studies, the tested factor was the toxicant, and time was the dimension along which repeated treatments were performed, and patterns of change were identified (Auber et al., 2017). Therefore, PRC has advantages over traditional ordination techniques enabling a formal and robust statistical analysis of temporal (long-term) data series from spatial gradients, providing community-level insights into anthropogenic disturbances effects. Additionally, PRC provide a quantitative assessment of individual species’ responses to stress agents. In this work, we selected reference sites (or less disturbed) as control. Other areas (treated or impacted areas) were compared to these reference sites, allowing changes in the environmental quality to be assessed over time. In general, Portuguese reservoirs have undergone significant eutrophication due to organic enrichment (Vasconcelos, 1991, 2001; Boavida & Gliwicz, 1996; INAG, 2006; Domingues & Galvão, 2007). This work assessed the phytoplankton composition across a disturbance gradient over 34 Portuguese hydropower reservoirs for eight years. We aimed to explore an efficient and user-friendly approach to monitor and assess water quality in two types of reservoirs using phytoplankton communities, a biological quality element used to classify the ecological potential (EP) according to the WFD.
We hypothesized that differences in phytoplankton communities would be expected (1) in different types of reservoirs due to species spatial–temporal variations caused by the hydric resource use regime, (2) across the disturbance gradient in each typology: Type1 (run of river), lowland reservoirs located in the main rivers (Douro and Tagus), with a very low residence time; Type2 (true reservoirs), deeper high-altitude reservoirs, primarily located in tributaries, with a high residence time. It is expected that PRC analysis will provide a concise summary of phytoplankton communities' temporal dynamics and be a comprehensive tool to assess ecosystem health for Mediterranean HMWB.
Materials and methods
Study area
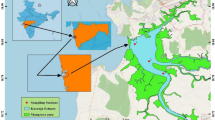
This study was carried out using data from 34 reservoirs in six catchments located in central and northern Portugal. The catchments were: Ave (1 reservoir), Cávado (6 reservoirs), Mondego (5 reservoirs), the Portuguese part of the international watersheds of Lima (2 reservoirs), Douro (11 reservoirs), and Tagus (9 reservoirs). The primary purpose of all these reservoirs is to provide hydroelectric power, although some secondary uses are also common, such as navigation, irrigation, water supply, and recreation. This extensive geographic area represents a wide range of physical and chemical characteristics, soil use, and anthropogenic pressure, including good and poor water quality conditions. Previous works have shown that phytoplankton community composition differed markedly between high altitude and lowland reservoirs (Cabecinha et al., 2009a). Based on the results of this work, we defined two types of reservoirs, comprising Type1 (n = 10)—“Run-of-river” reservoirs located in the main rivers (Douro and Tagus), with a very short residence time (1–3 days); Type2 (n = 24)—“True reservoirs” deeper high altitude reservoirs, located mainly in tributaries, with long residence time (65–300 days) (Fig. 1). “Run-of-river reservoirs” were generally situated at lower altitudes, had larger catchments, lower residence time, and were higher in water mineral content (hardness and conductivity) than higher altitude reservoirs (Tables 1, 2). In general, Type1 was more nutrient-rich, with watersheds dominated by industries and agriculture that occupied about 50% of the total area (> 15% of intensive agriculture). Type2 was characterized by watersheds with extensive natural areas (> 80%) and smaller agriculture areas (nearly 16%, but only 3% of intensive agriculture) (Cabecinha et al., 2009a, b, c, d).
Location of the 34 reservoirs studied and their distribution through six catchments: Ave, Cávado, Mondego, and the Portuguese part of the international watersheds of Lima, Douro, and the Tagus.  and
and  represent reservoirs of Type1 (Run-of-river reservoirs) and Type2 (True reservoirs); Red and Blue symbols represent impaired and reference sites on each type, respectively
represent reservoirs of Type1 (Run-of-river reservoirs) and Type2 (True reservoirs); Red and Blue symbols represent impaired and reference sites on each type, respectively
Environmental parameters and chlorophyll a
The Laboratory of Environment and Applied Chemistry (LABELEC) conducted the sampling of environmental and biological parameters during the period from 1996 to 2004. The samples were taken four times per year, which corresponded to the seasons of spring (April/May), summer (July/August), autumn (October/November), and winter (January/February). The sampling periodicity is indicated in Table 1. Not all reservoirs had the same sampling frequency; those with greater variability in physical, chemical and hydromorphological parameters were sampled more frequently (annually). The remaining reservoirs were sampled every 2 or 3 years. All samples were collected at 100 m from the reservoirs’ crest at two different depths: (a) near the surface at approximately 0.5 m depth, and (b) near the bottom at 2 m above the bottom, (this depth was only considered for environmental parameters).
Turbidity, conductivity, water temperature, dissolved oxygen and pH were measured directly at the sampling sites using a YSI handheld multiparameter probe (Yellow Spring Instruments). The remaining environmental variables were determined following the methodologies outlined by APHA (APHA, 1995).
A geographic information system database was created (ESRI, ArcGIS 9.0) with 12 spatial variables to determine the ecological status of each reservoir watershed. These variables were classified into four categories of anthropogenic stress measures that are prominent in the study area:
-
1.
Land cover—6 land use/land cover variables using Corine Land Cover (CLC 1990, 2000—IGEOE, 2006). Road density (km/ha watershed) and urban areas, intensive and extensive agriculture, natural and semi-natural areas, and burned areas ratio;
-
2.
Organic contamination load—Human population pressure (g BOD5/hab.eq.day by ha watershed) and domestic animal pressure (g BOD5/animal.eq.day by ha watershed) (INE, 2006);
-
3.
Industrial contamination load- Point source pollution, including the number of quarries, mines, and transformation industries (number of point source/ha watershed) (INE, 2006);
-
4.
Hydrometric variations—yearly water level changes were determined by the relative average and maximum theoretical water level differences.
Most variables were represented on a per-unit-area basis (for more detailed information, see Cabecinha et al., 2009a,c). For each variable, a 5-point scale was developed (from 1—High status to 5—Low status). Thus, the aggregate of these 5-score scales represented the ultimate ecological quality of the reservoir's watershed and was categorised as follows: I—< 18; II—18 to 22; III—22 to 26; IV—26 to 30 and V—> 30 (see Table 2). Classes I and II represented reference reservoirs, and classes III, IV, and V represented impaired sites.
Phytoplankton analysis
The environmental parameters and phytoplankton samples were collected from 1996 to 2004 using a Van Dorn bottle. Phytoplankton community composition was analyzed using inverted microscopy, following Utermohl’s method (Lund et al., 1958). To quantify and identify the phytoplankton, the samples were fixed in Lugol's solution (1% v/v) and, whenever possible, identified to the species level. The abundance of each taxon was estimated on a 5-score ordinal scale (0–20; 20–40; 40–60; 60–80; > 80%). A minimum of 50 random visual fields and at least 100 cells of the most common taxa were counted. Assuming that the cells were randomly distributed, the counting precision was ± 10% (Venrick, 1978). EDP Labelec provided all the data—Environment Laboratory is accredited according to the NP EN ISO/IEC 17025:2005 standard.
Statistical analysis
The spatial and temporal dynamics of phytoplankton communities along the perturbation gradient were analysed by the PRC ordination technique. PRC is based on RDA, the constrained form of Principal Component Analysis (Van den Brink & ter Braak, 1998, 1999; Cuppen et al., 2000; Van den Brink et al., 2000, 2003). The PRC method is a multivariate technique designed for data analysis from microcosm and mesocosm experiments. Due to its novelty, this method was mainly applied in aquatic ecotoxicology (Van den Brink & ter Braak, 1999; Van den Brink et al., 2003), with some incursions into ecology (Frampton et al., 2000, 2001). However, this approach has potential for a broader application in community ecology and the evaluation of ecosystem integrity.
The method analyzes differences in species composition between sites at each time point, similar to other ordination techniques. However, one advantage of this method is that any temporal changes in the ‘control’ (the reference sites in the present study) are constrained in the plot to a horizontal line. Thus PRC creates a graphical display with time (sampling dates) as a horizontal line and the basic response pattern (cdt) of each site (d) at each time (t) in relation to the control site on the vertical axis (by definition, the control site al-ways has a cdt of zero for every time). When these coefficients are plotted for each time point, a principal response curve of the community is obtained for each site compared to the control site (Van den Brink & ter Braak, 1999). This allows an easily understandable representation of the temporal changes in the phytoplankton assemblages at each site with the reference control site. An additional advantage of the PRC technique is that it allows detecting effects at the species level. Derived species weight (bk) is the factor by which the basic response pattern is multi-plied to attain the fitted response of species k. Species weights thus measure the affinity of a particular species to the community response pattern and can be used to estimate relative species abundance for each sampling date in each site compared to the control, using the expression exp (bk * cdt). When the coefficients cdt are plotted against sampling date t, the resulting PRC diagram displays a curve for each site that can be interpreted as the principal response curve of the community (Van den Brink & ter Braak, 1999).
In addition to providing a concise graphical summary of changes in community structure, PRC analysis allows an estimate of the variance in the data set explained per site. A PRC diagram aims to maximize the amount of variance due to sites; the higher the proportion of the variance displayed, the more closely will the fitted relative abundance of individual taxa inferred from the diagram matches the observed relative abundance. The null hypothesis assumes that the differences between impacted and reference sites are absent. To test these hypotheses Monte Carlo permutation tests were used (Van den Brink & ter Braak, 1999).
In the present study, the differences in phytoplankton community composition between the different ecological classes for the two Portuguese reservoir types were visualized by PRC (Principal Response Curves; Van den Brink and ter Braak (1998, 1999) using the CANOCO software package version 5.1 (ter Braak & Šmilauer, 2018). The analysis results in a diagram showing years on the x-axis and the first Principal Component of the differences in community structure between class I and the other classes on the y-axis. This yields a diagram showing the deviations in time between classes, with class I as reference (for Type2) and class II (for Type1). The species weights are shown in a separate diagram, indicating the species’ affinity with this stated difference. The taxa with high positive weight are indicated to show a response similar to the deviations indicated by PRC, those with negative weight, one that is opposite to the response indicated by PRC. Taxa with a near-zero weight show a response very dissimilar to the response indicated by PRC or no response.
The data sets of the physical and chemical variables associated with the water column were also analysed using PRC. In these analyses, the variables were cantered and standardized to account for differences in measurement scale (Kersting & Van den Brink, 1997). The variables related to chemical concentrations (TColf, FColf, NH4+, Fe2+, Mn2+, Cl−, NO3−, PO43−, TP, SiO2) were Ln(ax + 1) transformed, a was determined following (Van den Brink et al., 2000) (see Table 3). This was done to down-weight high abundance values and approximate normal distribution of the data (for rationale, see van den Brink et al., 1995). The scale used was adjusted to focus on inter-sample distances. Default values were chosen for all remaining options (ter Braak & Šmilauer, 2018).
This is one of the first examples in which monitoring data are analysed by PRC; how PRC can be used to analyse monitoring data is described more in detail in Van den Brink et al. (2008).
Results
From the 633 phytoplankton samples, a total of 250 taxa were identified. From these, 93 taxa occurred less than four times in each reservoir and were excluded from the dataset (see “Methods”). The 157 remaining taxa belonged to 6 divisions. The most important in terms of taxa number were Chlorophyta (41% of the taxa), Bacillariophyta (29%), and Cyanophyta (20%). There were 8 taxa of Crysophyta (5%) and 3 taxa of Pyrrophyta and Euglenophyta (representing each 2% of the total taxa).
The diversity of the phytoplankton communities in the two types of reservoirs is presented in Fig. 2a. These clearly show that the impaired sites of both types always had greater species richness than the less or non-impaired sites. Additionally, the diversity of the phytoplankton communities was determined to be associated only with well-known tolerant taxa, namely Chlorophyta, Cyanophyta, and mesoeutraphentic to hypereutraphentic diatoms (Fig. 2b, c). These figures clearly show that the greater species richness of impaired sites in both types of reservoirs was always associated with greater species richness of tolerant taxa. Over the past 8 years, there was a decline in biodiversity in 1998 and 1999, showing higher species richness (Fig. 2a).
Comparison of species richness from reference and impaired sites of both reservoir types (a). b and c For reference and impaired sites, the comparison between the diversity of the phytoplankton communities associated only with well-known tolerant taxa, namely Chlorophyta, Cyanophyta, and mesoeutraphentic to hypereutraphentic diatoms, of Type1 (Run-of-river reservoirs) and Type2 (True reservoirs), respectively
For both reservoir types, the PRC analysis shows a clear spatial gradient related to eutrophication (Figs. 3, 4). In our analysis, for Type1 reservoirs, in respective of the differences in phytoplankton communities between the different ecological classes, sampling date accounted for 29% of the total variation in species composition could be attributed to differences between sampling dates, 12% exclusively to the differences between years, 4% exclusively to differences between season, while the interaction between season and year explained the remaining 13%. Differences in species composition between the reservoirs with different ecological status explained 46% (P ≤ 0.001) of the variation in species composition; 20% (P ≤ 0.001) of the latter is displayed in the diagram (Fig. 3a). The remaining 25% of the total variance is related to differences between reservoirs with the same ecological status.
Diagram showing the first component of the PRC of the differences in taxa composition of the phytoplankton (a) and measured physical and chemical parameters (b) between the Type1 (Run-of-river reservoirs) having different ecological status (II through V). The taxa and parameter weights shown in the right part of the diagram represent the affinity of each taxon and parameter, respectively, with the response shown in the diagram. For clarity, only species with a weight larger than 1 or smaller than − 1 and parameters with a weight larger than 0.25 or smaller than − 0.25 are shown. In sampling date, 1, 2, 3, and 4 represent each year's spring, summer, autumn, and winter
Diagram showing the first component of the PRC of the differences in taxa composition of the phytoplankton (a) and measured physical and chemical parameters (b) between the Type2 (True reservoirs) having different ecological status (I through V). The taxa and parameter weights shown in the right part of the diagram represent the affinity of each species and parameter, respectively, with the response shown in the diagram. For clarity, only taxa with a weight larger than 1 or smaller than -1 and parameters with a weight larger than 0.25 or smaller than − 0.25 are shown. In sampling date, 1, 2, 3, and 4 represent each year's spring, summer, autumn, and winter
The most affected taxa were the diatoms Melosira distans (Ehrenberg) Kützing and Fragilaria capucina Desmazières, both with negative weights, indicating a reduced abundance compared to that in the reference site (Fig. 3a). In contrast, the taxa with the highest positive weight (i.e., which increased in abundance) were the diatoms Cyclotella meneghiniana Kützing and Melosira ambigua (Grunow) O.Müller (Fig. 3a). However, other taxa are also shown to have increased in time (e.g., Diatoma vulgaris Bory, Nitzschia sp., Navicula cryptocephala Kützing, and Ulnaria ulna (Nitzsch) Compère. Thus, the sign of the species scores indicates the direction of the changes in abundance, while the magnitude of the score reflects the size of the changes.
Also, for Type1 reservoirs, the differences between reference and impaired sampling sites measured by physical and chemical parameters are shown in Fig. 3b. 39% of the total variation in environmental variables could be attributed to differences between sampling dates, 17% exclusively to the differences between years, 12% exclusively to differences between seasons, while the interaction between season and year explains the remaining 10%. Differences in environmental variables between the reservoirs with different ecological status explained 41% (P > 0.05) of the variation in species composition; 25% (P > 0.05) of the latter is displayed in the diagram (Fig. 3b). The remaining 20% of the total variance is due to differences between reservoirs with the same ecological status. The Monte Carlo permutation test indicated no significant differences between reservoirs of the different classes. The major differences between reference and impaired sites were observed in 1998 and 1999. For these impaired sites, compared to the less disturbed ones, lower levels of chlorophyll a, Cl, and phosphorus (TP and PO43−) are indicated together with higher levels of NO3− and Secchi disk depth. Larger or smaller differences between class I and other ecological classes seem to be associated with higher or lower abundances of diatoms Melosira ambigua and Cyclotella meneghiniana (Fig. 3a), probably related to higher or lower levels of nutrients, namely NO3−.
The PRC diagram showing the differences in taxa composition between the Type2 reservoirs having different ecological status is shown in Fig. 4a. 9% of the total variation in species composition could be attributed to differences between sampling dates, 4% exclusively to the differences between years, 2% exclusively to differences between season, while the interaction between season and year explains the remaining 3%. Differences in species composition between the reservoirs with different ecological status explained 27% (P ≤ 0.001) of the variation in species composition; 21% (P ≤ 0.001) of the latter is displayed in the diagram (Fig. 4a). The remaining 64% of the total variance is due to differences between reservoirs with the same ecological status.
The PRC diagram shows fewer differences between phytoplankton communities of reservoirs belonging to status II and III than the reference sites. Contrarily, for reservoirs of status IV and V, more impaired, these differences were more significant. The most affected taxa were Tabellaria fenestrata (Lyngbye) Kützing (diatom) and the Dinobryon sp. (Crysophyta), both with positive weights, indicating a reduced abundance compared to that in the reference site (Fig. 4a). In contrast, the taxon with the highest negative weight (i.e., increased in abundance) was the Cyanophyta Aphanizomenon flos-aquae Ralfs ex Bornet & Flahault (Fig. 4a). Other species' abundance seems to have also increased in time (e.g., Microcystis flos-aquae (Wittrock) Kirchner and Anabaena spp. (Fig. 4a).
The differences between reservoirs with different ecological status and reference sampling sites measured by physic-chemical parameters are shown in Fig. 4b. 18% of the total variation in environmental variables could be attributed to differences between sampling dates, 7% exclusively to the differences between years, 8% exclusively to differences between season, while the interaction between season and year explains the remaining 3%. Differences in environmental variables between the reservoirs with different ecological status explained 31% (P ≤ 0.001) of the variation in species composition; 52% (P ≤ 0.001) of the latter is displayed in the diagram (Fig. 4b). The remaining 51% of the total variance is due to differences between reservoirs with the same ecological status. As in PRC based in phytoplankton communities, the PRC based on physical and chemical parameters shows fewer differences between reservoirs of status II and III when compared to the reference sites and more significant differences for reservoirs of status IV and V. For these impaired sites, compared to the reference, lower levels of dissolved oxygen in the hypolimnion and Secchi disk depth are indicated together with higher conductivity, hardness and levels of Cl, nutrients (NO3−, TP and PO43−) (Fig. 4b).
Discussion
The 34 studied reservoirs were identified and delimited into two types of dammed water bodies, characterized by different hydromorphological features, water chemistry characteristics, and a specific species composition (Cabecinha et al., 2009a). Community structure changes with pollution or stress. In the WFD, high ecological status through biological parameters is defined as a slight or minor deviation from the reference community. A PRC methodology was used to assess the importance of this deviation and measure the degradation of ecological status in the two types of reservoirs over time. The PRC analyses showed significant differences between the reference and impaired sites of each dam Type, reflecting the levels of disturbance over the study period.
In explaining the variance in species composition and environmental variables, time, namely differences in sampling dates, assumed higher importance in Type1 than in Type2 reservoirs. This was expected since Type1 is “riverine reservoirs” that resemble a river more than a lake, with short hydraulic retention times (Anderson et al., 2015; Munasinghe et al., 2021), good mixing, and relatively higher water velocities. Type2 are “artificial lake reservoirs” where water storage and release cycles are long and operate on at least seasonal cycles, but generally on multi-year cycles (Klaver et al., 2007; Hsu et al., 2015).
Most disturbed reservoirs of Type1 had larger watersheds belonging to international basins like Douro and Tagus, dominated by agriculture and having significant urban areas. These reservoirs were positively associated with an anthropogenic pressure gradient (Fig. 3b) and associated with mesoeutraphentic to hypereutraphentic taxa (Van Dam et al., 1994; Tavassi et al., 2004), namely Melosira ambigua, Cyclotella meneghiniana, Diatoma vulgaris, Navicula cryptocephala and Ulnaria ulna (Fig. 3a). These species are known to be tolerant or need periodic high concentrations of N, contrarily to Melosira distans (Van dam et al., 1994; Barinova & Chekryzheva, 2014).
Most impacted reservoirs of Type2 lay in densely populated, industrialized, or agricultural areas, receiving high organic matter inputs and industrial discharge. These findings clearly show that human activity significantly affects the trophic status of aquatic ecosystems. Therefore, these sites were positively correlated with water mineral content (Cl, hardness, and conductivity), nutrients (P and N), and organic pollution gradients (Fig. 4b). These disturbed sites were dominated by tolerant taxa, namely blue-green algae such as Aphanizomenon flos-aquae and Anabaena spp. These blue-green algae belonged to a genus whose ability to produce toxins that can affect various organisms, including humans, is known to increase in eutrophic conditions (Vasconcelos, 2001; Visser et al., 2016; Lürling et al., 2018). These taxa appear associated with meso- to hypereutrophic taxa like Fragilaria crotonensis Kitton, Diatoma vulgaris, Pediastrum duplex Meyen, Melosira ambigua, and Aulacoseira granulata (Ehrenberg) Simonsen (Fig. 4a) (Van Dam et al., 1994; Tavassi et al., 2004). Reference sites of Type2 presented in general large forested areas and were mainly dominated by intolerant taxa, Tabellaria fenestrata, Tabellaria floculosa (Roth) Kützing, and Crysophytas like Dinobryon sp. and Dinobryon bavaricum Imhof (Fig. 4a).
When reservoirs become more eutrophic, the diversity of phytoplankton assemblage decreases, ultimately leading to the dominance of Cyanobacteria (see Fig. 4a). The PRC diagram allowed analysing the changes in the community structure over time. In general, the most significant deviations from impaired sites to reference reservoirs of Type2 are associated with seasonality (namely in summer periods) and bloom formations. Bloom formation (as observed in 1997, 2002, and 2003—see Fig. 4a) could result in surface scums, producing unpleasant taste and odour, becoming an unsatisfactory food source for many organisms in the food web, and production of toxins (Reynolds & Petersen, 2000; Lürling et al., 2018).
Rarely will a single factor be responsible for the mass appearance of Cyanobacteria, but a combination of several of them, including hydrodynamic effects, oxygen depletion in the water column, anoxic conditions at the sediment–water interface, elevated temperatures, and low TN/TP-ratios (Dokulil & Teubner, 2000; Vasconcelos, 2001; Islam et al., 2012). This corroborates the results obtained in this study (see Fig. 4). Besides nutrients, the morphology of reservoirs is of decisive importance for cyanobacterial development. The dominance of colony-forming species such as Microcystis and Aphanizomenon is more commonly in deeper reservoirs (Dokulil & Teubner, 2000; Xiao et al., 2018; Christensen et al., 2019), as Type2 reservoirs that belong to the IV ecological status class.
For HMWBs, the reference conditions on which status classification is based are within the range of “Maximum Ecological Potential,” representing the maximum ecological quality that could be achieved for these systems (Lyche Solheim, 2005; GIG, 2007). Therefore, only sites showing nearly undisturbed physical and chemical, hydromorphological, and biological conditions were chosen as reference sites, as explained in the “Materials and methods” section (see “Environmental parameters and chlorophyll a”).
It was challenging to find many reference sites for Type1 reservoirs, with only 10 sampling sites. Only 2 sites were selected as reference sites. Most “run-of-river” reservoirs in Portugal lie in densely populated regions, representing rather impacted sites. Additionally, all these reservoirs belong to international river watershed, subject to significant anthropogenic pressures due to upstream intensive agriculture practiced in Spain, reflected by the mesoeutraphentic taxa that characterized these sites. This might indicate that the Type1 reservoirs investigated here as “best available” do not represent good reference sites. The PRC method could be helpful in this “scenario”, hence enables researchers to contrast the time series of impacted sites or treated sites with the time series of reference sites, but also has the advantage that an external particular starting position can be introduced as a reference, namely less impacted reservoirs or flushed lakes in other European countries.
However, according to Auber et al. (2017), when community/ecosystem dynamics have a gradual change, the PRC is less adapted since the community reference has less sharp differences, although contiguous, community structures that will difficult to determine a baseline and generally the discretization of time.
Many multivariate methods have been used to analyse biological time series of communities, with the ordination method PCA being the most frequently used. (e.g. Li & Kafatos, 2000; Cabecinha et al., 2018). Other methods, like non-metric multidimensional scaling and clustering, have also been proposed but have some disadvantages compared to ordination, as discussed by (Van den Brink & ter Braak, 1998; Van den Brink et al., 2008; Pardal et al., 2004). The PRC analysis results in a diagram where the time vector runs straight from left to right and differences are displayed on the y-axis. This presentation mode is very powerful, especially for non-experts, since this is the same type of display we would use to disseminate univariate information (Van den Brink et al., 2008; Auber et al., 2017).
Biomonitoring data sets often comprise biological and environmental data, like physical and chemical data, land-use data, etc. Trends and relationships between the biological and environmental data set can be displayed in a triplot, showing samples, species, and environmental variables. When interested in relationships between biology and environmental variables and their changes in time, one can imagine that these triplots could get very complex and only show a part of the variation (Van den Brink et al., 2008). A possibility to obtain a clearer overview is to perform separate PRC analyses on the biological and environmental data sets, as shown in Figs. 3 and 4. In this way, the dynamics of the differences between the sites for biological communities and environmental factors are displayed in separate diagrams. Their relationships over time can be easily inferred.
This ordination technique can summarize complex responses because it is not restricted to a single dimension [as (dis)similarity analysis]. When combined with Monte Carlo permutation testing is a graphical summary of the structure present in the obtained data set and the statistical significance of hypothesized differences (Van den Brink et al., 2003; ter Braak & Šmilauer, 2018).
Pardal et al. (2004) summarize how PRC analysis can be applied to several common environmental scenarios, independently of the number of analysed sites, namely in a standard disturbance gradient to a recovery scenario of the environmental quality after management or mitigation measures that might lead to a better environmental quality or even the establishment of threshold values/levels necessary for qualitative evaluation of ecosystem health. Therefore, PRC analysis could be a powerful tool to reach and implement WFD objectives since it allows to know in time the ecological status of a site and compare the deviations with the reference. Consequently, to assess “Maximum Ecological Potential” for all reservoirs until 2015, as a requisite of WFD, several mitigation measures could be implemented, and the monitoring results easily analysed, interpreted, and compared by PRC. These mitigation measures could reduce the nutrient load from the catchment to the reservoir, altering the hydrodynamic conditions (e.g., artificial mixing or intermittent turbulence of the water column), or even apply in reservoir eco-technologies.
The PRC diagrams also provide meaningful information on the species contributing to these trends. Combining this with knowledge about the ecological requirements of these species will provide decision-makers with a diagnostic tool for the ecological functioning of their water systems, e.g., as required by the EU Water Framework Directive.
Conclusions
In this study, PRC analysis was used effectively to explore a suitable way of monitoring and assessing the water quality of two types of Mediterranean reservoirs using phytoplankton communities. This method was used to analyse changes in species composition and environmental variables between sites with different ecological status over time, allowing to estimate the degree of impairment at a particular site by contrasting it with a reference site, as proposed by WFD.
For both reservoirs’ types, the PRC analysis showed significant differences between the reference and impaired sites, reflecting the levels of disturbance that they experienced over the study period, mostly associated with a clear spatial gradient related to eutrophication.
This methodology proved to be capable of concisely summarizing the complex data set of the phytoplankton community while permitting information to be displayed with visual clarity and easy to interpret by non-experts, namely decision-makers, politicians, and the general public. This PRC application clearly provides a highly accurate synthesis of community-level and environmental parameters dynamics at numerous sampling sites to easily characterize and quantify the spatio-temporal dynamics of ecosystems.
Moreover, PRC gives higher readability and interpretation of spatial patterns in temporal changes useful for spatial management decisions, reinforcing the relevance of this application for the ecosystem approach.
In conclusion, we believe that PRC will provide a powerful tool for environmental quality assessment in the future and should be incorporated into monitoring and assessment programs along with the existing range of univariate and multivariate tools presently used. Also, this approach can help policymakers to manage the natural capital to achieve multiple objectives, namely the ecosystem services provided by nature that can contribute to SDG targets.
Data availability
The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
References
Almeida, R., N. E. Formigo, I. Sousa-Pinto & S. C. Antunes, 2020. Contribution of zooplankton as a biological element in the assessment of reservoir water quality. Limnetica 39(1): 245–261. https://doi.org/10.23818/limn.39.16.
Anderson, D., H. Moggridge, P. Warren & J. Shucksmith, 2015. The impacts of ‘run-of-river’ hydropower on the physical and ecological condition of rivers. Water & Environment Journal 29(2): 268–276. https://doi.org/10.1111/wej.12101.
APHA, 1995. Standard Methods for the Examination of Water And Wastewater, 19th ed. Amer-ican Public Health Association, Washington:
Auber, A., M. Travers-Trolet, M. C. Villanuev & B. Ernande, 2017. A new application of principal response curves for summarizing abrupt and cyclic shifts of communities over space. Ecosphere 8(12): e02023. https://doi.org/10.1002/ecs2.2023.
Barinova, S. & T. Chekryzheva, 2014. Phytoplankton dynamic and bioindication in the Kondopoga Bay, Lake Onego (Northern Russia). Journal of Limnology. https://doi.org/10.4081/jlimnol.2014.820.
Bieroza, M. Z., R. Bol & M. Glendell, 2021. What is the deal with the green deal: will the new strategy help to improve European freshwater quality beyond the water framework directive? Science of the Total Environment 791: 148080. https://doi.org/10.1016/j.scitotenv.2021.148080.
Boavida, M. J. & Z. M. Gliwicz, 1996. Limnological and biological characteristics of the Alpine lakes of Portugal. Limnetica 12(2): 39–45.
Boeuf, B. & O. Fritsch, 2016. Studying the implementation of the Water Framework Directive in Europe: a meta-analysis of 89 journal articles. Ecology and Society 21: 19.
Brock, T. C. M., I. Roessink, D. M. Belgers, F. Bransen & S. J. Maund, 2009. Impact of a benzoyl urea insecticide on aquatic macroinvertebrates in ditch mesocosms with and without non-sprayed sections. Environmental to-Xicology and Chemistry 28: 2191–2205.
Cabecinha, E., R. Cortes, J. A. Cabral, T. Ferreira, M. Lourenço & M. A. Pardal, 2009a. Multi-scale approach using phytoplankton as a first step towards the definition of the ecological status of reservoirs. Ecological Indica-Tors 9(2): 240–255. https://doi.org/10.1016/j.ecolind.2008.04.006.
Cabecinha, E., R. Cortes, M. A. Pardal & J. A. Cabral, 2009b. A stochastic dynamic methodology (StDM) for reservoir’s water quality management: validation of a multi-scale approach in a south European basin (Douro, Portugal). Ecological Indicators 9(2): 329–345. https://doi.org/10.1016/j.ecolind.2008.05.010.
Cabecinha, E., M. Lourenço, J. P. Moura, M. A. Pardal & J. A. Cabral, 2009c. A multi-scale approach to modelling spatial and dynamic ecological patterns for reservoir’s water quality management. Ecological Modelling 220(19): 2559–2569. https://doi.org/10.1016/j.ecolmodel.2009.06.011.
Cabecinha, E., P. J. Van den Brink, J. A. Cabral, R. Cortes, M. Lourenço & M. A. Pardal, 2009d. Ecological relation-ships between phytoplankton communities at different spatial scales in European reservoirs: implications at catchment level monitoring programmes. Hydrobiologia 628: 27–45. https://doi.org/10.1007/s10750-009-9731-y.
Cabecinha, E., S. Hughes & R. Cortes, 2018. Consistent, congruent or redundant? Lotic community and organisational response to disturbance. Ecological Indicators 89: 175–187. https://doi.org/10.1016/j.ecolind.2018.01.060.
Cardoso, P. G., D. Raffaelli, A. I. Lillebø, T. Verdelhos & M. A. Pardal, 2008. The impact of extreme flooding events and anthropogenic stressors on the macrobenthic communities’ dynamics. Estuarine, Coastal and Shelf Science 76(3): 553–565. https://doi.org/10.1016/j.ecssj.2007.07.026.
Christensen, V. G., R. P. Maki, E. A. Stelzer, J. Norland & E. Khan, 2019. Phytoplankton community and algal toxici-ty at a recurring bloom in Sullivan Bay, Kabetogama Lake, Minnesota, USA. Scientific Reports 9: 16129. https://doi.org/10.1038/s41598-019-52639-y.
CNEGP, 2017. Dams and the sustainable development goals. Technical Commission on Engineering Activities associated with Water Resource Planning, Technical Work Document. pp. 40. https://www.spancold.org/wp-content/uploads/2019/03/181220_DAMS-AND-SDGs.-Technical-Working-Document.pdf
Cuppen, J. G. M., P. J. Van den Brink, K. F. Uil, E. Camps & T. C. M. Brock, 2000. Impact of the fungicide carbendazimin freshwater microcosms. II Water quality, breakdown of Particulate Organic Matter and responses of macro-invertebrates. Aquatic Toxicology. 48: 233–250.
Dehini, G. K. & P. I. A. Gomes, 2022. Deriving optimal hydraulic, water quality and habitat quality criteria against a predefined reference state of urban canals via an analytical method: Implications on ecological rehabilitation. Ecological Engineering 182: 106697. https://doi.org/10.1016/j.ecoleng.2022.106697.
Denys, L., J. Van Wichelen, J. Packet & G. Louette, 2014. Implementing ecological potential of lakes for the Water Framework Directive – approach in Flanders (northern Belgium). Limnologica 45: 38–49. https://doi.org/10.1016/j.limno.2013.10.004.
Dokulil, M. T. & K. Teubner, 2000. Cyanobacterial dominance in lakes. Hydrobiologia 438: 1–12.
Domingues, R. B. & H. Galvão, 2007. Phytoplankton and environmental variability in a dam regulated temperate estuary. Hydrobiologia 586: 117–134.
Domis, L., J. Elser, A. Gsell, V. Huszar, B. Ibelings, E. Jeppesen, S. Kosten, W. Mooij, F. Roland, U. Sommer, E. Van Donk, M. Winder & M. Lürling, 2013. Plankton dynamics under different climatic conditions in space and time. Freshwater Biology 58: 463–482.
Dziock, F., K. Henle, F. Foeckler, K. Follner & M. Scholz, 2006. Biological indicator systems in floodplains – a review. International Review of Hydrobiology 91: 271–291.
Ekdahl, E. J., J. L. Teranes, C. A. Wittkop, E. F. Stoermer, E. D. Reavie & J. P. Smol, 2007. Diatom assemblage response to Iroquoian and Euro-Canadian eutrophication of Crawford Lake, Ontario, Canada. Journal of Paleolimnology 37: 233–246.
EU, 2019. Guidance Document No. 37 Steps for defining and assessing ecological potential for improving comparability of Heavily Modified Water Bodies. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Helsinki, 134 pp.
European Environment Agency, 2018. European Waters, Assessment of Status and Pressures. EEA Report, Luxembourg:, 1–90.
Fetahi, T., M. Schagerl & S. Mengistou, 2014. Key drivers for phytoplankton composition and biomass in an Ethiopian highland lake. Limnologica 46: 77–83.
Frampton, G., P. J. Van den Brink & P. J. L. Gould, 2000. Effects of spring precipitation on a temperate arable collembolan community analysed using principal response curves. Applied Soil Ecology 421: 1–18.
Frampton, G., P. J. Van den Brink & S. D. Wratten, 2001. Diel activity patterns in an arable collembolan community. Applied Soil Ecology 17: 63–80.
Fuentes, S., E. Van Nood, S. Tims, I. Heikamp-de Jong, C. J. F. Ter Braak, J. J. Keller, E. G. Zoetendal & W. M. De Vos, 2014. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. The International Society for Microbial Ecology Journal 8(8): 1621–1633. https://doi.org/10.1038/ismej.2014.13.
Gunkel, G., D. Lima, F. Selge, M. Sobral & S. Calado, 2015. Aquatic ecosystem services of reservoirs in semi-arid areas: sustainability and reservoir management. WIT Transactions on Ecology and the Environment. https://doi.org/10.2495/RM150171.
Hsu, N. S., C. L. Huang & C. C. Wei, 2015. Multi-phase intelligent decision model for reservoir real-time flood control during typhoons. Journal of Hydrology 522: 11–34.
IGEOE - Instituto Geográfico do Exército (Geografic Military Institute), 2006. Corine Land Cover 1990 and 2000. http://www.igeoe.pt/
INAG - Instituto Nacional da Água (National Water Institute), 2006. Relatório intercalar do projecto “Qualidade ecológica e gestão integrada de albufeiras”. INAG, (in portuguese).
INAG I.P. 2009. Critérios para a Classificação do Estado das Massas de Água Superficiais - Rios e Albufeiras. Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento RegionaL.29 pp.
INE - Instituto Nacional de Estatística (National Statistics Institute), 2006. http://www.ine.pt.
Islam, M. N., D. Kitazawa & H. D. Park, 2012. Numerical modeling on toxin produced by predominant species of cyanobacteria within the ecosystem of Lake Kasumigaura, Japan. Procedia Environmental Sciences 13: 166–193. https://doi.org/10.1016/j.proenv.2012.01.017.
Kersting, K. & P. J. Van den Brink, 1997. Effects of the insecticide Dursban®4E (active ingredient chlorpyrifos) in outdoor experimental ditches: III. Responses of ecosystem metabolism. Environmental Toxicology and Chemistry 16: 251–259.
Klaver, G., B. van Os, P. Negrel & E. Petelet-Giraud, 2007. Influence of hydropower dams on the composition of the suspended and riverbank sediments in the Danube. Environmental Pollution 148(3): 718–728.
GIG. Lake Mediterranean GIG. Joint Research Centre, 2007. European Commission. http://circa.europa.eu/Public/irc/jrc/jrc_eewai/library?l=/milestone_reports/milestone_reports_2007/lakes&vm=detailed&sb=Title
Li, Z. & M. Kafatos, 2000. Interannual variability of vegetation in the United States and its relation to El Niňo/Southern Oscillation. Remote Sensing of Environment 71: 239–247.
Litchman, E., P. Pinto, F. Kyle, E. Christopher, A. Klausmeier, C. Kremer & M. Thomas, 2015. Global biogeochemical impacts of phytoplankton: a trait-based perspective. Journal of Ecology 103: 1384–1396.
Liu, C., X. Sun, L. Su, J. Cai, L. Zhang & L. Guo, 2021. Assessment of phytoplankton community structure and water quality in the Hongmen Reservoir. Water Quality Research Journal 56(1): 19–30. https://doi.org/10.2166/wqrj.2021.022.
Lund, J. W. G., C. Kipling & E. D. Le Cren, 1958. The inverted microscope methods of estimating algal numbers and the statistical basis of estimation by counting. Hydrobiologia 11: 143–170.
Lürling, M., M. M. Mello, F. van Oosterhout, L. S. Domis & M. M. Marinho, 2018. Response of natural Cyanobacteria and algae assemblages to a nutrient pulse and elevated temperature. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2018.01851.
Lyche Solheim, A., 2005. Reference Conditions of European Lakes. Indicators and methods for the Water Framework Directive Assessment of Reference conditions. Version 5. REBECCA Working Group. pp. 105.
Lyche Solheim, A., G. Phillips, S. Drakare, G. Free, M. Jarvinen, B. Skjelbred, D. Tierney & W. Trodd, 2014. Water Framework Directive Intercalibration Technical Report: Northern Lake Phytoplankton ecological assess-ment methods. EUR 26503. Luxembourg (Luxembourg): Publications Office of the European Union, JRC88307.
MEA, 2005. Millennium Ecosystem Assessment. Ecosystems & Human Well-Being: Wetlands and Water Synthe-sis; World Resources Institute. Washington, DC.
Munasinghe, D., M. Najim, S. Quadroni & M. M. Musthafa, 2021. Impacts of streamflow alteration on benthic macroinvertebrates by mini-hydro diversion in Sri Lanka. Scientific Reports 11: 546. https://doi.org/10.1038/s41598-020-79576-5.
Padedda, B. M., N. Sechi, G. G. Lai, M. A. Mariani, S. Pulina, M. Sarria, C. T. Satta, T. Virdis, P. Buscarinu & A. Lugliè, 2017. Consequences of eutrophication in the management of water resources in Mediterranean reservoirs: a case study of Lake Cedrino (Sardinia, Italy). Global Ecology and Conservation 12: 21–35.
Paliy, O. & V. Shankar, 2016. Application of multivariate statistical techniques in microbial ecology. Molecular Ecology 25(5): 1032–1057. https://doi.org/10.1111/mec.13536.
Pardal, M. A., P. G. Cardoso, J. P. Sousa, J. C. Marques & D. Raffaelli, 2004. Assessing environmental quality: a novel approach. Marine Ecology Progress Series 267: 1–8.
Reynolds, C. S. & A. C. Petersen, 2000. The distribution of planktonic Cyanobacteria in Irish lakes in relation to their trophic states. Hydrobiologia 424: 91–99.
Simboura, N., P. Panayotidis & E. Papathanassiou, 2005. A synthesis of the biological quality elements for the implementation of the European Water Framework Directive in the Mediterranean ecoregion: the case of Saronikos Gulf. Ecological Indicators 5(3): 253–266.
Sittenthaler, M., L. Koskoff, K. Pinter, U. Nopp-Mayr, R. Parz-Gollner & K. Hackländer, 2019. Fish size selection and diet composition of Eurasian otters (Lutra lutra) in salmonid streams: picky gourmets rather than op-portunists? Knowledge and Management of Aquatic Ecosystems 420: 29. https://doi.org/10.1051/kmae/2019020.
Statzner, B., B. Bis, S. Dolédec & P. Usseglio-Polatera, 2001. Perspectives for biomonitoring at large spatial scales: a unified measure for the functional composition of invertebrate communities in European running waters. Basic and Applied Ecology 2: 73–85.
Tavassi, M., S. S. Barinova, O. V. Anissimova & E. Nevo, 2004. Algal indicators of environment in the Nahal Yarqon basin. Central Israel. International Journal on Algae 6(4): 355–382.
ter Braak, C. J. F. & P. Šmilauer, 2018. Canoco reference manual and user's guide: software for ordination (ver-sion 5.10). Biometris, Wageningen University & Research.
Van Dam, H. & A. Merten, 1994. Coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherlands Journal of Aquatic Ecology 28(1): 117–133.
Van den Brink, P. J. & C. J. F. ter Braak, 1998. Multivariate analysis of stress in experimental ecosystems by principal response curves and similarity analysis. Aquatic Ecology 32: 161–178.
Van den Brink, P. J. & C. J. F. ter Braak, 1999. Principal response curves: analysis of time-dependent multivariate responses of biological community to stress. Environmental Toxicology and Chemistry 18(2): 138–148.
Van den Brink, P. J. & P. J. den Besten, 2008. Principal response curves technique for the analysis of multivariate biomonitoring time series. Environmental Monitoring & Assessment. https://doi.org/10.1007/s10661-008-0314-6.
van den Brink, P. J., E. van Donk, R. Gylstra, S. J. H. Crum & T. C. M. Brock, 1995. Effects of chronic low concentrations of the pesticides chlorpyrifos and atrazine in indoor freshwater microcosms. Chemosphere 31(5): 3181–3200.
Van den Brink, P. J., J. Hattink, F. Bransen, E. Van Donk & T. C. M. Brock, 2000. Impact of the fungicide car-bendazim in freshwater microcosms. II. Zooplankton, primary producers and final conclusions. Aquatic Toxicology 48: 251–264.
Van den Brink, P. J., N. W. Van den Brink & C. J. F. ter Braak, 2003. Multivariate analysis of ecotoxicological data using ordination: demonstrations of utility on the basis of various examples. Australian Journal of Soil Re-Search 9: 141–156.
van Paassen, J. G., A. J. Britton, R. J. Mitchell, L. E. Street, D. Johnson, A. Coupar & S. J. Woodin, 2020. Legacy effects of nitrogen and phosphorus additions on vegetation and carbon stocks of upland heaths. New Phytologist. https://doi.org/10.1111/nph.16671.
Vasconcelos, V. M., 1991. Species composition and dynamics of phytoplankton in a recently commissioned res-ervoir (Azibo – Portugal). Archiv Für Hydrobiologie 121: 67–78.
Vasconcelos, V. M., 2001. Toxic freshwater cyanobacteria and their toxins in Portugal. In Chorus, I. (ed), Cyanotoxins – Occurrence, Effects, Controlling Factors Springer, Berlin: 64–69.
Vendrig, N. J., L. Hemerik & C. J. F. ter Braak, 2017. Response variable selection in principal response curves using permutation testing. Aquatic Ecology 51: 131–143. https://doi.org/10.1007/s10452-016-9604-1.
Venrick, E. L., 1978. Statistical considerations. In Sournia, A. (ed), Monographs on Oceanographic Methods 6: Phytoplankton Manual United Nations Educational, Scientific and Cultural Organization, Paris: 238–250.
Verdonschot, R. C. M., A. M. Van Oosten-Siedlecka, C. J. F. ter Braak & P. F. M. Verdonschot, 2015. Macroinvertebrate survival during cessation of flow and streambed drying in a lowland stream. Freshwater Biology 60(2): 282–296. https://doi.org/10.1111/fwb.12479.
Visser, P. M., B. W. Ibelings, M. Bormans & J. Huisman, 2016. Artificial mixing to control cyanobacterial blooms: a review. Aquatic Ecology 50: 423–441. https://doi.org/10.1007/s10452-015-9537-0.
Warwick, R. M. & K. R. Clarke, 1991. A comparison of some methods for analysing changes in benthic community structure. Journal of the Marine Biological Association 71: 225–244.
Xiao, M., M. Li & C. S. Reynolds, 2018. Colony formation in the cyanobacterium Microcystis. Biological Reviews 93: 1399–1420. https://doi.org/10.1111/brv.12401.
Zhang, S., H. Xu, Y. Zhang, Y. Li, J. Wei & H. Pei, 2020. Variation of phytoplankton communities and their driving factors along a disturbed temperate river-to-sea ecosystem. Ecological Indicators 118: 106776. https://doi.org/10.1016/j.ecolind.2020.106776.
Acknowledgements
We would like to thank the LABELEC staff for the environmental and phytoplankton data, namely Engineer Lourenço Gil.
Funding
Open access funding provided by FCT|FCCN (b-on). This research was funded by the Portuguese Foundation for Science and Technology (FCT) and the Operational Competitiveness Programme (COMPETE) under the Project UIDB/04033/2020 (CITAB-Inov4Agro-UTAD).
Author information
Authors and Affiliations
Contributions
In the overall context of this paper, the contributions are as follows: E.C. and P.V.B. conceived, designed, and drafted the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Handling editor: Andrew Dzialowski
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cabecinha, E., Pardal, M.Â., Cabral, J.A. et al. Assessing the ecological potential of reservoirs: a principal response curve (PRC) analysis approach. Hydrobiologia 851, 25–44 (2024). https://doi.org/10.1007/s10750-023-05310-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05310-7