Abstract
Despite the potential adverse consequences they can impart on biotopes, cyanobacteria are an understudied component of lake plankton communities in Ireland. This study, in summer 2019, examined cyanobacterial diversity and associated environmental variables in a lake previously impacted by algal blooms, in north-west Ireland. Vertical profiling showed that photosynthetic pigment concentrations were spatially heterogenous for the majority of the survey. However, water column homogeneity was observed towards the end of the survey, coinciding with increases in pigment concentrations. Sunshine hours, nutrient concentrations and wind speed were stable throughout the survey. Spatial homogeneity in pigments was attributed to an increase in minimal temperatures observed in the water column. PCR-DGGE analysis identified patterns in community composition, indicating a rise in cyanobacterial richness concomitant to increase in temperature and photosynthetic pigments. Band sequencing analysis identified phylotypes similar to the toxigenic species Microcystis sp. These were prominent at the end of the survey and coincided with the presence of the mcyE gene and low levels of microcystins, as indicated by real time PCR and ELISA analyses. The summer background presence of toxigenic cyanobacteria with potential bloom-forming capacity highlights the importance of annual lake cyanotoxin monitoring to inform resource management to protect human and animal health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Favourable water quality status is imperative to protect an ecosystem’s ecological status and the general public (Lileikyte & Belous, 2011). Due to the significant value of natural water bodies in terms of ecosystem services, protection has been afforded to them through legislative instruments. In the European Union, surface waters are monitored and controlled according to the Water Framework Directive (WFD) (20/60/EC) with the overall aim of the directive to achieve water quality of at least “good status” by 2027 for rivers, lakes, estuaries and coastal waters (Carvalho et al., 2019). Under the WFD, the structure, biomass and abundance of phytoplankton have been identified as important assessors of ecological condition for water bodies (Lileikyte & Belous, 2011).
Cyanobacteria, a phylum of prokaryotic photosynthetic organisms, are commonly associated with aquatic habitats (Brand, 2009) where they are an important component of the plankton community (Sivonen, 2009) with an ecological niche similar to that of some eukaryotic algae (Wood, 2016). Under favourable extrinsic conditions, cyanobacteria can proliferate in aquatic environments (Backer, 2002). Algal blooms, which can result in the mass accumulation of cyanobacteria, are considered one of the most adverse consequences of freshwater pollution (Jančula & Maršálek, 2011). These events can cause a cascade of negative effects which impair ecosystem functionality, consequently impacting organisms inhabiting the biotope (Sellner et al., 2003; Quiblier et al., 2013) and leading to a reduction in aquatic biodiversity (Sanseverino et al., 2016). One of the most serious consequences of some cyanobacterial proliferations is the synthesis of potentially toxic secondary metabolites commonly referred to as cyanotoxins, which can elicit the mortality of organisms in contact either directly or indirectly with cyanobacterial blooms (Sellner et al., 2003; Johnk et al., 2008). In addition, blooms can negatively impact economic activities linked to the water system (Sellner et al., 2003).
Contemporarily, eutrophication has been associated with increased nutrient enrichment due to anthropogenic activities associated with agricultural practices (Bláha et al., 2009), which have been further exacerbated by changes in climatic conditions (Carey et al., 2012). Due to the volatility of cyanobacterial blooms and the adverse consequences their occurrences incur, water authorities in some countries have adopted management strategies (Zamyadi et al., 2016) with effective monitoring being deemed pivotal for their management (Ouellette & Wilhelm, 2003; Bastien et al., 2011; Moreira et al., 2014). Monitoring is important to document cyanobacterial bloom occurrences and their associated toxicity potential (Baker et al., 2002). However, differing sampling methodologies and analysis techniques can impact (Ahn et al., 2007, 2008) the quality of information attained (Chorus & Bartham, 2005). In situ monitoring of cyanobacterial dynamics in water bodies is often routinely limited to one sampling location with intermittent sampling typically occurring once or twice monthly (Brand et al., 2010; Pobel et al., 2011). This can impede the evaluation of several factors relevant to cyanobacterial dynamics in terms of capturing both spatial and temporal patterns in their abundance, diversity and toxicity potential (Brand et al., 2010). These sampling restrictions are primarily due to budgetary or logistical constraints, restricting the resolution of in situ surveys and subsequent analytical techniques applied (Bertani et al., 2017).
A multifaceted approach to monitoring, which can document the dynamics of both the biological and environmental drivers of proliferation, has been emphasised as important when implementing monitoring programmes for cyanobacteria (Caldwell Eldridge et al., 2017). Additionally, high frequency monitoring regimes have been considered useful as they enable a better understanding of plankton dynamics in an ecosystem (Le Vu et al., 2011).
In the present study, we aimed to characterise the dynamics of cyanobacteria in an Irish context. Scientific studies of this group in Irish lakes are scarce, with Touzet et al. (2013, 2016) being two of the few examples. This study in Lough Arrow in North-West Ireland used multiple analytical techniques complimented by high frequency but intermittent sampling at multiple sites across the lake. This site has historically been impacted by cyanobacteria (Lucy et al., 2009) with contemporary occurrences of blooms occurring at times in the lake with blooms having been cited as a driver of habitat degradation. As such, the abundance, diversity and microcystin production potential of the cyanobacterial community in the lake were investigated to assess the prevalence of toxigenic phylotypes and examine their relationship with environmental variables.
Methods
Study area
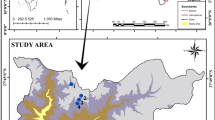
Lough Arrow, a lake situated in the North-West of Ireland (54° 03′ 36.7″ North and 8° 19′ 39.1″ West) (Fig. 1) was where in situ sampling was conducted for the purposes of this research. The lake is 14.58 Km2 in size and its average depth is 9 m (maximum 33 m) (National Parks and Wildlife Services, 2015).
This lake is a water abstraction resource for several adjacent urbanised areas and two sewage treatment plants are located in close proximity to the lough. It is also managed as a recreational brown trout (Salmo trutta) fishery. The lough has a number of European directives protecting its biodiversity. It is designated under both EU Habitats Directive (92/43/EEC) (site code: 001673) and the EU Birds Directive (2009/147/EC) (site code: 004050). It contains a designated representative example of the Annex I habitat “Hard oligo- mesotrophic waters with benthic vegetation of Chara spp. (3140)”. Additionally, the site supports “Nationally” important populations of bird species referred to in Article IV of the EU Birds Directive (National Parks and Wildlife Service, 2014). Under the Habitats Directive (92/43/EEC) Article 17 conservation assessment of the lough, nutrient pollution has been cited as a driver of degradation for this Annex I habitat with its current conservation condition being recently classified as Unfavourable-Bad (Roden & Murphy, 2020). Historically, this lake has been impacted by eutrophication events in the past (Lucy et al., 2009).
Sampling and data acquisition
In situ sampling commenced on the 19th of July 2019 and ended on the 15th of August 2019. Meteorological data was retrieved from Knock weather station, County Mayo over the course of the sampling period.
A transect through the centre of the lake from North to South consisting of five sites was selected for sampling, which took place every 3 days during this period. Each site was sampled on 10 occasions.
Data collection was undertaken from a boat. A Garmin® GPS 12 was used to locate individual sampling sites. At each sampling site, a Hawkeye H22PX sonar depth sounder was used to determine the water column depth and Secchi depth measurement was taken. Following this, a depth profile was taken utilising both an Algae Wader Pro System probe (Chelsea Technologies Group LTD) and a YSI MultiQuatroPro probe, taking measurements from the water surface down to 1 m above the lake bed at 1 m intervals. Parameters recorded per profile included phycocyanin, phycoerythrin, chlorophyll-a, temperature, oxygen concentration and conductivity. Upon completion of water column profiling at each sampling site, four 2000 ml water samples were collected: one from the surface (depth 1), one from one metre above the lakebed (depth 4) and two elsewhere in the water column depending on where the highest phycocyanin signals were recorded during the real time profiling (depths 2 & 3). A Duncan & Associates 2-l Niskin sampler was used to collect the subsurface samples while a 5-l bucket was used to collect the surface water sample. All water samples were individually stored in 2-l sample containers prior to processing in the laboratory (room temperature, processed within 2 h of sampling). Each individual 2-l sample was homogenised then partitioned into 1000 ml, 500 ml, 350 ml and 100 ml volumes. The first three volumes were filtered onto individual Whatmann GF/F filters. The 100 ml sample was filtered onto a Whatmann cellulose nitrate filter (1 μm pore size). All filters were then individually placed in 15 ml centrifuge tubes while filtrates were kept in 50 ml centrifuge tubes. All samples were stored in a freezer in the laboratory at − 20°C until further processing.
Nutrient analysis
A HACH DR3900 spectrophotometer was used to determine the concentration of nutrients in the filtered water samples obtained in situ. Compatible cuvette kits from HACH (LCK304, LCK 349. LCK 339) for ammonium NH4–N (0.015–2 mg/l), nitrate NO3–N (0.23–13.5 mg/l) and phosphate PO4–P (0.05–1.5 mg/l) were used for the analyses following the manufacturers’ guidelines.
DNA extraction
Extraction of genomic DNA from the frozen Whatmann cellulose nitrate filters was undertaken utilising E.Z.N.A. ® Plant DNA Kits (Omega Bio-Tek) following the manufacturer’s guidelines for Gram-negative bacteria. The protocol also included a bead beating step in a Ribolyser using 180-µm-diameter glass beads for two consecutive 20 s shaking cycles at maximum velocity. The eluted DNA had a final volume of 100 µl and was stored at − 20°C until further analysis. Prior to complete extraction of all filters, select samples were tested utilising a nanodrop to determine successful extraction of targeted DNA.
16S rDNA DGGE community fingerprinting analysis
A semi-nested PCR approach was used for the amplification of environmental DNA. An initial amplification was undertaken with cyanobacterial specific primers CYA359f (5′- ggggaattttccgcaatggg-3′) and 23S30r (5′-cttcgcctctgtgtgcctaggt-3′) (0.2 μM) (Touzet et al., 2016). The PCR cocktail for each individual reaction consisted of 25 µl DreamTaq Green PCR Master Mix (THERMO SCIENTIFIC), 1 µl of forward and reverse primers, 22 µl of PCR grade water and 1 µl of previously eluted DNA per reaction.
The thermocycling conditions for amplification were as follows: an initial denaturation step (94°C, 5 min) followed by 15 cycles of amplification (denaturation) (94°C, 0.5 min), annealing (58°C, 1 min) and extension (72°C, 2 min), and a final extension step (72°C, 10 min).
The subsequent amplification utilised 1 µl of the first PCR reaction with a PCR cocktail consisting of a similar composition with differing primers being utilised. CYA359f and CYA781r(a) (5′ -gactactggggtatctaatcccatt-3′) or CYA781r(b) (5′ gactacaggggtatctaatcccttt- 3′) were used to amplify filamentous and spherical cyanobacteria, respectively (Touzet et al., 2016).
Thermocycling conditions for the second amplification involved an initial denaturation step (94°C, 5 min), 35 cycles of amplification denaturation (94°C, 1 min), annealing (62°C, 1 min) and extension (72°C, 2 min) and a final extension step (72°C, 30 min).
Visualisation of the PCR products was carried out via agarose gel electrophoresis using 1X GelRed® and a Bio-Rad Gel Doc System.
Amplicons deemed suitable for further processing were separated using Denaturing Gradient Gel Electrophoresis (DGGE). Gels consisted of 8% acrylamide, 7 M urea, 40% formamide, deionised water and they had a denaturant gradient of 25–55%. 35 µl of PCR product was loaded into individual wells and electrophoresis was carried out for 16.5 h at 60°C with a constant voltage of 60 V.
Gels were stained with SYBR® Gold Nucleic Acid Gel Stain (Invitrogen) and subsequently viewed under a Bio-Rad Gel Doc System. Bands of interest were excised from the gel using sterile pipette tips and eluted overnight at 5°C in 50 μl of PCR grade water.
After 24 h, the eluted bands were reamplified with CYAseq-f (5′ -gcgaaagcctgacggagc-3′) and CYAseq-r (5′-ggggtatctaatcccattcgct-3′) (0.2 μM) primers with a PCR cocktail consisting of 1 µl of forward and reverse primers, 25 µl DreamTaq Green PCR Master Mix (THERMO SCIENTIFIC), 22 µl of PCR grade water and 1 µl of previously eluted excised band per reaction.
The amplification conditions followed an initial denaturation step (94°C, 5 min) followed by 15 cycles of amplification (denaturation) (94°C, 0.5 min), annealing (58°C, 1 min) and extension (72°C, 2 min), and a final extension step (72°C, 10 min). Agarose gel electrophoresis was undertaken to confirm the successful re-amplification of the excised bands.
The purification of successfully reamplified bands of interest was carried out using E.Z.N.A.® and E-Z 96® Cycle-Pure Kits (Omega Bio-Tek) following the manufacturer’s guidelines and were stored at − 20°C prior to external sequencing (Eurofins-MWG, Germany).
Determination of DGGE migration patterns of sequenced bands of interest was carried out with TOTAL-LAB TL120 software (Nonlinear Dynamics, UK). A densitometric scan of the gels was created and background noise was subtracted using a rolling disc algorithm. Band matching matrices were constructed using peak height values prior to further analysis.
Phylogenetic inference
BLASTN software was used to orientate the identification of the sequenced DGGE bands. Then, Mega 11 software was utilised with imported sequences which were aligned to reference cyanobacterial sequences. Maximum likelihood analysis was undertaken to determine the best fit substitution model for phylogenetic inference, which was followed by bootstrap analysis (500 replicates). The Gram-positive species Clostridium lavalense (strain CCRI-9929, GenBank entry: EF564278) (Domingo et al., 2009) was selected as out-group for rooting the phylogenetic tree.
Real time PCR analysis of cyanobacteria in environmental samples
The quantification of 16S rRNA and mcyE gene copies was conducted utilising Multiplex quantitative PCR (qPCR) Phytoxigene™ CyanoDTec kits with primers specific to cyanobacteria following the manufacturer’s instructions. Sample selection was determined based on the presence of potentially toxin producing cyanobacterial species as per the band sequencing analysis. The master mix was resuspended in 80 µl of PCR grade water and vortexed for 10 s. The resuspended master mix was added to individual wells of a Microamp Fast 96 well reaction plate (Applied Biosystems) at a volume of 20 µl. 5 µl of sample or standards were added to individual wells. The plate was sealed and spun at 25 rpm for 10 s, then placed in an Applied biosystems 7500 Fast Real Time PCR Instrument. HID Real Time Analysis was utilised for data analysis. The amplification conditions were as follows, an initial denaturation step (95°C, 2 min) followed by 40 cycles of amplification (denaturation) (95°C, 0.25 min), annealing (optics on) (60°C, 30 s). Ct values were imported upon completion and interpolation was utilised from the standard curve to determine gene copies in environmental samples for each target gene.
ELISA for the determination of cyanotoxins in environmental samples
Microcystin concentrations in extracts were determined utilising the Microcystins-ADDA Enzyme-linked Immunosorbent Assay (Catalog No. ALX-850-319, ENZO Scientific, Switzerland). The assay is based on an indirect competitive ELISA format for the congener independent detection of microcystins using specific antibodies. The assay generates a colour signal in which intensity is inversely proportional to the concentration of microcystins present in the sample. This colour signal is evaluated utilising a microplate reader at 450 nm. Toxin concentration was estimated by interpolation using a standard curve.
Extracts were obtained by placing the Whatmann filters in 2 ml of 100% methanol with mechanical disruption via bead beating in a Fastprep FP120 cell disruptor. The homogenates were then passed through a 3 ml syringe fitted with a 0.45 \(\upmu\)m pore size filter. The filtrate solution was then evaporated to dryness using a stream of nitrogen gas and stored at − 20°C. Prior to analysis, samples were resuspended in 50 µl of 50% methanol and diluted in deionised water as necessary. The assay was carried out according to the manufacturer’s guidelines in 96-well plates prior to absorbance measurement with an iMark™ Microplate Absorbance Reader.
Data treatment and analysis
Statistical analysis was conducted using IBM SPSS 26. Data collected was tested for homoscedasticity and normality to meet the assumptions of the tests applied. A value of α = 0.05 was used to denote significance with mean and standard deviation reported as appropriate. Significant differences in spatial and temporal variation in environmental and biological parameters recorded in Lough Arrow were determined utilising Kruskal–Wallis tests. Post hoc Mann–Whitney pairwise multiple comparisons adjusted with Bonferroni correction were utilised to determine individual differences where a significant difference was observed. Spearman correlation analysis was undertaken where appropriate to identify patterns between variables.
Results
Secchi depth and meteorological data
The Secchi depth across all sampling sites over the course of the sampling period ranged from 3.3 (site 4, 09/08/19) to 5.8 m (site 4, 06/08/19) with an average of 4.2 m (± 0.48) across all sites. Individual sites did not significantly differ in Secchi depth over the course of the survey (P > 0.05). Furthermore, there was also no significant difference spatially between sites on any of the sampling dates (P > 0.05).
Mean wind speed in the region fluctuated between 8.32 and 30.41 km/h with an average of 16.03 (± 5.1) Km/h for the period (Supplementary File 1). In comparison of the first five sampling occasions which occurred in July and the final five which occurred in August, there were no significant differences in mean wind speed (P > 0.0.05) with averages of 17.21 and 14.90 km/h respectively.
Daily sunshine hours fluctuated from 0.0 to 8.90 with an average of 2.98 (± 2.47) h of daily sunshine over the sampling period (measured via a Cambell-Strokes recorder). The sunshine hours occurring between the July and August sampling periods did not significantly differ (P > 0.05) with an average of 2.20 h for July and 3.67 h for August.
Nutrient analysis
Nutrient concentrations were low for nitrate NO3–N, ammonium NH4–N and phosphate PO4–P both spatially and temporally during the survey. The concentrations were at the lower limit of the LCK kits threshold for each parameter. The maximum recorded ammonium NH4–N concentration was 0.068 mg/l, with the majority of measurements being less than 0.015 mg/l. A similar pattern was observed with nitrate NO3–N concentrations, the majority being < 0.23 mg/l. Phosphate PO4–P levels did not exceed 0.05 mg/l during the survey.
Water column profiling
Water temperature ranged from 15.30 to 19.10°C. The average temperature of the water column across the five sites varied significantly both temporally and spatially (P < 0.05) (Fig. 2). The minimum temperatures at the beginning of the survey were lower in comparison to the minimum temperatures recorded towards the end of the survey (range: 15.30–17.60°C). In contrast, the maximum temperatures were less variable throughout the sampling period (range: 18.10–19.10°C). Greater homogeneity of the water column was apparent at the end of the survey.
A similar trend was identified for conductivity in the water column profiles, which differed significantly over time (P < 0.05). The average conductivity readings ranged from 232.43 to 238.69 µS/cm. Site comparisons on individual sampling days showed that conductivity measurements differed significantly, revealing spatial patterns (P < 0.05).
In contrast to temperature and conductivity, dissolved oxygen concentrations showed limited variation between sites (P > 0.05). However, significant differences occurred over time (P < 0.05).
Significant variations were identified for pigment concentrations for all individual sites over time (P < 0.05). As such, higher average and maximum pigment concentrations were observed towards the end of the survey. For instance, average chlorophyll-a concentrations observed across all sites ranged from 4.23 to 5.91 µg/l with the lower average concentration occurring on the 22/07/19 and the highest concentration occurring on the 09/08/19 (7.48 µg/l).
A similar trend was observed for phycocyanin with the lowest average concentration occurring on the 19/07/19 (2.03 µg/l) and the highest on the 09/08/19 (3.64 µg/l). This pattern was also observed for phycoerythrin. Peak average concentrations occurred on the 09/08/19 (3.81 µg/l) while the lowest averages were on the 19/07/19 (2.43 µg/l). The patterns of gradual increase in the concentrations of the monitored pigments over time are visualised in Fig. 3.
Molecular profiling of cyanobacteria
DGGE analysis of 16S rRNA gene amplicons encompassed sites 1 (north) & 5 (south) at depths 1 (surface) and 4 (near lakebed) for all sampling dates (Fig. 4). A greater focus was then placed on the samples collected on 15/08/19 due to the observed lake wide increases in photosynthetic pigments (particularly phycocyanin) observed towards the end of the survey (Fig. 4).
DGGE gel derived migration patterns and relative intensities (proxy for abundance) of individual bands occurring in Lough Arrow during the summer of 2019. Site 1 and site 5 (D1–10) for depths 1 (D1) (surface, left) and 4 (D4) (near lake bed, right). The bottom panel focuses on the 15/08/19, encompassing all sites (1–5) and depths (1–4) on that sampling occasion
Initial PCR analysis and rapid light microscopy based screening of water samples indicated that filamentous cyanobacteria were not present. However, non-filamentous cyanobacteria were successfully amplified.
Cyanobacterial abundance and richness varied between sites 1 and 5 both spatially and temporally. At site 1, up to 5 bands were detected. The surface samples showed on average 2.6 (± 0.9) bands per sampling occasion while the 10 m deep sample had on average 3.4 (± 1.1) bands occurring.
Greater richness was observed at site 5, with up to 10 bands being detected. Overall, there were on average 3.6 bands (± 1.1) at the 13 m sampling depth and 3.0 bands (± 1.1) at the surface.
The DGGE gel for the samples collected on 15/08/19 showed greater richness (14 bands), which was highest within the euphotic zone between 0 and 5 m depth, with an average of 7.4 (± 1.5) bands. Overall, no significant difference in richness was observed when comparing all depths and all sites (P > 0.05).
Band sequencing and phylogenetic inference
Successfully excised and sequenced DGGE bands were initially screened in BLASTN NCBI database to identify potential matches. Their positioning in the 16S rDNA cyanobacterial phylogeny was ascertained from maximum likelihood analysis with a best fit substitution model determined in Mega 11, which returned a phylogenetic tree (Fig. 5). This identified the excised bands from reference lanes 4, 5, 6, and 9, which occurred on 15/08/19 to be closely related to the hepatotoxin-producing species Microcystis sp.
Detection of mcyE gene and microcystin concentration estimation in 15/08/19 samples
On 15/08/19, no spatial pattern in DGGE band intensity was identified for the potentially toxic phylotypes (RLs 4, 5, 6, 9) across all sites and depths (P > 0.05).
Analysis by qPCR of the mcyE gene in the DNA extracts showed positive detection in the 15/08/19 samples (N = 10) in Lough Arrow, both at depths 1 and 4. The mcyE gene copy number peaked at depth 1 of site 5 (63,210 copies) and was the lowest at site 2 depth 4 (14 m) (106 copies). Although mcyE gene copy numbers appeared more elevated near the surface, this distribution pattern was not statistically supported between depths 1 and 4 across all sites (P > 0.05).
Likewise, the ELISA-based analysis of microcystin toxins in the samples followed a similar pattern to the abundance of the sequenced Microcystis sp. bands and the estimated mcyE gene copy numbers. Microcystin concentrations were overall very low (maximum concentration of 0.087 µg/l). Concentrations between depths 1 and 4 did not vary significantly (P > 0.05) (N = 10).
Discussion
Cyanobacteria can have a profound impact on their environment due to their bloom forming capacity (Izydorczyk et al., 2005). Their biomass and community composition can be inconstant both spatially and temporally. Analytical approaches which characterise this variability are important (Zamyadi et al., 2016). However, in Ireland studies characterising these factors are limited, more so studies describing toxicity potential and synthesis. Given the adverse impacts these members of the plankton community can impart on their respective biotope in terms of environmental health and ecosystem service delivery, it is important to describe their dynamics in an Irish context.
Lough Arrow, a renowned trout fishery protected under multiple EU directives and used locally as a resource and amenity, has been cited as under pressure from nutrient pollution (Roden & Murphy, 2020) with cyanobacterial blooms being recorded in the past. This study attempted to delineate patterns in cyanobacterial activity both spatially and temporally over the course of mid to late summer 2019, identified toxicity potential and production occurring in the absence of a bloom. Thus providing a baseline which can be utilised to inform future conservation measures in the lough and provide a reference point for cyanobacterial dynamics in Irish lakes.
16S rRNA gene based cyanobacterial diversity and associated environmental variables
Analysis into the diversity of cyanobacteria in Lough Arrow via 16S rRNA gene DGGE analysis revealed both spatial and temporal changes, highlighting dynamic ecological processes.
Variation in time as determined via DGGE band intensity (proxy for abundance) and occurrence was observed at both sites 1 (north) and 5 (south), indicating shifts in cyanobacteria community composition in the lake. Furthermore, the relative intensity of individual bands also changed over time between the surface and bottom depths sampled at each site, potentially indicating vertical migration in the water column.
The 15/08/19 samples were further focused upon based on the higher concentrations of phycocyanin measured near the end of the survey. The community of spherical cyanobacteria was profiled by DGGE fingerprinting at the multiple stations and depths on this date, revealing an increase in diversity (14 phylotypes detected), which was in line with previous results (Touzet et al., 2016). This increase in diversity may represent a bias associated with an increase in the spatial resolution of sampling in the lake on the day. However, no significant differences in richness were observed between sites and depths on this occasion, indicating homogeneity across the lake.
Fluorometric probes were utilised to monitor in real time photochemically active pigments associated with cyanobacteria, including chlorophyll a, phycocyanin and phycoerythrin. Phycocyanin in particular is often used to estimate the in situ density of cyanobacteria (Ahn et al., 2007; Izydorczyk et al., 2005; Zamyadi et al., 2016). Significant changes were observed over time in the pigment concentrations measured in Lough Arrow. These typically increased over time, peaking towards the end of the survey. The pigment profiles at this time indicated homogeneity throughout the lake, which may have been partly attributed to the synergistic influences of extrinsic environmental factors. Lough Arrow is a well exposed lake with a significant fetch due to its hydromorphology. Wind speed is often cited as a driver of plankton distribution in lakes, with greater wind speeds deemed less suitable for cyanobacterial proliferations (Blottiere, 2015). In aquatic environments, the majority of cyanobacteria can deploy passive and active mechanisms to control their positioning in the water column (Oren, 2014). These attributes are particularly useful for their prevalence when stratification develops (Carey et al., 2012; Mantzouki et al., 2016). Data obtained from a weather station in the region identified that wind speeds were stable throughout the sampling period, indicating that mixing conditions did not significantly shift. However, average wind speeds were in excess of 10.78 km/h, which are indicative of conditions less suitable for cyanobacterial proliferation (Blottiere, 2015).
Mixing can also potentially increase water turbidity, thereby hindering algal growth and potentially resuspending nutrients (Blottiere, 2015). That was not observed in this study, as supported by Secchi depth measurements. Nutrient levels also remained low throughout the survey (which was corroborated by state agency data for statutory WFD monitoring) (Environmental Protection Agency, 2023), even though the cyanobacterial biomass, estimated via phycocyanin, progressively increased.
Irradiance, another driver behind the growth rate and proliferation of plankton (Edwin et al., 2007) was also indirectly monitored via sunshine hours. No significant difference in sunshine hours occurred over the course of the sampling period. However, it must be noted that optimum intensity and duration of irradiance is species-specific and highly variable in cyanobacteria (Havens et al., 1998) as some species can tolerate low underwater irradiance (Havens et al., 2003) while other species require sufficient irradiation to prosper in their environment (Havens et al., 2003).
Temperature, which is another important environmental variable for cyanobacterial growth (Robarts & Zohary, 1987) fluctuated in Lough Arrow throughout the sampling period providing some indication in regards to the mixing patterns occurring in the lakes water column. Maximum recorded temperatures in the lake remained stable throughout the survey. In contrast, the average minimum recorded temperature fluctuated between July and August with a notable average increase from 15.9 to 17.4°C with limited variation between surface and lakebed recordings in August, indicating a progressively warming homogeneous water column, leading to temperatures as high as 18.5°C being recorded at depths of 14 m in August. Given that temperature increases of 2°C have been cited as sufficient to significantly promote the growth of certain cyanobacterial species (Blottiere, 2015), their proliferation may have been hampered in the lake by other factors.
Overall, the observed changes in photosynthetic pigments associated with cyanobacteria and the corresponding temporal patterns in environmental variables during the 2019 summer assessment indicate an interplay of multiple factors influencing cyanobacterial growth and abundance in the lough.
Microcystin toxin production potential
Sequencing and phylogenetic analyses confirmed the presence of potentially toxic species linked to Microcystis sp. (RLs 4, 5, 6 and 9) on the 15/08/19, with no differences in their individual band intensities occurring across all sites, indicating their homogenous distribution and abundance in Lough Arrow on the day. Their presence coincided with warmer conditions, which is well aligned with this taxonomic groups competitive success (Deutsch et al., 2020). Microcystis sp. have the potential to synthesise microcystins, noxious compounds that can elicit multiple toxicity endpoints on various organisms (Svirčev et al., 2019). However, the presence of Microcystis sp. does not directly indicate if toxin-producing strains are present or whether or not the toxins are expressed. Toxigenicity potential was determined through qPCR analysis of the mcyE microcystin synthesis gene (Davis et al., 2009). A similar pattern to the peak height intensities retrieved from the DGGE gel was observed here, indicating spatially homogenous Microcystis sp. forms capable of producing microcystins throughout the lake on the 15/08/19.
Direct measurement of microcystin concentrations in Lough Arrow indicated that the distribution of microcystins followed a similar pattern spatially to both the band intensities of Microcystis sp. phylotypes and the mcyE gene copy numbers estimated in the DNA extracts. However, microcystin concentration values were low (max conc. 0.087 µg/l), falling well below the risk threshold recommended by the World Health Organisation (1 μg/l) for the prevalent microcystin congener microcystin-LR (Bláha et al., 2009; Carmichael & Boyer, 2016).
Implications for water quality monitoring
Historically, many Irish lakes have been exposed to the adverse consequences of cyanobacterial blooms. Lough Arrow is no different having been subjected to cyanobacterial blooms in the past. However, Irish lakes are poorly studied in regards to cyanobacterial dynamics with few studies describing spatial and temporal dynamics collectively. Furthermore, toxicity potential and synthesis are poorly characterised in an Irish context. Cyanobacteria in Irish lakes are monitored under the Water Framework Directive (2000/60/EC) as a smaller element of a plankton assessment. Direct measurement of toxicity production and potential are not utilised in the assessment. Furthermore, the sampling intensity deployed may be insufficient in identifying cyanobacterial blooms as some lakes are only sampled four times per year.
Regardless, it is generally accepted that informed conservation and resource management is considered pivotal in mitigating the potential adverse consequences of cyanobacterial blooms. Effective monitoring systems are important but can also be limited due to costs and feasibility, restricting the spatial and temporal resolution of in situ surveys and the suite of analytical tools subsequently used (Bertani et al., 2017). This study highlights the importance of adequate monitoring as Microcystis sp. with toxicity potential were actively synthesising cyanotoxins in the lake in the absence of an algal bloom and perceived low nutrient concentrations. Cyanobacteria, such as Microcystis sp. have the capacity to form high-density cyanobacterial blooms quickly and contemporary anthropogenic pressures may lead to transient eutrophication-like events through nutrient enrichment, thus promoting the development of cyanobacterial blooms that may impart adverse consequences to the lake.
Conclusion
In Lough Arrow, an increase in cyanobacterial abundance was observed during a 30-day survey in summer 2019, as indicated from DGGE analysis and pigment concentrations in the water column. This coincided with marked increases in the minimal temperatures recorded. However, no bloom occurred, which was likely due to wind-driven mixing, suboptimal temperatures and low nutrient concentrations at the time. Microcystis sp., a cyanobacterial genus with bloom forming and cyanotoxin-producing capacities, was nevertheless detected together with low levels of microcystins. Increasing summer temperatures in Lough Arrow and other temperate lakes in Europe due to forecasted global climate change may facilitate proliferation events in the future. This study strengthens the rationale for the necessity of a tailored environmental monitoring strategy in lakes dependent on ecosystem services to potentially prevent and mitigate public health risks associated with the occurrence of toxigenic cyanobacteria.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available online but are available from the corresponding author on reasonable request.
References
Ahn, C., S. Joung, S. Yoon & H. Oh, 2007. Alternative alert system for cyanobacterial bloom, using phycocyanin as a level determinant. The Journal of Microbiology 45(2):98–104.
Ahn, C., C. Park, H. Kim, B. Yoon & H. Oh, 2008. Comparison of sampling and analytical methods for monitoring of cyanobacteria-dominated surface waters. Hydrobiologia 596(1): 413–421. https://doi.org/10.1007/s10750-007-9125-y.
Backer, L., 2002. Cyanobacterial harmful algal blooms (CyanoHABs): developing a public health response. Lake and Reservoir Management 18: 20–31. https://doi.org/10.1080/07438140209353926.
Baker, J., B. Entsch, B. Neilan & D. McKay, 2002. Monitoring changing toxigenicity of a cyanobacterial bloom by molecular methods. Applied and Environmental Microbiology 68: 6070–6076. https://doi.org/10.1128/AEM.68.12.6070-6076.2002.
Bastien, C., R. Cardin, É. Veilleux, C. Deblois, A. Warren & I. Laurion, 2011. Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. Journal of Environmental Monitoring 13: 110–118. https://doi.org/10.1039/C0EM00366B.
Bertani, I., C. Steger, D. Obenour, G. Fahnenstiel, T. Bridgeman, T. Johengen, M. Sayers, R. Shuchman & D. Scavia, 2017. Tracking cyanobacteria blooms: do different monitoring approaches tell the same story? Science of the Total Environment 575: 294–308. https://doi.org/10.1016/j.scitotenv.2016.10.023.
Bláha, L., P. Babica & B. Marsalek, 2009. Toxins produced in cyanobacterial water blooms—toxicity and risks. Toxicology 2(2): 36–41. https://doi.org/10.2478/v10102-009-0006-2.
Blindow, I., G. Anderson, A. Hargeby & S. Johansson, 1993. Long term pattern of alternative stable states in two shallow eutrophic lakes. Journal of Freshwater Biology 30: 159–167.
Blottiere, L., 2015. The effects of wind-induced mixing on the structure and functioning of shallow freshwater lakes in a context of global change. Université Paris Saclay, https://tel.archives-ouvertes.fr/tel-01258843/document. Accessed 23 March 2023.
Brand, L., 2009. Human exposure to cyanobacteria and BMAA. Amyotrophic Lateral Sclerosis 10: 85–95. https://doi.org/10.3109/17482960903273585.
Brand, L., J. Pablo, A. Compton, N. Hammerschlag & D. Mash, 2010. Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 9(1): 620–635.
Caldwell Eldridge, S., C. Driscoll & T. Dreher, 2017. Using High-Throughput DNA Sequencing, Genetic Fingerprinting, and Quantitative PCR as Tools for Monitoring Bloom-Forming and Toxigenic Cyanobacteria in Upper Klamath Lake, Oregon, 2013 and 2014. Scientific Investigations Report. U.S Department of the Interior Reston, Virginia.
Carey, C., B. Ibelings, E. Hoffmann, D. Hamilton & J. Brookes, 2012. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Research 46: 1394–1407. https://doi.org/10.1016/j.watres.2011.12.016.
Carmichael, W. & G. Boyer, 2016. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 54(1): 194–212.
Carvalho, L., E. Mackay, A. Cardoso, A. Baattrup-Pedersen, S. Birk, K. Blackstock, G. Borics, A. Borja, C. Feld, M. Ferreira, L. Globevnik, B. Grizzetti, S. Hendry, D. Hering, M. Kelly, S. Langaas, K. Meissner, Y. Panagopoulos, E. Penning, J. Rouillard, S. Sabater, U. Schmedtje, B. Spears, M. Venohr, W. van de Bund & A. Solheim, 2019. Protecting and restoring Europe’s waters: an analysis of the future development needs of the Water Framework Directive. Science of the Total Environment 658: 1228–1238.
Chorus, I. & J. Bartham, 2005. Toxic Cyanobacteria in Water, 2nd ed. E & F Spoon, London:
Davis, T., D. Berry, G. Boyer & C. Gobler, 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8: 715–725. https://doi.org/10.1016/j.hal.2009.02.004.
Deutsch, E., I. Alameddine & S. Qian, 2020. Using structural equation modeling to better understand microcystis biovolume dynamics in a mediterranean hypereutrophic reservoir. Ecological Modelling 435: 109282.
Domingo, M.-C., A. Huletsky, M. Boissinot, M.-C. Helie, A. Bernal, K. A. Bernard, M. L. Grayson, F. J. Picard & M. G. Bergeron, 2009. Clostridium lavalense sp. nov., a glycopeptide-resistant species isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology 59: 498–503. https://doi.org/10.1099/ijs.0.001958-0.
Edwin, W., A. Kardinaal, L. Tonk, I. Janse, S. Hol, P. Slot, J. Huisman & P. Visser, 2007. Competition for light between toxic and nontoxic strains of the harmful cyanobacterium microcystis. Applied and Environmental, Microbiology 73(9): 2939–2946.
Environmental Protection Agency, 2023. Lough Arrow Water Framework Directive chemistry data. https://www.catchments.ie/data/?_gl=1*1d6huh2*_ga*MTYxNDc3NTQyNS4xNjg4OTA4MzUw*_ga_TPK2CK9KEX*MTY5MjI2MTMwNS4zMS4xLjE2OTIyNjEzMTUuMC4wLjA.#/waterbody/IE_WE_35_159?_k=41mmu5. Accessed 22 June 2023.
Havens, K., E. Phlips, M. Cichra & B. Li, 1998. Light availability as a possible regulator of cyanobacteria species composition in a shallow subtropical lake. Freshwater Biology 39: 547–556. https://doi.org/10.1046/j.1365-2427.1998.00308.x.
Havens, K., R. James, T. East & V. Smith, 2003. N:P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environmental Pollution. 122(3): 379–390.
Izydorczyk, K., M. Tarczynska, T. Jurczak, J. Mrowczynski & M. Zalewski, 2005. Measurement of phycocyanin fluorescenceas an online early warning system for cyanobacteria in reservoir intake water. Environmental Toxicology 20: 425–430. https://doi.org/10.1002/tox.20128.
Jančula, D. & B. Maršálek, 2011. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85: 1415–1422. https://doi.org/10.1016/j.chemosphere.2011.08.036.
Johnk, K., J. Huisman, J. Sharples, B. Sommeijer, P. Visser & A. Strooms, 2008. Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biology 14(3): 495–512.
Le Vu, B., B. Vinçon-Leite, B. Lemaire, N. Bensoussan, M. Calzas, C. Drezen, J. Deroubaix, N. Escoffier, Y. Dégrés, C. Freissinet, A. Groleau, J. Humbert, G. Paolini, F. Prévot, C. Quiblier, E. Rioust & B. Tassin, 2011. High-frequency monitoring of phytoplankton dynamics within the European water framework directive: application to metalimnetic cyanobacteria. Biogeochemistry 106: 229–242. https://doi.org/10.1007/s10533-010-9446-1.
Lileikyte, D. & O. Belous, 2011. Water quality evaluation: toxic cyanobacteria in surface water. Environmental Research, Engineering and Management 1(55): 43–48.
Lucy, F., T. Graczyk & M. Connolly, 2009. Lake Risk Assessment for Cryptosporidium and Other Human Enteric Pathogens in Lough Arrow, Counties Sligo and Roscommon, Environmental Protection Agency, Wexford, Ireland:
Mantzouki, E., P. Visser, M. Bormans & B. Ibelings, 2016. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquatic Ecology 50: 333–350. https://doi.org/10.1007/s10452-015-9526-3.
Moreira, C., V. Ramos, J. Azevedo & V. Vasconcelos, 2014. Methods to detect cyanobacteria and their toxins in the environment. Applied Microbiology and Biotechnology 98: 8073–8082. https://doi.org/10.1007/s00253-014-5951-9.
National Parks and Wildlife Service, 2014. Site Synopsis: Lough Arrow SPA (0004050). Department of Arts, Heritage and the Gaeltacht, Dublin, Ireland.
National Parks and Wildlife Service, 2015. Natura 2000 Standard Data Form: Lough Arrow SAC (001673). Department of Arts, Heritage and the Gaeltacht, Dublin, Ireland.
Oren, A., 2014. Cyanobacteria: Biology, Ecology and Evolution. In Sharma, N., A. Rai & L. Stal (eds), Cyanobacteria: An Economic Perspective 1st ed. Wiley, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK.
Ouellette, A. & S. Wilhelm, 2003. Toxic cyanobacteria: the evolving molecular toolbox. Frontiers in Ecology and the Environment 1(7): 359–366.
Pobel, D., J. Robin & J. Humbert, 2011. Influence of sampling strategies on the monitoring of cyanobacteria in shallow lakes: Lessons from a case study in France. Water Research 45(3): 1005–1014.
Quiblier, C., W. Susanna, E.-S. Isidora, H. Mark, V. Aurélie & H. Jean-François, 2013. A review of current knowledge on toxic benthic freshwater cyanobacteria—ecology, toxin production and risk management. Water Research 47: 5464–5479. https://doi.org/10.1016/j.watres.2013.06.042.
Robarts, R. & T. Zohary, 1987. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom forming cyanobacteria. New Zealand Journal of Marine and Freshwater Research. 21(3): 391–399.
Roden, C., & P. Murphy, 2020. Sub littoral vegetation of Lough Arrow in 2019. Report to the INTERREG VA CANN project. Sligo, Ireland, p. 20.
Sanseverino, I., D, Conduto, L. Pozzoli, S Dobricic & T. Lettieri, 2016. Algal bloom and its economic impact. European Commission. Joint Research Centre, Publications Office, LU.
Sellner, K., G. Doucette & G. Kirkpatrick, 2003. Harmful algal blooms: causes, impacts and detection. Journal of Industrial Microbiology and Biotechnology 30: 383–406. https://doi.org/10.1007/s10295-003-0074-9.
Sivonen, K., 2009. Cyanobacterial Toxins. Encyclopaedia of Microbiology, 1st ed. Elsevier, Amsterdam.
Svirčev, Z., D. Lalić, G. Bojadžija Savić, N. Tokodi, D. Drobac Backović, L. Chen, J. Meriluoto & G. Codd, 2019. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of Toxicology 93: 2429–2481. https://doi.org/10.1007/s00204-019-02524-4.
Touzet, N., D. McCarthy, G. Fleming, 2013. Molecular fingerprinting of lacustrian cyanobacterial communities: regional patterns in summer diversity. FEMS Microbiol. Ecol. 86, 444–457. https://doi.org/10.1111/1574-6941.12172.
Touzet, N., D. McCarthy, A. Gill & G. Fleming, 2016. Comparative summer dynamics of surface cyanobacterial communities in two connected lakes from the west of Ireland. Science of the Total Environment 553: 416–428.
Wood, R., 2016. Acute animal and human poisonings from cyanotoxin exposure—a review of the literature. Environment International 91: 276–282. https://doi.org/10.1016/j.envint.2016.02.026.
Zamyadi, A., F. Choo, G. Newcombe, R. Stuetz & R. K. Henderson, 2016. A review of monitoring technologies for real-time management of cyanobacteria: recent advances and future direction. TrAC Trends in Analytical Chemistry 85: 83–96. https://doi.org/10.1016/j.trac.2016.06.023.
Funding
Funding for this Research was provided to the Atlantic Technological University, Sligo by the INTERREG VA programme, for the Collaborative Actions for the Natura Network project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The research was conceptualised by DG and NT. The experiment was designed by DG and NT. Field sampling preparation and in situ data collection was carried out by DG. Analytical sample processing was carried out by DG. Figures for the purposes of this manuscript were prepared by DG. Data analysis and interpretation was carried out by DG and reviewed by NT. The original draft manuscript was prepared by DG, reviewed by NT and subsequently edited by both NT and DG. FL was a reviewing author during this process. All authors contributed to the final submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Handling editor: Luigi Naselli-Flores
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garland, D., Lucy, F. & Touzet, N. Summer dynamics of cyanobacteria in an oligo-mesotrophic temperate lake in Northwest Ireland. Hydrobiologia 850, 4327–4341 (2023). https://doi.org/10.1007/s10750-023-05307-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05307-2