Abstract
Anthropogenic salinisation of inland waters worldwide causes diverse social, economic and ecological impacts, including shifts in the composition of biological communities. I used published data on occurrence of aquatic invertebrate taxa in relation to salinity to develop a novel Invertebrate-Based Salinity Index (IBSI) suitable for purposes such as indicating the ecological impact of anthropogenic salinisation and charting faunal recovery after salinity mitigation. Testing of the index with data independent of those used in its derivation showed a stronger association with salinity than prior Australian salinity indices based on macroinvertebrates. IBSI is applicable to both running and standing inland waters across Australia, and could be extended to other countries. Potential limitations of IBSI and its underlying salinity tolerance values are discussed, and suggestions are made for future index refinement and testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unnatural elevation of the salt content of inland waters, or secondary salinisation, results from a variety of human influences including changes in land cover and land use, irrigation, wastewater disposal, anthropogenic climate change and the application of salts to roads for de-icing (Williams, 2001; Herbert et al., 2015). Salinisation has many social, economic and ecological consequences, including shifts in the composition of aquatic biological communities resulting from the highly variable ion requirements and tolerances of individual species and their life-history stages (Hintz & Relyea, 2019; Cañedo-Argüelles, 2020). Secondary salinisation is a worldwide problem (Cañedo-Argüelles et al., 2013; Herbert et al., 2015; Thorslund et al., 2021) that is especially acute in parts of Australia (Peck & Hatton, 2003; Timms, 2005; Rengasamy, 2006). Most notably, extensive land clearing for agricultural production in the south-west of Western Australia has resulted in greater penetration of rainfall to groundwater and consequent rises in water tables, mobilising salt stored in saline groundwater and the soil profile. These processes have led to greater accession of saline groundwater to surface water bodies, causing local and regional decline and extinction of species with lower salinity tolerances (Halse et al., 2003; Pinder et al., 2005).
The salinisation of water bodies can be assessed directly by chemical analysis of ion concentrations, or else by estimating salinity from gravimetric determination of total dissolved solids (TDS) or physical measurement of electrical conductivity (EC). Alternatively, salinity and its ecological impacts can be inferred from the structure and composition of biological assemblages. For example, the occurrence of fossil remains of organisms such as diatoms in lake sediments can be used to reconstruct past salinity regimes via transfer functions based on taxon-specific salinity associations (e.g., Fritz et al., 1991; Tibby & Reid, 2004). Indices can also be applied to data on contemporary biological assemblages to infer recent salinity exposure and impacts (Williams et al., 1999; Ziemann & Schulz, 2011; Stenger-Kovács et al., 2018). Invertebrate assemblages are appropriate for this purpose because of the wide range of salt tolerances within the diverse invertebrate fauna of inland waters (Zinchenko & Golovatyuk, 2013; Pickwell et al., 2022).
Three salinity-oriented invertebrate indices have been developed specifically for Australian application. Initially, Horrigan et al. (2005) calculated a Salinity Index (SI) as an unweighted average of ‘salinity sensitivity scores’ (SSSs) of invertebrate taxa present in a sample. Sensitivity was assessed by statistical analysis of field data from the state of Queensland. Subsequently, Horrigan et al. (2007) developed an Acute Salinity Index (ASI), similar to the SI but based on ‘acute tolerance scores’ (ATSs) derived from laboratory toxicity testing rather than field data. Horrigan et al. (2007) also provided further SSSs, as well as ‘suggested generalised scores’ (SGSs) based on both field and laboratory data. More recently, Schäfer et al. (2011) developed a salinity-oriented version of the SPEAR (SPEcies At Risk) index, named SPEARsalinity, for the states of South Australia and Victoria. This index is described as a ‘trait-based indicator for the impact of salinisation on south-east Australian streams’, and is based on a binary classification of aquatic macroinvertebrate taxa as either ‘at risk’ or ‘not at risk’ from salinisation. Taxa were initially classified as ‘at risk’ or ‘not at risk’ on the basis of four variables: ‘food source’, ‘reproduction type’, ‘respiration mode’ and ‘physiological sensitivity to salinity’. However, after testing of index versions based on various combinations of these variables, only the last was retained.
These indices contribute to the toolset available for salinity monitoring and assessment in Australia, but their use is problematic because of uncertain assignment of many taxa to the tolerance categories that they incorporate. Horrigan et al. (2005, 2007) provide SSS and/or ATS and/or SGS values for only 70 taxa, mostly families, and in some cases the various scores for the same family span the entire range of 1–10. Schäfer et al. (2011) include 172 taxa, again mostly families, but do not specify which of these taxa they classified as ‘at risk’ and ‘not at risk’. Instead, they tabulate taxon-specific ‘physiol. sensitivity to salinity’ in supplementary information. However, the table entries are frequently ambiguous with respect to their criterion for classifying a taxon as ‘at risk’, which is ‘majority of taxa in family with 72 h LC50 < 35 mS cm−1.’ For example, the entry may be simply a category (e.g., ‘low’), a category range (e.g., ‘low-medium’), a range of EC values that overlaps the criterion threshold (e.g., 15–60 mS cm−1), or a censored EC value that could signify an LC50 either above or below the threshold (e.g., > 13.5 mS cm−1).
In addition, the ability of the SI, the ASI and SPEARsalinity to indicate salinity appears to be only moderate. Horrigan et al. (2007) calculated coefficients of determination (R2) for non-linear regressions of the SI and ASI on EC of 0.33 and 0.36 respectively for Queensland streams, and Schäfer et al. (2011: Supplementary Information) reported R2 values for regression of the SI and SPEARsalinity on the logarithm of EC for Victorian and South Australian streams of 0.38–0.53 and 0.11–0.50, respectively. These R2 values are little different from values obtained using the SIGNAL (Stream Invertebrate Grade Number—Average Level) index, which is an invertebrate-based indicator of general physical and chemical contamination or enrichment, and not intended to the specific to salinity or any other particular stressor (Chessman, 2003). For example, R2 values for the relationship between SIGNAL and stream EC have been reported as 0.30 (from Pearson correlation of -0.55) Australia-wide (Chessman, 2003) and 0.30–0.57 in Victoria (Schäfer et al., 2011: Supplementary Information). Similarly, Kath et al. (2018) found that proportional variation in SPEARsalinity in relation to variation in EC in Victorian streams was equivalent to proportional variation in SIGNAL and less than proportional variation in EPT (Ephemeroptera, Plecoptera and Trichoptera) richness, another non-stressor-specific invertebrate-based indicator.
Salinity indices based on invertebrates have also been developed elsewhere than Australia. Such indices include the Canadian Chloride Contamination Index (CCI: Williams et al., 1999), the German weighted average salinity (xj: Wolf et al., 2009) and the British Salinity Association Group Index (SAGI: Pickwell et al., 2022). However, these indices are difficult to apply in Australia because available information is insufficient to assign most Australian aquatic invertebrate taxa to the salinity-association, salinity-preference and salinity-tolerance categories that they incorporate.
Considering the limitations of existing salinity indices based on invertebrates for Australian application, I developed a new index using published information on the invertebrate fauna of both standing and running inland waters of the whole Australian continent. My aim was to provide an alternative to the existing Australian indices that may be more responsive to salinity variation and therefore better suited to purposes such as indicating the ecological impact of anthropogenic salinisation and charting faunal recovery after salinity mitigation. I also tested the performance of the new index with data independent of those used in its derivation.
Methods
Index development
I developed an Invertebrate-Based Salinity Index (IBSI), incorporating taxon-specific salinity tolerance, indicator strength (importance or value as an indicator of salinity), and abundance. The index score for an invertebrate sample is calculated by the following equation:
where Ti is the maximum salinity tolerance of the ith of n taxa present in the sample, Si is the indicator strength of the ith taxon, and Ai is a measure of the abundance of the ith taxon. The index is thus an average of the tolerances of the taxa present in the sample, weighted by indicator strength and taxon abundance. If only presence/absence data are available, Ai can be omitted.
I estimated taxon-specific values of T and S by compiling raw data from 118 publicly available journal articles, reports and theses that reported occurrences or abundances of invertebrate taxa in Australian inland waters together with spatially and temporally coincident values of salinity, EC or TDS (Supplementary Information A). All invertebrate life-history stages were included except ephippia and dead individuals. Taxon names were corrected for spelling errors where applicable and updated where necessary to current taxonomy, generally following the Australian Faunal Directory (https://biodiversity.org.au/afd/home). Suspect identifications such as taxa recorded well outside of their accepted geographic ranges were downgraded to a plausible higher-level taxon. Obvious physical and chemical data errors were also corrected: for example, EC values reported in mS cm−1 that were obviously actually measured in μS cm−1 given their range and TDS values in the same article.
EC values (in μS cm−1) were converted to approximate salinity values (in mg l−1) by multiplying the former by 0.7, the factor reported by Walton (1989) for sea water. TDS values (in mg l−1) were converted to approximate salinity values (in mg l−1) by multiplying the former by 0.9, the average value reported by Bayly and Williams (1966) for 17 Australian salt lakes. These conversions are only approximate because the exact relationships among salinity, EC and TDS vary according to the chemical composition of a water body and, in the case of EC, temperature. However, errors resulting from the use of standard conversion factors were considered negligible in relation to the overall range of salinity in Australian inland waters.
Values of tolerance (T) were estimated at the genus level. Variation in salinity tolerance can sometimes be appreciable among congeneric species (Carbonell et al., 2012; Lawrie et al., 2021), but derivation of robust T values at species level was considered impractical because of the absence or paucity of records for most species, the greater likelihood of misidentification at that level, and the likely occurrence of unrecognised cryptic species. The value of T for each genus was initially based on the square root of the maximum salinity at which it had been recorded in any data source. The square-root transformation was used because the statistical distribution of maximum salinity values had high positive skew, i.e., many genera had low maxima and few genera had high maxima. The square-root transformation therefore better differentiated genera with lower maxima relative to those with higher maxima. After square-root transformation, values were linearly re-scaled and rounded to the nearest integer so that they ranged from T = 1 (lowest tolerance) to T = 10 (highest tolerance). The minimum value was not set to zero because all aquatic invertebrates have some tolerance of (and indeed a requirement for) salts.
Initial values of T were adjusted in a few instances to deal with suspected anomalies. For three essentially marine genera, the initial value of T was < 4, the applicable value if seawater salinity was the limit of tolerance. The value of T for these genera was therefore increased to 4. For an additional 17 genera, the maximum salinity of occurrence was an extreme value that was inconsistent with other data for the same genus, and might have resulted from misidentifications, errors in environmental measurements, mistakes in data handling, or records of dead or dying individuals (Lawrie et al., 2021). In these cases, T was changed to the average of the T values derived from the highest and second-highest salinity of occurrence, rounded to the nearest integer.
Values of indicator strength (S) were assigned on the logic that the strongest indicators of salinity would be genera with the highest T values (extremely tolerant) as well as those the lowest T values (extremely sensitive), whereas the weakest salinity indicators would be genera with intermediate tolerance. The value of S for genus i (Si) was therefore calculated as follows:
This equation converts T values to S values such that both maximum and minimum T values (10 and 1, respectively) translate to an S value of 5 (maximum indicator strength), and mid-range T values (5 and 6) translate to an S value of 1 (minimum indicator strength). Other T values translate to S values of 2–4 depending on how much they deviate in either direction from the mid-range T values.
Index testing
The index was tested with data from published studies that were not used in the derivation of T and S values because they reported chemical and/or biological data combined for multiple sampling points or times. These studies encompassed a diverse range of water bodies (11 lakes, four ponds, two claypans, two streams and a rain pool) spread across northern, southern, eastern and western Australia (Table 1). They also included sampling of benthic, littoral, nektonic and planktonic invertebrate assemblages. The published data were used to compile a genus list for each water body, with taxon names corrected and updated where necessary as for index development. The average salinity of each water body was calculated from reported data, with EC and TDS values converted as for index development.
The IBSI score was calculated for each water body, either with some form of abundance weighting, or without abundance weighting, depending on the degree and nature of quantification in each study (Table 1). The forms of abundance weighting included frequency of occurrence (number of occasions on which a genus was recorded or proportion of sampling points at which it was recorded), proportional abundance (percentage of specimens), and abundance as ranked by the authors on a scale of 1–3 or 1–5. Any recorded genera without assigned T and S values were excluded from calculations. In order to assess the effect of incorporating indicator strength in the index, IBSI values were re-calculated with all values of S set to 1. Linear, logarithmic, power and exponential regression models were generated to see which best approximated the IBSI-salinity relationship. These analyses were done with XLSTAT version 2022.3.1 (Addinsoft, 2022).
Results
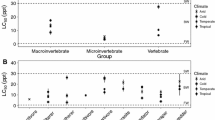
Values of T and S were derived for 764 genera (Supplementary Information B). However, values for 211 of these genera were based on a single study, and values for an additional 98 were based on only two studies. The great majority of genera had low T values (Fig. 1a), with only the brine shrimp genera Artemia and Parartemia achieving the maximum value of 10, and only five insect and crustacean genera attaining a value of 9 (Australocypris, Berosus, Culicoides, Diacypris and Platycypris). Most genera had high S values (Fig. 1b). For the independent test data, IBSI values were closely related to salinity (Fig. 2), with a power regression providing the best fit (R2 = 0.95; P < 0.001). When IBSI values were re-calculated with S set to 1 for all genera, the power relationship of IBSI to salinity was weaker (R2 = 0.93).
Discussion
The independent test data for IBSI were multifarious, involving a variety of environments (large and small standing water bodies as well as running waters), invertebrate assemblage types (benthic, littoral, nektonic and planktonic), sampling methods and degrees of quantification. Despite this disparity, the association between IBSI scores and salinity was robust (R2 = 0.95). This association strength exceeds that reported between the SI or ASI and EC for stream benthos in Australia (R2 = 0.33–0.53: Horrigan et al., 2007; Schäfer et al., 2011) and the United Kingdom (R2 = 0.43–0.87: Pickwell et al., 2022). Zhao et al. (2018) reported a higher association strength between the SI and EC in China (R2 = 0.95), but their study appears to have used the same data for derivation of taxon SSSs and index testing. The association between IBSI scores and salinity also exceeds that reported between SPEARsalinity and EC for stream benthos in Australia (R2 = 0.11–0.53: Schäfer et al., 2011), South Africa (no significant relationship: Malherbe et al., 2022), and the United Kingdom (R2 = 0.34–0.88: Pickwell et al., 2022).
IBSI differs in several respects from the SI, the ASI and SPEARsalinity (Table 2). For example, IBSI operates at a finer level of taxonomic resolution, allowing for the wide variation in salinity tolerance that can occur among cofamilial genera (Castillo et al., 2018). Taxonomic resolution may be a contributor to IBSI’s close association with salinity, because finer resolution generally produces stronger associations with pertinent environmental variables for stressor-specific invertebrate indices (e.g., Monk et al., 2012; Turley et al., 2014; Chessman et al., 2022). In contrast, the strength of association between the pesticide-oriented version of SPEAR and environmental variables appears to be little affected by taxonomic resolution in the family–species range (Beketov et al., 2009; Liebmann et al., 2022). However, this result may be influenced by the practice of sometimes assigning SPEAR’s family-level trait values to constituent species instead of deriving species-specific values (Beketov et al., 2009). A similar practice is apparent in the case of SPEARsalinity, with many nominally family-level sensitivity values simply being order-level values assigned to constituent families. For example, the order Amphipoda and five amphipod families all have identical sensitivity values of 15–52 mS cm−1, and the order Coleoptera and eight beetle families all have identical values of 15–60 mS cm−1 (Schäfer et al., 2011: Supplementary Information). Thus, the real level of taxonomic resolution of SPEARsalinity is order rather than family in many cases.
Another point of difference is that IBSI operates at a single taxonomic level rather than a mixture of levels, thereby avoiding the issue of overlapping taxa (e.g., the inclusion of an order as well as families within that order) that applies to SPEARsalinity. The inclusion of mixed taxonomic levels and overlapping taxa will likely result in index values and performance being affected by the degree of taxonomic resolution achieved for particular taxonomic groups. For example, King and Richardson (2002) found that identifying chironomids at family level and other taxa at genus or species level produced significantly weaker biota-environment relationships than identifying chironomids at species level and other taxa at family level. Laini et al. (2022) reported that a flow-oriented invertebrate index had a stronger association with current velocity and mesohabitat type when calculated with mixed taxonomic levels (family, genus and species) than when calculated at family level only. Such differences complicate comparisons among studies with inconsistent patterns of taxonomic resolution.
In addition, the salinity-tolerance values compiled for IBSI encompass many more taxa than those tabulated for the SI, the ASI and SPEARsalinity (Table 2). More than 365 families of invertebrates inhabit Australian inland waters (author’s unpublished compilation), but the various score lists for the SI and ASI (Horrigan et al., 2005, 2007) cover only 26–53 of them, while the ‘physiol. sensitivity to salinity’ tabulation for SPEARsalinity (Schäfer et al., 2011) includes 141. The 764 genera for which IBSI T and S values are provided here represent 256 families, but still omit many of the > 1630 invertebrate genera that inhabit Australian inland waters (author’s unpublished compilation). Because salt lakes have been the primary focus of studies of the distribution of Australian invertebrate taxa in relation to salinity (Supplementary Information A), many of the omissions are taxa confined to running waters. Targeted studies would be needed to generate T and S values for such taxa.
Another distinguishing feature of IBSI is that it is based on ten levels of salinity tolerance rather than the two levels of SPEARsalinity and the three levels of the SI and ASI (Table 2). The Canadian CCI uses only two levels of salinity tolerance (Williams et al., 1999), but the British SAGI uses five levels of salinity association (Pickwell et al., 2022), and the German weighted average salinity uses six levels of salinity preference or tolerance (Wolf et al., 2009). Conceptually, resolution of salinity association or tolerance into a larger number of levels should improve index performance, but the extent of any such effect requires empirical testing.
IBSI also differs from the SI, the ASI and SPEARsalinity in that it incorporates weighting of the contribution of each taxon present in a sample according to its inferred strength as a salinity indicator. Weighting of taxa according to indicator strength or value is a component of many other stressor-specific indices for aquatic invertebrates (e.g., Armanini et al., 2011; Chalar et al., 2011; Gieswein et al., 2019; Dorić et al., 2021; Sundermann et al., 2022). When IBSI values for the test data were re-calculated without weighting by indicator strength, IBSI’s association with salinity was weakened, albeit not greatly.
Like SPEARsalinity, but unlike the SI and ASI, IBSI also incorporates weighting of taxon contributions by taxon abundance. Such weighting is not essential to IBSI if the data do not permit it, but abundance-weighted versions of stressor-specific indices for aquatic invertebrates are typically more responsive to environmental variation than unweighted versions (e.g., Haase et al., 2019; Chessman et al., 2022; Liebmann et al., 2022). If abundance weighting based on counts of individuals is included, it may be preferable to apply a transformation such as the cube or fourth root that constrains the influence of the dominant taxa on the index value (Smith et al., 2001; Teixeira et al., 2012).
Although results for the independent test data suggested that IBSI is a viable alternative to the SI, the ASI and SPEARsalinity, IBSI has some limitations at its present stage of development. The use of documented field occurrences to estimate T and S values was suboptimal because many values were based on only one or two studies, which may not have sampled the full salinity range that a taxon can occupy. There is considerable scope to refine T and S values through systematic invertebrate surveys across a range of salinities, and more sophisticated methods of determining tolerance limits from field data than simply the highest recorded salinity of occurrence could also be trialled (e.g., Cormier et al., 2018). However, field occurrences may never be sufficient to determine salinity tolerances of genera whose distributions are severely limited by factors other than salinity. For example, aquatic invertebrate genera that require flowing water (Chessman et al., 2022) cannot occupy the most saline aquatic environments, salt lakes, regardless of their salinity tolerance. Tolerance testing under controlled laboratory conditions may therefore be needed to determine reliable T and S values for such genera. Experimentally derived salinity tolerances (e.g., LC50 values) are available for some Australian freshwater invertebrates, but were not used in the present study because of the small number of taxa covered and the variable relativity of field-derived and laboratory-derived tolerance values (Kefford et al., 2004).
The use of the genus level to calculate IBSI is a constraint for potential users who may not be able to achieve that level of taxonomic resolution for major components of the invertebrate fauna. This constraint should be greatly alleviated as molecular methods for specimen identification become more widely adopted (Pawloski et al., 2018; Carew et al., 2022), but family-level IBSI scores could if necessary be calculated using the maximum or average T and S values of constituent genera.
Further testing of IBSI across a range of geographic regions and settings would be beneficial for refinement of the index and its interpretation. For example, it would be useful to determine how IBSI scores are influenced by factors other than salinity, such as ionic composition, environment type (estuarine, lacustrine, palustrine or riverine), and invertebrate assemblage type (littoral, benthic or planktonic). In particular, it is likely that the current T values principally represent tolerance to salinity dominated by Na+ and Cl−, because such dominance prevails in Australian inland waters (Williams, 1967). IBSI might therefore behave anomalously in waters with different ionic dominance, given the effect of ionic composition on salt toxicity to Australian freshwater invertebrate species (Zalizniak et al., 2006; Hills et al., 2019). The salinity tolerance of particular taxa may also vary geographically independently of ionic composition (Arnott et al., 2023).
Potential uses of IBSI include prediction and monitoring of the ecological impact of salinisation or remedial management. In addition, it is possible that IBSI could be used in combination with other stressor-specific indices as a diagnostic tool to indicate predominant stressors impacting on the invertebrate fauna at monitoring sites. Such an approach was used by Clews and Ormerod (2009) to differentiate the effects of flow variation and nutrient enrichment in the Wye River system in the United Kingdom, but has received only limited testing in Australia (Chessman & McEvoy, 1998, 2012). Although developed specifically for Australian application, IBSI could be trialled elsewhere in the world after determination of T and S values for genera that have not been rated here, or have been rated but may have different tolerances in other regions. Such international application could also compare IBSI with analogous indices such as SAGI (Pickwell et al., 2022).
Data availability
Data are available in the cited documents.
Code availability
Not applicable.
References
Addinsoft, 2022. XLSTAT statistical and data analysis solution. Addinsoft, Long Island, NY, U.S.A. [available on internet at https://www.xlstat.com/en].
Armanini, D. G., N. Horrigan, W. A. Monk, D. L. Peters & D. J. Baird, 2011. Development of a benthic macroinvertebrate flow sensitivity index for Canadian rivers. River Research and Applications 27: 723–737. https://doi.org/10.1002/rra.1389.
Arnott, S. E., V. Fugère, C. C. Symons, S. J. Melles, B. E. Beisner, M. Cañedo-Argüelles, M.-P. Hébert, J. A. Brentrup, A. L. Downing, D. K. Gray, D. Greco, W. D. Hintz, A. McClymont, R. A. Relyea, J. A. Rusak, C. L. Searle, L. Astorg, H. K. Baker, Z. Ersoy, C. Espinosa, J. M. Franceschini, A. T. Giorgio, N. Göbeler, E. Hassal, M. Huynh, S. Hylander, K. L. Jonasen, A. Kirkwood, S. Langenheder, O. Langvall, H. Laudon, L. Lind, M. Lundgren, E. R. Moffett, L. Proia, M. S. Schuler, J. B. Shurin, C. F. Steiner, M. Striebel, S. Thibodeau, P. U. Cordero, L. Vendrell-Puigmitja, G. A. Weyhenmeyer & A. M. Derry, 2023. Widespread variation in salt tolerance within freshwater zooplankton species reduces the predictability of community-level salt tolerance. Limnology and Oceanography Letters 8: 8–18. https://doi.org/10.1002/lol2.10277.
Bayly, I. A. E. & W. D. Williams, 1966. Chemical and biological studies on some saline lakes of south-east Australia. Australian Journal of Marine and Freshwater Research 17: 177–228. https://doi.org/10.1071/MF9660177.
Beketov, M. A., K. Foit, R. B. Schäfer, C. A. Schriever, A. Sacchi, E. Capri, J. Biggs, C. Wells & M. Liess, 2009. SPEAR indicates pesticide effects in streams - Comparative use of species- and family-level biomonitoring data. Environmental Pollution 157: 1841–1848. https://doi.org/10.1016/j.envpol.2009.01.021.
Bunn, S. E. & P. M. Davies, 1992. Community structure of the macroinvertebrate fauna and water quality of a saline river system in south-western Australia. Hydrobiologia 248: 143–160. https://doi.org/10.1007/BF00006082.
Cañedo-Argüelles, M., 2020. A review of recent advances and future challenges in freshwater salinization. Limnetica 39: 185–211. https://doi.org/10.23818/limn.39.13.
Cañedo-Argüelles, M., B. J. Kefford, C. Piscart, N. Prat, R. B. Schäfer & C.-J. Schulz, 2013. Salinisation of rivers: an urgent ecological issue. Environmental Pollution 173: 157–167. https://doi.org/10.1016/j.envpol.2012.10.011.
Carbonell, J. A., A. Millán & J. Velasco, 2012. Concordance between realised and fundamental niches in three Iberian Sigara species (Hemiptera: Corixidae) along a gradient of salinity and anionic composition. Freshwater Biology 57: 2580–2590. https://doi.org/10.1111/fwb.12029.
Carew, M. E., W. K. Yow, K. L. Robinson, R. A. Coleman & A. A. Hoffman, 2022. DNA barcoding and metabarcoding of highly diverse aquatic mites (Acarina) can improve their use in routine biological monitoring. Marine and Freshwater Research 73: 900–914. https://doi.org/10.1071/MF21291.
Castillo, A. M., D. M. T. Sharpe, C. K. Ghalambor & L. F. De León, 2018. Exploring the effects of salinization on trophic diversity in freshwater ecosystems: a quantitative review. Hydrobiologia 807: 1–17. https://doi.org/10.1007/s10750-017-3403-0.
Chalar, G., R. Arocena, J. P. Pacheco & D. Fabián, 2011. Trophic assessment of streams in Uruguay: A Trophic State Index for Benthic Invertebrates (TSI-BI). Ecological Indicators 11: 362–369. https://doi.org/10.1016/j.ecolind.2010.06.004.
Chessman, B. C., 2003. New sensitivity grades for Australian river macroinvertebrates. Marine and Freshwater Research 54: 95–103. https://doi.org/10.1071/MF02114.
Chessman, B. C. & P. K. McEvoy, 1998. Towards diagnostic biotic indices for river macroinvertebrates. Hydrobiologia 364: 169–182. https://doi.org/10.1023/A:1003142819625.
Chessman, B. C. & P. K. McEvoy, 2012. Insights into human impacts on streams from tolerance profiles of macroinvertebrate assemblages. Water, Air, & Soil Pollution 223: 1343–1352. https://doi.org/10.1007/s11270-011-0949-8.
Chessman, B. C., L. Metzeling & D. P. Robinson, 2022. Development of a flow-sensitive macroinvertebrate index for Australian rivers. River Research and Applications 38: 846–862. https://doi.org/10.1002/rra.3950.
Clews, E. & S. J. Ormerod, 2009. Improving bio-diagnostic monitoring using simple combinations of standard biotic indices. River Research and Applications 25: 348–361. https://doi.org/10.1002/rra.1166.
Cormier, S. M., L. Zheng & C. M. Flaherty, 2018. A field-based model of the relationship between extirpation of salt-intolerant benthic invertebrates and background conductivity. Science of the Total Environment 633: 1629–1636. https://doi.org/10.1016/j.scitotenv.2018.02.044.
Dorić, V., I. Pozojević, N. Vučković, M. Ivković & Z. Mihaljević, 2021. Lentic chironomid performance in species-based bioassessment proving: high-level taxonomy is not a dead end in monitoring. Ecological Indicators 121: e107041. https://doi.org/10.1016/j.ecolind.2020.107041.
Fritz, S. C., S. Juggins, R. W. Battarbee & D. R. Engstrom, 1991. Reconstruction of past changes in salinity and climate using a diatom-based transfer function. Nature 352: 706–708. https://doi.org/10.1038/352706a0.
Gieswein, A., D. Hering & A. W. Lorenz, 2019. Development and validation of a macroinvertebrate-based biomonitoring tool to assess fine sediment impact in small mountain streams. Science of the Total Environment 652: 1290–1301. https://doi.org/10.1016/j.scitotenv.2018.10.180.
Haase, P., F. Pilotto, F. Li, A. Sundermann, A. W. Lorenz, J. D. Tonkin & S. Stoll, 2019. Moderate warming over the past 25 years has already reorganized stream invertebrate communities. Science of the Total Environment 658: 1531–1538. https://doi.org/10.1016/j.scitotenv.2018.12.234.
Halse, S. A., J. K. Ruprecht & A. M. Pinder, 2003. Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia. Australian Journal of Botany 51: 673–688. https://doi.org/10.1071/BT02113.
Herbert, E. R., P. Boon, A. J. Burgin, S. C. Neubauer, R. B. Franklin, M. Ardón, K. N. Hopfensperger, L. P. M. Lamers & P. Gell, 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6: e206. https://doi.org/10.1890/ES14-00534.1.
Hills, K. A., R. V. Hyne & B. J. Kefford, 2019. Species of freshwater invertebrate that are sensitive to one saline water are mostly sensitive to another saline water but an exception exists. Philosophical Transactions of the Royal Society B 374: e20180003. https://doi.org/10.1098/rstb.2018.0003.
Hintz, W. D. & R. A. Relyea, 2019. A review of the species, community, and ecosystem impacts of road salt salinisation in fresh waters. Freshwater Biology 64: 1081–1097. https://doi.org/10.1111/fwb.13286.
Horrigan, N., S. Choy, J. Marshall & F. Recknagel, 2005. Response of stream macroinvertebrates to changes in salinity and the development of a salinity index. Marine and Freshwater Research 56: 825–833. https://doi.org/10.1071/MF04237.
Horrigan, N., J. E. Dunlop, B. J. Kefford & F. Zavahir, 2007. Acute toxicity largely reflects the salinity sensitivity of stream macroinvertebrates derived using field distributions. Marine and Freshwater Research 58: 178–186. https://doi.org/10.1071/MF05241.
Kath, J., J. R. Thomson, R. M. Thompson, B. J. Kefford, F. J. Dyer & R. Mac Nally, 2018. Interactions among stressors may be weak: implications for management of freshwater macroinvertebrate communities. Diversity and Distributions 24: 939–950. https://doi.org/10.1111/ddi.12737.
Kefford, B. J., P. J. Papas, L. Metzeling & D. Nugegoda, 2004. Do laboratory salinity tolerances of freshwater animals correspond with their field salinity? Environmental Pollution 129: 355–362. https://doi.org/10.1016/j.envpol.2003.12.005.
King, R. S. & C. J. Richardson, 2002. Evaluating subsampling approaches and macroinvertebrate taxonomic resolution for wetland bioassessment. Journal of the North American Benthological Society 21: 150–171. https://doi.org/10.2307/1468306.
Laini, A., G. Burgazzi, R. Chadd, J. England, I. Tziortzis, M. Ventrucci, P. Vezza, P. J. Wood, P. Viaroli & S. Guareschi, 2022. Using invertebrate functional traits to improve flow variability assessment within European rivers. Science of the Total Environment 832: e155047. https://doi.org/10.1016/j.scitotenv.2022.155047.
Lawrie, A. D., J. Chaplin & A. Pinder, 2021. Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes. Marine and Freshwater Research 72: 1553–1576. https://doi.org/10.1071/MF21088.
Liebmann, L., P. Vormeier, O. Weisner & M. Liess, 2022. Balancing effort and benefit – How taxonomic and quantitative resolution influence the pesticide indicator system SPEARpesticides. Science of the Total Environment 848: e157642. https://doi.org/10.1016/j.scitotenv.2022.157642.
Malherbe, W., J. H. J. van Vuren & V. Wepener, 2022. The application of a macroinvertebrate indicator in Afrotropical regions for pesticide pollution. Journal of Toxicology 2018: e2581930. https://doi.org/10.1155/2018/2581930.
Monk, W. A., P. J. Wood, D. M. Hannah, C. A. Extence, R. P. Chadd & M. J. Dunbar, 2012. How does macroinvertebrate taxonomic resolution influence ecohydrological relationships in riverine ecosystems. Ecohydrology 5: 36–45. https://doi.org/10.1002/eco.192.
Pawlowski, J., M. Kelly-Quinn, F. Altermatt, L. Apothéloz-Perret-Gentil, P. Beja, A. Boggero, A. Borja, A. Bouchez, T. Cordier, I. Domaizon, M. J. Feio, A. F. Filipe, R. Fornaroli, W. Graf, J. Herder, B. van der Hoorn, J. I. Jones, M. Sagova-Mareckova, C. Moritz, J. Barquín, J. J. Piggott, M. Pinna, F. Rimet, B. Rinkevich, C. Sousa-Santos, V. Specchia, R. Trobajo, V. Vasselon, S. Vitecek, J. Zimmerman, A. Weigand, F. Leese & M. Kahlert, 2018. The future of biotic indices in the ecogenomic era: integrating (e)DNA metabarcoding in biological assessment of aquatic ecosystems. Science of the Total Environment 637–638: 1295–1310. https://doi.org/10.1016/j.scitotenv.2018.05.002.
Peck, A. J. & T. Hatton, 2003. Salinity and the discharge of salts from catchments in Australia. Journal of Hydrology 272: 191–202. https://doi.org/10.1016/S0022-1694(02)00264-0.
Pickwell, A., D. Constable, R. Chadd, C. Extence & S. Little, 2022. The development of a novel macroinvertebrate indexing tool for the determination of salinity effects in freshwater habitats. River Research and Applications 38: 522–538. https://doi.org/10.1002/rra.3914.
Pinder, A. M., S. A. Halse, J. M. McRae & R. J. Shiel, 2005. Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543: 1–24. https://doi.org/10.1007/s10750-004-5712-3
Pinder, A., K. Quinlan, D. Cale & R. Shiel, 2013. Invertebrate Communities and Hydrological Persistence in Seasonal Claypans of Drummond Nature Reserve, Western Australia, Department of Parks and Wildlife, Perth:, 34.
Pinder, A. M., D. J. Cale & L. Lewis, 2020. Aquatic Invertebrate Diversity of Lake McLarty in 2019, Department of Biodiversity, Conservation and Attractions, Perth:, 25.
Rengasamy, P., 2006. World salinization with emphasis on Australia. Journal of Experimental Botany 57: 1017–1023. https://doi.org/10.1093/jxb/erj108.
Schäfer, R. B., B. J. Kefford, L. Metzeling, M. Liess, S. Burgert, R. Marchant, V. Pettigrove, P. Goonan & D. Nugegoda, 2011. A trait database of stream invertebrates for the ecological risk assessment of single and combined effects of salinity and pesticides in south-east Australia. Science of the Total Environment 409: 2055–2063. https://doi.org/10.1016/j.scitotenv.2011.01.053.
Smith, R. W., M. Bergen, S. B. Weisberg, D. Cadien, A. Dalkey, D. Montagne, J. K. Stull & R. G. Velarde, 2001. Benthic Response Index for assessing infaunal communities on the Southern California mainland shelf. Ecological Applications 11: 1073–1087. https://doi.org/10.1890/1051-0761(2001)011[1073:BRIFAI]2.0.CO;2.
Stenger-Kovács, C., K. Körmendi, E. Lengyel, A. Abonyi, E. Hajnal, B. Szabó, K. Buczkó & J. Padisák, 2018. Expanding the trait-based concept of benthic diatoms: development of trait and species-based indices for conductivity as the master variable of ecological status in continental saline lakes. Ecological Indicators 95: 63–74. https://doi.org/10.1016/j.ecolind.2018.07.026.
Sundermann, A., A. Müller & M. Halle, 2022. A new index of a water temperature equivalent for summer respiration conditions of benthic invertebrates in rivers as a bio-indicator of global climate change. Limnologica 95: e125980. https://doi.org/10.1016/j.limno.2022.125980.
Teixeira, H., S. B. Weisberg, A. Borjac, J. A. Ranasinghe, D. B. Cadien, R. G. Velarde, L. L. Lovell, D. Pasko, C. A. Phillips, D. E. Montagne, K. J. Ritter, F. Salas & J. C. Marques, 2012. Calibration and validation of the AZTI’s Marine Biotic Index (AMBI) for Southern California marine bays. Ecological Indicators 12: 84–95. https://doi.org/10.1016/j.ecolind.2011.05.025.
Thorslund, J., M. F. P. Bierkens, G. H. P. O. Essink, E. H. Sutanudjaja & M. T. H. van Vliet, 2021. Common irrigation drivers of freshwater salinisation in river basins worldwide. Nature Communications 12: e4232. https://doi.org/10.1038/s41467-021-24281-8.
Tibby, J. & M. A. Reid, 2004. A model for inferring past conductivity in low salinity waters derived from Murray River (Australia) diatom plankton. Marine and Freshwater Research 55: 597–607. https://doi.org/10.1071/MF04032.
Timms, B. V., 1973. A limnological survey of the freshwater coastal lakes of East Gippsland, Victoria. Australian Journal of Marine and Freshwater Research 24: 1–20. https://doi.org/10.1071/MF9730001.
Timms, B. V., 1975. Basic limnology of two crater lakes in western Victoria. Proceedings of the Royal Society of Victoria 87: 159–166.
Timms, B. V., 1981. Animal communities in three Victorian lakes of differing salinity. Hydrobiologia 81: 181–193. https://doi.org/10.1007/BF00048715.
Timms, B. V., 2005. Salt lakes in Australia: present problems and prognosis for the future. Hydrobiologia 552: 1–15. https://doi.org/10.1007/s10750-005-1501-x.
Timms, B. V., P. Coleman & J. Cooper, 2014. Seagull Lake, western Eyre Peninsula, South Australia: a saline lake to benefit from climate change? Transactions of the Royal Society of South Australia 138: 161–180. https://doi.org/10.1080/03721426.2014.11649007.
Turley, M. D., G. S. Bilotta, C. A. Extence & R. E. Brazier, 2014. Evaluation of a fine sediment biomonitoring tool across a wide range of temperate rivers and streams. Freshwater Biology 59: 2268–2277. https://doi.org/10.1111/fwb.12429.
Walton, N. R. G., 1989. Electrical conductivity and total dissolved solids - what is their precise relationship? Desalination 12: 275–292. https://doi.org/10.1016/0011-9164(89)80012-8.
Watson, G. F., M. Davies & M. J. Tyler, 1995. Observations on temporary waters in northwestern Australia. Hydrobiologia 299: 53–73. https://doi.org/10.1007/BF00016886.
Williams, W. D., 1967. The chemical characteristics of lentic surface waters in Australia: a review. In Weatherley, A. H. (ed), Australian Inland Waters and their Fauna: Eleven Studies A.N.U. Press, Canberra: 18–77.
Williams, W. D., 1975. A note on the macrofauna of a temporary rainpool in semi-arid Western Australia. Australian Journal of Marine and Freshwater Research 26: 425–429. https://doi.org/10.1071/MF9750425.
Williams, W. D., 1995. Lake Corangamite, Australia, a permanent saline lake: conservation and management issues. Lakes & Reservoirs 1: 55–64. https://doi.org/10.1111/j.1440-1770.1995.tb00006.x.
Williams, W. D., 2001. Anthropogenic salinisation of inland waters. Hydrobiologia 466: 329–337. https://doi.org/10.1023/A:1014598509028.
Williams, D. D., N. E. Williams & Y. Cao, 1999. Road salt contamination of groundwater in a major metropolitan area and development of a biological index to monitor its impact. Water Research 34: 127–138. https://doi.org/10.1016/S0043-1354(99)00129-3.
Wolf, B., E. Kiel, A. Hagge, H.-J. Krieg & C. K. Feld, 2009. Using the salinity preferences of benthic macroinvertebrates to classify running waters in brackish marshes in Germany. Ecological Indicators 9: 837–847. https://doi.org/10.1016/j.ecolind.2008.10.005.
Zalizniak, L., B. J. Kefford & D. Nugegoda, 2006. Is all salinity the same? I. The effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates. Marine and Freshwater Research 57: 75–82. https://doi.org/10.1071/MF5103.
Zhao, Q., F. Guo, Y. Zhang, Z. Yang & S. Ma, 2018. Effects of secondary salinisation on macroinvertebrate functional traits in surface mining-contaminated streams, and recovery potential. Science of the Total Environment 640–641: 1088–1097. https://doi.org/10.1016/j.scitotenv.2018.05.347.
Ziemann, H. & C.-J. Schulz, 2011. Methods for biological assessment of salt-loaded running waters - fundamentals, current positions and perspectives. Limnologica 41: 90–95. https://doi.org/10.1016/j.limno.2010.09.005.
Zinchenko, T. D. & L. V. Golovatyuk, 2013. Salinity tolerance of macroinvertebrates in stream waters (review). Arid Ecosystems 3: 113–121. https://doi.org/10.1134/S2079096113030116.
Acknowledgements
I thank Stuart Halse and Brian Timms for help in obtaining their published data, and all those who contributed to the studies that informed the current research. I am also grateful to two anonymous reviewers whose comments helped to improve the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: Stuart Halse
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chessman, B.C. A new salinity index for the invertebrate fauna of Australian inland waters. Hydrobiologia 850, 3539–3550 (2023). https://doi.org/10.1007/s10750-023-05252-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05252-0