Abstract
Apalachicola Bay, a river-fed estuary in the northeast Gulf of Mexico is experiencing impacts from anthropogenic and climate-induced changes in freshwater inputs and sea level rise. These synergistic pressures have resulted in a historic oyster fishery collapse and the expansion of mangroves, which are displacing native salt marsh. Understanding how these natural and man-made-driven changes have affected the nekton community is essential for the sustainable management of this coastal region. To evaluate changes in nekton occurrence and distribution in Apalachicola Bay, we analyzed 20 years of biological monitoring data. Our results indicated that spatial variation in species’ populations was driven by differences in salinity, while seasonal changes were driven by temperature. Freshwater inflow is the primary driver of salinity, and these differences demonstrated that river flow reductions have the potential to alter nekton communities. The effects of climate-driven droughts and anthropogenic freshwater flow reductions on estuarine community structure highlight the importance of maintaining sufficient river flow for nursery areas, which provide ecosystem services for the region. Ultimately, these findings support the continued need for monitoring programs that track accelerating ecosystem change and provide a clearer understanding for how community composition will respond to global change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate and anthropogenically driven pressures are changing the dynamics of estuarine systems globally. Climate change is causing sea level rise, extreme rainfall variability, and increasing water temperatures in almost all coastal systems (Poff et al., 2002; von Storch et al., 2021). These changes can result in habitat loss and/or changes in abiotic characteristics of the system that can impact the abundance of estuarine organisms (Field et al., 2014). Shifts in hydrological cycles are commonly driven by upstream reallocation of water for anthropogenic use (Vest, 1993; Ruhl, 2005; Surratt et al., 2008; Petes et al., 2012), which can result in changes in freshwater flow downstream (Dutterer et al., 2013) and alterations to salinity gradients in the receiving basins (Rozas & Minello, 2011). The environmental changes associated with hydrological alterations and climate change are also known to cause shifts in estuarine nekton community structure (Maes et al., 2005; Lugendo et al., 2007; Boucek & Rehage, 2014; Colombano et al., 2021). That is, the distribution and abundance of species are intrinsically linked to the abiotic aspects of the system, which have been shown to include factors such as pH, salinity, temperature, and dissolved oxygen (Piazza & La Peyre, 2011; Colombano et al., 2021). The influence of these environmental drivers can result in a range of outcomes from restructuring of estuarine nekton communities to a complete collapse of fisheries; either end of the spectrum can have implications on overall estuarine productivity and the ecosystem services these systems provide to humans (Scavia et al., 2002; Gibson et al., 2005).
The Apalachicola National Estuarine Research Reserve is located within the Apalachicola Bay estuary and is currently experiencing the impacts of climate change, such as sea level rise and changes in rainfall, in addition to anthropogenically caused changes in river flow (Wang et al., 2013; Huang et al., 2016). In particular, freshwater inflow has been identified as a primary environmental influence on the productivity of the estuary, and these flows have been decreasing over the past 45 years (Livingston et al., 1997). Apalachicola Bay receives most of its freshwater from the Apalachicola River, the largest river in the state of Florida, USA, which is estimated to account for as much as 35% of the freshwater discharge on the west coast of Florida (McNulty et al., 1972).
The Apalachicola, Chattahoochee, and Flint (ACF) River basin, located in the southeastern United States, encompasses 51,281 km2 across the states of northern Georgia and Alabama, and terminates at Apalachicola Bay, Florida. Historically, the state of Georgia has used the water produced in the northern portion of the watershed to support municipal water supply for the city of Atlanta (current population 6 million), and agricultural activities for farming, which occur along the river basin. Water diversion and damming to support these activities began in the middle of the twentieth century, with the construction of Lake Lanier near Atlanta, Georgia, USA to hold water back for the city, and the Jim Woodruff dam, which was constructed near the Florida and Georgia border in 1959. For the past few decades, Georgia and Florida were entrenched in a legal battle over Georgia’s continued reallocation of drinking water from the ACF watershed to supply their growing urban population. Florida argued that Georgia’s increasing upstream freshwater needs caused significant ecological harm downstream, the most notable being the collapse of the Apalachicola Bay oyster fishery in 2012 (Hallerman, 2021). As of March 2021, the Supreme Court unanimously ruled to dismiss the case, in favor of Georgia, allowing the state to maintain its current water use practices (Hallerman, 2021). Future projections suggest that Georgia will double its upstream freshwater use from the ACF river basin by the year 2040, which presents continued challenges for both the health of the Apalachicola Bay estuarine system and Florida’s seafood industry (LoCascio, 2015). In the face of these hydrological changes, it becomes increasingly important to understand the impacts of freshwater inflow on Apalachicola Bay water quality, and the estuary’s biological communities.
Changes in environmental and biological characteristics in response to altered freshwater input have already impacted the ecological and economic productivity of the Apalachicola Bay system. Apalachicola Bay provides nursery habitat for both commercially and recreationally important fishes and macroinvertebrates, including a shrimp fishery (Litopenaeus setiferus, Farfantepenaeus duorarum, Farfantepenaeus aztecus), blue crab fishery (Callinectes sapidus), and one of the most historically productive oyster fisheries (Crassostrea virginica) in the US. (Berrigan, 1990; Wang et al., 2008; Oczkowski et al., 2011). In 2007, the total commercial fishing industry attributed to Apalachicola Bay was estimated to contribute $134 million in economic output to the state of Florida (Crist, 2007). However, in recent years, drought conditions and low river flow are thought to be two primary reasons for observed increases in oyster mortality (Livingston et al. 2000; Petes et al., 2012), changes in estuarine trophic organization (Livingston et al., 1997), and reduced fish species richness and diversity (Livingston et al., 1997). The most notable of these changes was the previously mentioned 2012 collapse of the Apalachicola Bay oyster fishery that led to the National Oceanic and Atmospheric Administration’s (NOAA) declaration of a federal fishery disaster for Apalachicola Bay in 2013 (Camp et al., 2015), coupled with a 5-year closure of the oyster fishery beginning in 2020 (McCawley, 2020). Previous studies suggest a variety of factors, all associated with reduced freshwater inflow, are contributing to the catastrophic decline in oyster populations. These factors include increased salinity (Camp et al., 2015), greater predation by euryhaline predators (Menzel et al., 1966; Kimbro et al., 2017; Pusack et al., 2019), and altered nutrient levels (Oczkowski et al., 2011). While the dynamics between freshwater inflow and oyster populations in Apalachicola Bay have been well studied, less is known about the relationship between freshwater inflow and estuarine nekton communities in this system.
There is limited information describing the linkages between environmental parameters and nekton community structure in Apalachicola Bay. Gorecki & Davis (2013) found seasonal and spatial variation in nekton community structure in the lower Apalachicola River, but the study did not extend into the bay proper. Large fish community structure has been shown to vary spatially with different salinity gradients across Apalachicola Bay (Hamilton et al., 2022). However, both these studies were conducted over a relatively short time frame (~ 4 years) and did not examine long-term patterns in environmental changes and nekton community structure. In fact, the last long-term assessment of the estuary’s trophic organization and overall response to environmental changes was published over 2 decades ago (Livingston, 1982; Livingston et al., 1997). Since that time, the Apalachicola Bay system has experienced a variety of continual climate and anthropogenic changes, and thus, an up-to-date investigation of how nekton communities may have responded to these synergistic pressures is needed. With the continued impacts of climate change (e.g., drought and sea level rise) and reduction of freshwater inflow, it is critical for natural resource managers to understand the long-term relationships between environmental parameters and nekton communities to conserve this highly productive system.
The objectives of this study were threefold: (1) identify seasonal and spatial patterns in the nekton community structure of Apalachicola Bay, (2) determine how environmental characteristics explain patterns in nekton community structure, and (3) investigate the role of freshwater inflow from Apalachicola River in driving patterns in nekton community structure in Apalachicola Bay. Results of this study provide a 20-year prospective of the relationship between estuarine nekton community structure and environmental variables, and results of this study will also support an ongoing food web modeling project in Apalachicola Bay. The findings described herein, along with the current and future analyses that leverage this important data resource, will guide future management decisions in the region in the face of climate and anthropogenic-driven change.
Methods
Study site
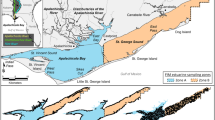
The study was conducted in the Apalachicola Bay estuary (29° 42′12.43″ N–84° 55′26.14’ W, Fig. 1), a subtropical, river-dominated system in Franklin County, Florida, USA. A portion of the watershed is managed by the NOAA, National Estuarine Research Reserves, a system of 30 reserves designated throughout the coastal regions and Great Lakes of the United States, which have been dedicated to protection and preservation of estuarine systems through research and education. The Apalachicola National Estuarine Research Reserve (ANERR) consists of a combined area of 94,986 hectares of submerged land and uplands. Livingston et al. (1975) reported that spatial differences in habitat characteristics such as salinity and bottom type contributed to variation in nekton community assemblages in Apalachicola Bay; therefore, the estuary was characterized into three distinct habitats. The Upper Bay (stations 1–3), East Bay portion of the system is near the river and its distributaries. Stations located in that portion of the system tend to be lower in salinity and have muddier substrates relative to other locations. Middle Bay (stations 4–6) tends to be transitional from the upper to the lower stations, while the Lower Bay, southern locations (stations 7 & 8) typically are characterized by the highest salinity levels with sand substrate covered by seagrass (Fig. 1).
The Apalachicola River and Estuary (29° 42′12.43″ N–84° 55′26.14’ W), located in the Florida panhandle, northern Gulf of Mexico, United States. There were eight biological sampling locations used in this study, each shown here and separated into regions (Upper-blue, Middle-red, Lower-dark gray). Locations of the USGS Apalachicola River Sumatra and Blountstown flow gages (#02359170 & #02358700) approximately 30 km and 70 km upstream of the estuary mouth are also indicated as these data are also used in the analyses. Biological Sampling occurred from 2000 to 2019 and were situated along the northeast-to-southwest axis of the estuary
Data collection and statistical analysis
Biological and environmental sampling occurred from 2000 to 2019 at eight sites situated along the northeast-to-southwest estuarine transect (Fig. 1). Stations were selected to follow a known salinity gradient, from river mouth to the Gulf of Mexico (Livingston et al., 1975). Monthly sampling occurred from 2000 to 2013, after which quarterly sampling (March, June, September, & December) was conducted from 2014 to 2019. Each sampling effort occurred over consecutive 2-to-3-day periods.
For each sampling event (month) and site (station), water quality parameters were collected prior to biological sampling. Water quality parameters included temperature (°C), salinity (Practical Salinity Scale), and dissolved oxygen (mg L−1 and percent saturation). Water quality parameters were measured using YSI 85 or YSI ProDSS multiparameter water quality meters. We also collected meteorological data including cloud cover (percent), wind direction (degrees), wind speed, and water depth (m). A visual assay of bottom type was recorded at each station, and trawls were deployed over multiple habitat types including sandy bottom, mud bottom, and sea grass. Time series of temperature, salinity, and dissolved oxygen were tested for significant trends using seasonal Kendall tests, which measure a monotonic trend over time while accounting for seasonal fluctuations (Hirsch & Slack, 1984). A Z-test statistic of less than 0.05 was considered significant in the Kendall test. Time series data were analyzed using the R programming package.
Biological sampling consisted of five two-minute trawls towed horizontally in a straight line and into the current while maintaining a ground speed of approximately 1 m s−1. The tows were performed using a 4.8 m wide otter trawl with 2.54 cm stretched mesh that was fitted with a 0.32 cm liner into the cod-end of the net. To standardize abundance measures for effort, individual tows at each station during a month were pooled to calculate nekton abundance-per-cubic-meter (# m−3) by summing the number of individuals for each taxon collected at a site within a month over all tows, divided by the total volume of water filtered through the net. These trawls typically catch slower swimming, young-of-the-year fishes and macroinvertebrates (ranging, on average, from 8 to 150 mm) and slower moving, larger adult species. Therefore, larger, faster swimming species were most likely underestimated in samples as they could effectively avoid the net. Upon capture, fishes and macroinvertebrates were identified to species and counted. When possible, all organisms were returned to the water immediately after being processed to minimize mortality; however, smaller specimens that were difficult to identify in the field were kept and identified using a dissection microscope.
To determine if there was a significant long-term trend between flow and salinity, we used a loess regression to calculate trend statistics using Apalachicola River flow data obtained from the United States Geological Survey (USGS) National Water Information System Web Interface (http://waterdata.usgs.gov/) from the Sumatra and Blountstown, Florida Apalachicola River gages (#02359170 & #02358700 respectively; Fig. 1). The Sumatra gage is located approximately 30 km upstream of the mouth of the river. This gage served as a proxy for river flow closest to the estuary and provide the best record of river discharge into Apalachicola Bay. The Blountstown gage is located approximately 70 km upstream and represented the longest record of flow for the lower watershed (1928 to present). Flow data from the Sumatra gage were used to examine the relationship between river flow and Apalachicola Bay salinity. Monthly averaged salinity and river flow data were used to develop the statistical model. These data were fitted using LOESS regression (locally estimated scatterplot smoothing), which is a non-parametric locally weighted regression technique that accounts for extreme high and low values to fit a smooth curve through points in a scatter plot. Significance level for the LOESS was set at the P < 0.05 level. In addition, we plotted the long-term trends in river flow from 1928 to 2019 using the Blountstown gage to provide an historical context of how river flow was impacted by the synergistic impacts of anthropogenic and climate-induced stressors such as dam construction and periodic droughts.
We used multivariate, non-parametric analyses to evaluate the relationship between nekton community structure and environmental data. The statistical approaches used to analyze the environmental data do not allow for missing data within sample matrices. Instances of incomplete datasets can mostly be attributed to poor weather, insufficient tides to access locations, and broken sampling instrumentation. To resolve instances where data were missing or unavailable, we used the MISSING routine in the PRIMER-E software (Clarke et al., 2014; PRIMER-E, 2021), which applies an expected maximization algorithm to estimate values from the environmental datasets in which less than 5% of the data in the matrix are unavailable. Environmental data were then normalized to adjust for skewness in the data using Euclidean distance square-root transformation. To investigate relationships between nekton communities over both time (season) and space (station or region), non-metric Multidimensional Scaling (nMDS) ordinations were used to visualize the communities in 3D space and were calculated and constructed using Bray–Curtis similarity matrices. In the plots, each point represents a nekton community during a season (month) and region (station). The closer the points are within the coordinate plane, the more similar the community structure is between those representative samples. The nMDS community plots were presented along with the monthly averaged trends in the environmental variables by region of the bay.
To test for statistically significant differences in nekton community structure between space (region) and time (season), a two-factored permutational analysis of variance (PERMANOVA) was used (Anderson, 2001). PERMANOVA is a non-parametric form of a generalized linear model providing the ability to include factors, groups and treatments in experimental design as well as evaluating interactions between factors. In this study, we included season, region, and a season x-region interaction as factors in the analysis. The PERMANOVA permutations were run for a total of 9,999 iterations and significance was determined with a P (permutated) < 0.05. For nekton data, regional patterns were defined by the resultant nMDS ordinations, and seasons were defined a priori by calendar months in the northern hemisphere as follows: Spring: March–May, Summer: June–August, Fall: September–November, Winter: December–February. A Similarity of Percentages (SIMPER) analysis was used to evaluate the percent contribution of taxa that were most influential in driving the differences among the regions of the bay. The RELATE routine was used to test for matched resemblance matrices between nekton community structure and environmental variables (temperature, salinity, and dissolved oxygen), and distance-based linear models (DistLM) determined which variable or combination of environmental variables best accounted for the variation in nekton communities that were observed.
To evaluate the influence of river flow on water quality and community structure, flow data from Apalachicola River (Sumatra gage: 2000–2019) were ranked from highest to lowest mean annual flows and the highest and lowest ranked flow years were selected the for comparison. Differences between the two flows scenarios and water quality (temperature, salinity, and oxygen) were statistically verified using a t test assuming unequal variance on monthly flow and water quality values. Parametric tests on flow and water quality were evaluated using R software. Non-metric MDS visualized community similarities between highest and lowest flow years for the upper region of the estuary. An Analysis of Similarity (ANOSIM) procedure was used to confirm statistical significance between community resemblance matrices for the two flow regimes, and SIMPER evaluated which species contributed most to differences in community structure between the two flow regimes. All non-parametric multivariate analyses were performed using PRIMER-e statistical software (Clarke et al., 2014; PRIMER-E, 2021).
Results
Environmental data
Water quality trends varied over the 20-year period. Bay-wide temperatures exhibited a cooling trend from 2000 to 2003 (Z < 0.0001) followed by a steady warming pattern through 2019 (Z = 0.03), with a net increase in temperature of + 2.52 °C over the 20-year period (Fig. 2A). Bay-wide salinities decreased between 2000 and 2004 (Z < 0.0001), increased from 2005 to 2007 (Z < 0.0001) and have since exhibited no significant change over time (Z = 0.23; Fig. 2B). Dissolved oxygen exhibited no significant change between 2000 and 2003 (Z = 0.20), a decrease between 2004 and 2008 (Z < 0.0001) and an increase since 2009 (Z < 0.0001; Fig. 2C).
The relationship between river flow and salinity shows a strong negative trend over the course of the study period (r = − 0.68; Fig. 3). Freshwater influx from the nearest Apalachicola River gage (#02359170) was the primary factor driving salinity in the estuary.
Long-term river flow over the previous nine decades showed large annual variations in flow. The overall trend for the 91-year period showed an upward trend in river flow from 1928 to 1975, after which flows decreased from 1975 to 2019 (Fig. 4). The highest recorded annual flow occurred in 1964 (1132.71 m3 s−1) and lowest occurred in 2012 (215.33 m3 s−1), respectively. The highest and lowest flows during the study period (2000–2019) occurred in 2005 (827.43 m3 s−1) and 2012, respectively (Fig. 2).
Mean annual Apalachicola River flow (m3 s−1) at the USGS gage site #02358700, near Blountstown, Florida USA from 1928 to 2019. Installation of the dam was completed in 1959 (red, long-dash vertical line). The highest and lowest flow years during the study from 2000 to 2019 occurred in the years 2005 (red, short dash vertical line) and 2012 (red, dotted vertical line), respectively. The black, curve linear, long-dashed line indicates the best fit trend line for annual river flow from 1928 to 2019 (y = − 1.6676x2 + 122.92x + 20,412, r = 0.0406)
Integrated biological and environmental data
From 2000 to 2019, a total of 98 taxa were sampled, with 16 species making up greater than 90% of the total catch. Fishes were the most abundant nekton (Supplementary Table 1). The five most common species (76.3%) were bay anchovy (Anchoa mitchilli), spot (Leiostomus xanthurus), Atlantic croaker (Micropogonias undulatus), menhaden (Brevoortia spp.), and pinfish (Lagodon rhomboides). The most abundant macroinvertebrates in the catches consisted of the aviu shrimp (Acetes americanus), white shrimp (Litopenaeus setiferus), arrow shrimp (Tozeuma carolinense), longtail grass shrimp (Periclimenes longicaudatus), the Atlantic brief squid (Lolliguncula brevis), and blue crab (Callinectes sapidus).
Seasonal monthly means for temperature, salinity, and dissolved oxygen showed distinct and predictable patterns. Mean temperatures during the study period varied seasonally with highest and lowest water temperatures during summer (29.50 °C) and winter (14.37 °C), respectively (Fig. 5A). Average salinities also varied seasonally, with summer (18.00 psu) and fall (18.84 psu) generally exhibiting higher salinity than winter (10.89 psu) and spring (12.47 psu, Fig. 5B). Dissolved oxygen was negatively correlated with both temperature and salinity, and the lowest and highest values occurred in the summer (6.46 mg L−1) and winter (7.16 mg L−1), respectively (Fig. 5B & C). Spatial patterns in environmental variables depended upon the variable and location in the estuary. Temperature (Fig. 5A) and dissolved oxygen (Fig. 5C) did not vary based upon the region of the bay they were sampled; however, salinity increased with distance to the river mouth (Fig. 5B). The lowest salinities were found in the Upper Bay (6.89 psu) and increased from the Middle Bay (16.70 psu) to Lower Bay regions (21.57 psu; Fig. 5B). Non-metric MDS plots indicated potential seasonal and spatial patterns in nekton communities (Fig. 5D–G). With exception of winter, regional nekton community structure grouped along the northeast-to-southwest estuary transect, which clustered the Upper Bay sites (Stations 1–3), the Middle Bay sites (Stations 4–6), and the Lower Bay sites (Stations 7 & 8; Fig. 5). Winter communities exhibited less regional separation in the ordinations. Community composition appeared to be similar for the Upper and Middle Bay sites but were distinct from the Lower Bay sites. Seasonal differences became more apparent during the spring, summer, and fall seasons, in comparison with more overlap between communities during winter Fig. 5 D–G).
Mean monthly (± 1 SE) temperature (A), salinity (B), and dissolved oxygen (C) by season (Spring: March–May, Summer: June–August, Fall: September–November, Winter: Dec–Feb) and region (Upper Bay stations 1–3, Middle Bay stations 4–6, and Lower Bay stations 7 & 8) in Apalachicola Bay from 2000 to 2019. Bottom: nMDS of nekton communities by station for each region of the bay (Upper Bay 1–3, Middle Bay stations 4–6, and Lower Bay stations 7 & 8) by season (D) Spring (March-May), (E) Summer (June-August), (F) Fall (September-November), and (G) Winter (December-February)
A two-factored PERMANOVA indicated that community structure varied significantly by season and region and among regions in the same season (Fig. 5D–G; Supplementary Table 2; Main Tests). Pairwise comparisons showed that communities were distinct among all combinations of season and region when directly compared to one another (Supplementary Table 2; Pairwise Tests).
Site specific SIMPER analysis showed that the relative abundances and the number of species contributing to each regional community were different (Supplementary Table 3A). Nekton community composition changed along the north–south axis of the estuary. There were increasingly more species contributing to the community composition from the north to south (Upper: 3 taxa, Middle: 6 taxa, Lower: 9 taxa respectively). Some of the same taxa were found in each region; however, their relative abundances were generally different. For example, Bay anchovy (Anchoa mitchilli) contributed to community structure in all regions of the bay. The Upper Bay region of the system was also characterized by higher abundances of Atlantic croaker (Micropogonias undulatus), and spot (Leiostomus xanthurus; Supplementary Table 3A). The Middle Bay region shared some of the same species as the Upper region; however, their relative abundances changed and supported higher abundances Atlantic brief squid (Lolliguncula brevis), sand sea trout (Cynoscion arenarius), and hardhead catfish (Ariopsis felis; Supplementary Table 3A). In the Lower Bay region, which generally exhibited the highest salinity, results showed a different community structure compared to the Upper and Middle regions. The Lower region was composed of more salt-tolerant species such as pinfish (Lagodon rhomboides), pigfish (Orthopristis chrysoptera), arrow shrimp (Tozeuma carolinense), and lizardfish (Synodus foetens; Supplementary Table 3A).
The RELATE analysis was used to test matched resemblance matrices between nekton community structure and the environmental data (temperature, salinity, and dissolved oxygen) and results indicated that water quality was a significant predictor of nekton community structure in Apalachicola Bay (Rho 0.617; P = 0.011; df = 1276). More specifically, distance-based linear models (DistLM) showed that of the three environmental variables considered, salinity was the variable that accounted for the most variation in the regional nekton communities; however, salinity was only able to account for about 14% of the variation in the community data alone (R2 = 0.139; Pseudo-F 77.755; P = 0.001; df = 1276).
Comparison of ranked years for highest and lowest Apalachicola River flows differed between 2005 (µ = 827.4; SE = 152.9; df = 23) and 2012 (µ = 215.3; SE = 29.5; df = 23; P < 0.01) with annual flows being nearly 4 times greater during 2005 (Fig. 6A). The observed differences in flows between the two years influenced East Bay salinity (Fig. 6B & C). Salinity was significantly lower in 2005 (µ = 8.4; SE = 2; df = 23) than 2012 (µ = 15.1; SE = 1.5; df = 23; P < 0.01; Fig. 6C). However, there were no differences in temperature (2005: µ = 22.8; SE = 1.6; df = 23; 2012: µ = 22.8; SE = 1.7; df = 23; P > 0.05) or dissolved oxygen (2005: µ = 7.29; SE = 7; df = 23; 2012: µ = 6.34; SE = 1.7; df = 23; P > 0.05). When we compared East Bay (stations 1–3; Fig. 6B) water quality and community composition between high and low flow years (2005 & 2012 respectively) we found that river flow did have an effect nekton community structure. Comparison of nekton communities between the two flow scenarios verified that community composition between the two years (2005 & 2012) were different (ANOSIM: Rho = 0.2; P = 0.002; df = 23; Fig. 6D). Therefore, the change in river flow and the resultant change in salinity had an influence on the community composition between the 2 years. Similarity of percentages (SIMPER) showed that bay anchovy (Anchoa mitchilli) was the most abundant taxon during both years (Supplementary Table 3B). However, during the low flow year fewer taxa were supported, with the Bay anchovy (Anchoa mitchilli) and sand seatrout (Cynoscion arenarius) contributing most to the community composition in the low flow year (Fig. 6; Supplementary Table 3B).
A Mean monthly river flows for the highest and lowest ranked flow years during to 2000–2019 study period for East Bay (Upper Region) only. B Map with Upper Region (stations 1–3) indicated by a circle, C a bar chart comparing mean ± 1 standard error salinities for East Bay (Upper Region) between the 2 years, and D an nMDS comparing nekton community composition between the 2 years
Discussion
Climate and anthropogenic pressures on estuarine systems are increasing globally (Syvitski et al., 2005; Romero et al., 2013; Jiang et al., 2014). These disturbances can alter nekton community structure, impair whole ecosystems, and cause entire fisheries to collapse (Jennerjahn & Mitchell; 2013). As these pressures continue to increase, natural resource managers must have consistent, long-term data that can be used to develop tools to make effective management decisions about the sustainability of their systems (McClatchie et al., 2014; Alizadeh et al., 2018; Newton et al., 2022). Here, we have provided the most up-to-date evaluation of nekton community structure and environmental change in Apalachicola Bay, a system experiencing synergistic pressures from climate change and reduced river flow from anthropogenic reduction upstream through damming and agricultural practices. Our results confirmed with previous studies that Apalachicola River discharge plays a role in determining the biological characteristics of the bay (Livingston, 1982; Livingston, 1997). The varying seasonal and spatial patterns in nekton community structure, driven by changes in river flow and drought, highlights the need for more detailed investigations in the Apalachicola estuary (and other estuaries worldwide) as climate change and other anthropogenic factors continue to alter global coastal dynamics. Data derived from long-term monitoring programs, such as those that were used in this study, can provide the necessary hindcasting ability to develop robust ecosystem models, which have the potential to implement forecasts of future impacts to an estuary (Kärnä & Baptista 2016; Georgas et al., 2016). Even though each system has its own individual characteristics, geological features, environmental and anthropogenic pressures, the more system-specific knowledge gained, the better we can manage these systems as a global collective.
Two important findings of the present study showed that Apalachicola Bay nekton communities differed spatially and seasonally. Nekton community structure changed along a north to south axis, likely driven by bay salinity, which tended to increase with increased distance from the river. However, the degree of change depended upon the time of year, or season that was sampled. Distinct seasonal patterns are common in temperate and subtropical estuaries, aligning with results from previous studies in the system (Livingston et al., 1975; Gorecki & Davis, 2013), other systems in North America (Shervette & Gelwick, 2008; Flaherty et al., 2013) and estuaries globally (Green et al., 2009; Becker et al., 2020; Zhang et al., 2022). In the present case, differences in nekton communities were distinguishable across all four seasons. Winter nekton communities showed the least regional differences, and in the cases where differences were apparent, the Lower Bay nekton communities consistently separated from the Upper and Middle Bay regions within the nMDS ordinations.
In addition to salinity, another potential driver of the spatial and seasonal variation observed was habitat and substrate type. Not only does the Lower Bay tend to be higher in salinity, that region also has the lowest turbidity (furthest from the river and closest to the Gulf of Mexico) with a sandy bottom-type, normally consistent with an environment that supports marine grasses. These habitat characteristics are quite different when compared to the Upper and Middle Bay regions, in which the water is much fresher and more turbid, and the bottom type is characterized muddy detrital sediments. In Apalachicola Bay, fish, and invertebrate taxa such as pinfish (Lagodon rhomboides), inshore lizardfish (Synodus foetens), and arrow shrimps (Tozeuma carolinense) typically are found in the high salinity sites in the Lower Bay with clear water that is suitable for the growth of marine grasses (Supplementary Table 3A). Bottom type has been shown to impact nekton community structure in other estuarine systems. For instance, previous studies show that sea grasses and mangroves (Yáñez-Arancibia et al., 1993), submerged aquatic vegetation (Potanogeton nodosus, Najas guadalupensis; Castellanos & Rozas, 2001), and submerged structural components such as oyster reefs (Stunz et al., 2010) all drive nekton community structure in coastal systems to some degree. Thus, our findings suggest that salinity and season impact nekton community composition throughout the Apalachicola Bay estuary, but the influence of river flow on the distribution of benthic vegetation may also contributes to the spatial variation of estuarine communities in the bay.
The winter community composition across all regions of the bay showed the most similarity. Winter months (December to February) are some of the main recruitment months for many species in the estuary as indicated by the appearance of post-larval nekton in the system (Gorecki & Davis, 2013). Because many of these estuarine dependent species are still somewhat planktonic and are not able to swim effectively against strong currents, their movements through the water column are likely controlled by wind and tidal currents. This physical limitation could account for the lack of spatial separation in the community composition during the winter months. As these young-of-year grow and become more able to swim effectively, they began to settle out into preferred habitats, likely driving the spatial separation in the other seasons. Previous studies also identify a seasonal pattern in Apalachicola Bay nekton community composition. More specifically, different species demonstrated predictable timing for recruitment into Apalachicola Bay during the recruitment season (Gorecki & Davis, 2013; Garwood, Personal Observation). Gorecki & Davis (2013) recognized seasonal variation between nekton communities of the lower Apalachicola River and upper Apalachicola Bay, with fish species such as menhaden, pinfish, and spot during winter-spring, contributing most to the seasonal differences in community composition. This pattern was strongly linked to the timing of juvenile recruitment to the estuary, as well as variations in salinity levels (Gorecki & Davis, 2013). These patterns are common in other estuarine systems in North America (Monteleone, 1992), including the Gulf of Mexico (Hernandez et al., 2010) and other temperate and subtropical systems around the globe (Drake & Arias, 1991; Franco-Gordo et al., 2003; Lopes et al., 2006).
Young-of-the-year estuarine nekton recruitment in Apalachicola Bay typically begins at the beginning of winter and continues through spring and summer (Potter & Claridge, 1986). This seasonality highlights the potential for climate-driven changes in abiotic factors like salinity and temperature to influence the timing of nekton recruitment and migration patterns (Roessig et al., 2004). These potential changes could have cascading effects on estuarine fish populations, thus impacting commercial and recreational fishers, forcing them to change their harvesting practices in accordance with each species’ physiological tolerance of environmental conditions (Roessig et al., 2004). Managers will need information and tools to track these trends closely and inform management decisions that will decrease impacts to the fisheries, and we contend that studies such as the one described in this paper can help provide the necessary context for adaptive management approaches.
Because water temperature and salinity are driving the seasonal differences in community composition in Apalachicola Bay, climate change will continue to be an important factor affecting these communities as the seasonal patterns within the region change and potentially become less distinct. For instance, during the study period bay temperatures increased by a net 2.52°C, and with global climate change expected to warm water temperatures in the Gulf of Mexico by as much as 4°C by the end of the century (Muhling et al., 2011; Biasutti et al., 2012), seasonal nekton community patterns in Apalachicola Bay and other Gulf of Mexico estuaries may change as winter-spring environmental conditions begin to resemble summer-fall conditions. From a regional perspective, winter-spring is regarded as the “wet season,” which typically corresponds to the time of the year in which river flows tends to be greater. If changes in climate resulted in less frequent rainfall events upstream (i.e., drought conditions), effectively reducing flows downstream, community composition between the wet and dry seasons will potentially become more similar.
We evaluated the influence of river flow on nekton community composition by investigating how community structure changed in the Upper Bay between high and low flow years. Results of our third hypothesis suggest that smaller, annual changes in flow can have significant shorter-term impacts on community composition (Supplementary Table 3B). Lower salinity species decreased in abundance in the Upper Bay and more species with an affinity to higher salinities moved into the traditional low salinity, upper regions of the bay. For instance, the popular commercial blue crab (Callinectes sapidus) and menhaden (Brevoortia spp.), which tend to prefer the lower salinity water conditions found in the Upper Bay were less abundant in the community when compared to the commercial pink shrimp (Farfantepenaeus duoarum) and silver perch (Bairdiella chrysoura), which tend to be found in the more southern, saltier portions of the bay during normal and higher flow years.
Consequently, certain species maybe be limited or gain more access to different regions of the bay depending on the volume-per-unit-time of freshwater input to the system. This finding is especially important to consider because the Upper Bay is valued as an important nursery ground for certain species (Livingston et al., 1975). A future decrease in river input would likely shift species abundances so that they resemble the community structure observed during 2012 low river flow event. These changes to community structure could then affect adult populations and thus have more economic and cultural impacts on the commercial and recreational fisheries respectively. More adaptive management strategies will likely be necessary to protect the integrity of the Apalachicola Bay ecology, which will in-turn have an impact on the local economy. Moreover, the combination of upstream water diversion during the same drought period was directly implicated in the oyster fishery disaster declaration in 2013 (Camp et al., 2015). With the potential for increased frequency and severity of drought likely in the future (Strzepek et al., 2010), other fisheries species could be impacted and restoration of the now collapsed oyster fishery may be unlikely if more management actions are not taken. Therefore, to evaluate the range of potential changes to estuarine community structure over the next 30 years, we advocate for an adaptive management strategy with continued biological monitoring in the system coupled with the development of ecological modeling tools to help managers track these changes in estuarine systems.
There were differences in abundance of important fisheries species during high and low flow years. For example, blue crab had a higher abundance during the high flow period. Both commercial shrimp species had greater abundances during the low flow period. These changes in abundance would likely result in differing levels of success for each fishery depending on river flow conditions. Our results suggest that species distributions are likely to shift to areas with more favorable environmental conditions as well, meaning fishers would need to adapt and potentially relocate to continue their practices (Roessig et al., 2004). Given this trend, commercially and recreationally important species could potentially compete for resources, causing changes in the trophic hierarchy of the system. For instance, ingestion of the copepod Acartia tonsa, an ecological important member of the food web, tends to be highest in low salinity regions of the bay (Putland & Iverson, 2007), so the reduction of low salinity areas would likely result in lower productivity for species that rely on A. tonsa (whether directly or indirectly). Increasing salinity throughout Apalachicola Bay would also lead to an influx of more salinity-tolerant predators, such as drills and stone crabs that prey on oysters (Menzel et al., 1966; Kimbro et al., 2017; Pusack et al., 2019). This increased salinity would only lead to more declines in oyster populations, exacerbating impacts on the many other organisms that depend on oyster reefs for foraging and refuge such as blue crab, spotted seatrout (C. nebulosus), and shrimp species (Penaeidae, Solomon et al., 2014). These likely outcomes become even more concerning given the ACF Supreme Court decision in 2021, which ended in favor of the state of Georgia (Hallerman, 2021). A consequence of this decision will require the federal government to hold back more water for the state of Georgia during periods of reduced freshwater input to the watershed requiring river flow to be maintained at historically low levels during these periods (Corn et al., 2008). The combined influence of anthropogenic upstream river flow reductions with climate-induced extreme droughts, rainfall events, and an increase in the frequency and severity of both high and low flow events, changes to nekton community structure appears likely (Wang et al., 2013; Chen et al., 2014).
Evaluating the connection between seasonal and spatial patterns in water quality and nekton community structure highlights the known influence of environmental characteristics on the biological composition of estuarine systems. The present study highlights the influence of river flow on nekton community structure as well as how nekton community composition can vary over time and space. Results from this study emphasize the importance for continuous long-term monitoring programs that can reveal biological community patterns in estuarine systems over short- and long-term time scales and across geographical gradients. While results provide a baseline understanding of current nekton distributions in Apalachicola Bay and emphasize the role of environmental conditions in driving community structure, understanding of potential future changes in the system is needed, as well as estuarine systems globally. With the continued impacts of climate change and man-made disturbances such as dams, dikes, river flow reduction, sea level rise, and increasing water temperature, the development of predictive models would allow for an assessment of the range of future changes to nekton communities in response to a variety of environmental change scenarios, fishing impacts, and spatial changes in habitat type. New projects are already underway to provide adaptive management tools for resource managers in Apalachicola Bay, and similar tools could be applied to other estuarine systems using long-term monitoring program data. This information will provide a range of potential outcomes that can inform monitoring plans, restoration efforts, and management strategies to measure and address these likely changes. Specifically, in the US, the development of food web models could be a guidepost for the use of available long-term monitoring data in informing sustainable ecosystem management across the National Estuarine Research Reserve network and other coastal systems.
Estuarine systems are highly dynamic, and changes in ecological structure and function are driven by multiple complex variables, all of which impact the system synergistically (Kennish, 2002; Vasconcelos et al., 2015). While these systems are dynamic and experience constant, short-term environmental change, they are also highly susceptible to long-term environmental pressures. This caveat provides an important rationale for continued investment in monitoring programs. Regardless of the difficulty of maintaining long-term estuarine monitoring programs and the issues surrounding the understanding highly complex datasets, there is still great investment potential because these systems have already been shown to be “recorders” of global environmental change (Bianchi & Allison, 2009). Even though the existing data from the present study remains vastly untapped, we must also recognize that more information is needed. For example, additional factors such as tides, short-term and seasonal wind patterns, stochastic events such as tropical storms, multi- to decadal climate events (e.g., El Nino Southern Oscillation), and changes in offshore nekton spawning events collectively contribute to the overall response of post-larval and juvenile nekton abundance in Apalachicola Bay. We have little recent information related to the external influence of the Gulf of Mexico on Apalachicola Bay, especially with respect to offshore stock spawning success. It is likely that environmental conditions for species offshore also have considerable effects on the nekton communities within bay, as many of these species use these protected habitats as nurseries. Increased sampling efforts and additional studies in the offshore and nearshore environments could provide more insight. Therefore, we postulate that collectively, the more information we can obtain on individual coastal systems will allow for a better understanding of our planet as a connected and integrated system.
Data availability
All data and materials are owned, stored, and managed in-house by the Apalachicola National Estuarine Research Reserve. All data, which are relevant to the manuscript are available to the public at the reasonable request of the corresponding author.
References
Alizadeh, M. J., M. R. Kavianpour, M. Danesh, J. Adolf, S. Shamshirband & K. W. Chau, 2018. Effect of river flow on the quality of estuarine and coastal waters using machine learning models. Engineering Applications of Computational Fluid Mechanics 12: 810–823.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. https://doi.org/10.1111/J.1442-9993.2001.01070.Pp.X.
Becker, A., M. B. Lowry & M. D. Taylor, 2020. Scales of spatial and temporal variation of small bodied nekton within intermittently closed/open lakes and lagoons (icolls) in South-Eastern Australia. Regional Studies in Marine Science 33: 100936.
Berrigan, M. E., 1990. Biological and economical assessment of an oyster resource development project in Apalachicola Bay, Florida. Journal of Shellfish Research 9: 149–158.
Bianchi, T. S. & M. A. Allison, 2009. Large-river delta-front estuaries as natural “recorders” of global environmental change. Proceedings of the National Academy of Sciences 106: 8085–8092.
Biasutti, M., A. H. Sobel, S. J. Camargo & T. T. Creyts, 2012. Projected changes in the physical climate of the Gulf Coast and Caribbean. Climatic Change 112: 819–845.
Boucek, R. E. & J. S. Rehage, 2014. Climate extremes drive changes in functional community structure. Global Change Biology 20: 1821–1831. https://doi.org/10.1111/Gcb.12574.
Camp, E. V., W. E. Pine Iii, K. Havens, A. S. Kane, C. J. Walters, T. Irani, A. B. Lindsey & J. G. Morris Jr., 2015. Collapse of a historic oyster fishery: diagnosing causes and identifying paths toward increased resilience. Ecology And Society. https://doi.org/10.5751/Es-07821-200345.
Castellanos, D. L. & L. P. Rozas, 2001. Nekton use of submerged aquatic vegetation, marsh, and shallow unvegetated bottom in the Atchafalaya River delta, A Louisiana tidal freshwater ecosystem. Estuaries 24: 184–197.
Chen, X., K. Alizad, D. Wang & S. C. Hagen, 2014. Climate change impact on runoff and sediment loads to the Apalachicola river at seasonal and event scales. Journal of Coastal Research 68: 35–42. https://doi.org/10.2112/Si68-005.1.
Clarke, K. R., R. N. Gorley, P. J. Somerfield & R. M. Warwick, 2014. Change in marine communities: An approach to statistical analysis and interpretation, 3rd ed. Primer-E Ltd., Plymouth:
Colombano, D. D., S. Y. Litvin, S. L. Ziegler, S. B. Alford, R. Baker, M. A. Barbeau, J. Cebrián, R. M. Connolly, C. A. Currin, L. A. Deegan, J. S. Lesser, C. W. Martin, A. E. Mcdonald, C. Mcluckie, B. H. Morrison, J. W. Pahl, L. M. Risse, J. A. M. Smith, L. W. Staver, R. E. Turner & N. J. Waltham, 2021. Climate change implications for tidal marshes and food web linkages to estuarine and Coastal Nekton. Estuaries and Coasts 44: 1637–1648. https://doi.org/10.1007/S12237-020-00891-1.
Corn, M. L., K. Alexander & E. H. Buck, 2008. Apalachicola-Chattahoochee-flint drought: species and ecosystem management congressional research service reports. Vol Rl34440, Washington D.C., Pp. 28.
Crist, C., 2007. Letter to president bush on georgia’s request to suspend reservoir operating rules.
Drake, P. & A. M. Arias, 1991. Composition and seasonal fluctuations of the ichthyoplankton community in a shallow tidal channel of Cadiz Bay (Sw Spain). Journal of Fish Biology 39: 245–263.
Dutterer, A. C., C. Mesing, R. Cailteux, M. S. Allen, W. E. Pine & P. A. Strickland, 2013. Fish recruitment is influenced by river flows and floodplain inundation at Apalachicola River, Florida. River Research and Applications 29: 1110–1118. https://doi.org/10.1002/Rra.2604.
Field, C.B., V.R. Barros, D.J. Dokken, K.J. Mach, M.D. Mastrandrea, T.E. Bilir, M. Chatterjee, K.L. Ebi, Y.O. Estrada, R.C. Genova, B. Girma, E.S. Kissel, A.N. Levy, S. Maccracken, P.R. Mastrandrea, And L.L.White, 2014: Climate change 2014: Impacts, adaptation, and vulnerability. Part a: Global and sectoral aspects. contribution of working group ii to the fifth assessment report of the intergovernmental panel on climate change (Eds)]. Cambridge University Press, Cambridge, United Kingdom And New York, Ny, Usa, Pp. 1132.
Flaherty, K. E., R. E. Matheson, R. H. Mcmichael & W. B. Perry, 2013. The influence of freshwater on nekton community structure in hydrologically distinct basins in northeastern Florida Bay, Fl, USA. Estuaries and Coasts 36: 918–939.
Franco-Gordo, C., E. Godınez-Domınguez, E. Suárez-Morales & L. Vásquez-Yeomans, 2003. Diversity of ichthyoplankton in the central Mexican Pacific: A seasonal survey. Estuarine, Coastal and Shelf Science 57: 111–121.
Georgas, N., L. Yin, Y. Jiang, Y. Wang, P. Howell, V. Saba, J. Schulte, P. Orton & B. Wen, 2016. An open-access, multi-decadal, three-dimensional, hydrodynamic hindcast dataset for the long island sound and New York/New Jersey Harbor Estuaries. Journal Of Marine Science And Engineering 4. https://www.mdpi.com/2077-1312/4/3/48
Gibson, C. A., J. L. Meyer, N. L. Poff, L. E. Hay & A. Georgakakos, 2005. Flow regime alterations under changing climate in two river basins: implications for freshwater ecosystems. River Research and Applications 21: 849–864. https://doi.org/10.1002/Rra.855.
Gorecki, R. & M. B. Davis, 2013. Seasonality and spatial variation in nekton assemblages of the lower Apalachicola River. Southeastern Naturalist 12: 171–196.
Green, B. C., D. J. Smith, S. E. Earley, L. J. Hepburn & G. J. Underwood, 2009. Seasonal changes in community composition and trophic structure of fish populations of five salt marshes along the Essex Coastline, United Kingdom. Estuarine, Coastal and Shelf Science 85: 247–256.
Hallerman, T., 2021. Georgia secures major victory in supreme court water wars case. The Atlanta Journal-Constitution. Atlanta, GA.
Hamilton, B. R., C. T. Peterson, A. Dawdy & R. D. Grubbs, 2022. Environmental correlates of elasmobranch and large fish distribution in a river-dominated estuary. Marine Ecology Progress Series 688: 83–98.
Hernandez, F. J., Jr., S. P. Powers & W. M. Graham, 2010. Detailed examination of ichthyoplankton seasonality from a high-resolution time series in the Northern Gulf of Mexico during 2004–2006. Transactions of the American Fisheries Society 139: 1511–1525.
Hirsch, R. M. & J. R. Slack, 1984. A nonparametric trend test for seasonal data with serial dependence. Water Resources Research 20: 727–732.
Huang, W., S. C. Hagen, D. Wang, P. A. Hovenga, F. Teng & J. F. Weishampel, 2016. Suspended sediment projections in Apalachicola Bay in response to altered river flow and sediment loads under climate change and sea level rise. Earth’s Future 4: 428–439. https://doi.org/10.1002/2016ef000384.
Jennerjahn, T. C. & S. B. Mitchell, 2013. Pressures, stresses, shocks and trends in estuarine ecosystems—an introduction and synthesis. Estuarine, Coastal and Shelf Science 130: 1–8. https://doi.org/10.1016/J.Ecss.2013.07.008.
Jiang, Z., J. Liu, J. Chen, Q. Chen, X. Yan, J. Xuan & J. Zeng, 2014. Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during The past 50 years. Water Research 54: 1–11.
Kärnä, T. & A. M. Baptista, 2016. Evaluation of a long-term hindcast simulation for the Columbia river estuary. Ocean Modelling 99: 1–14.
Kennish, M. J., 2002. Environmental threats and environmental future of estuaries. Environmental Conservation 29: 78–107.
Kimbro, D. L., J. W. White, H. Tillotson, N. Cox, M. Christopher, O. Stokes-Cawley, S. Yuan, T. J. Pusack & C. D. Stallings, 2017. Local and regional stressors interact to drive a salinization-induced outbreak of predators on oyster reefs. Ecosphere 8: E01992. https://doi.org/10.1002/Ecs2.1992.
Livingston, R. J., 1982. Trophic organization of fishes in a coastal seagrass system. Marine Ecology Progress Series 7: 1–12.
Livingston, R. J., 1997. Trophic response of estuarine fishes to long-term changes of river runoff. Bulletin of Marine Science 60: 984–1004.
Livingston, R. J., R. L. Iverson, R. H. Estabrook, V. E. Keys & J. Taylor, 1975. Major features of the apalachicola bay system: physiography, biota, and resource management. Florida Scientist 37: 245–271.
Livingston, R. J., X. Niu, F. G. Lewis Iii & G. C. Woodsum, 1997. Freshwater input to a gulf estuary: long-term control of trophic organization. Ecological Applications 7: 277–299.
Livingston, R. J., F. G. Lewis Iii, G. C. Woodsum, X.-F. Niu, B. Galperin, W. Huang, J. D. Christensen, M. E. Monaco, T. A. Battista, C. J. Klein, R. L. Howell Iv & G. L. Ray, 2000. Modelling oyster population response to variation in freshwater input. Estuarine, Coastal and Shelf Science 50: 655–672.
Locascio, S., 2015. The Apalachicola-Chattahoochee-flint river dispute: Atlanta vs. Apalachicola, water apportionments’ real version of David Vs. Goliath. Journal of Land Use & Environmental Law 30: 331–362.
Lopes, R. M., M. Katsuragawa, J. F. Dias, M. A. Montú, J. H. Muelbert, C. Gorri & F. P. Brandini, 2006. Zooplankton and Ichthyoplankton distribution on the southern Brazilian shelf: an overview. Scientia Marina 70: 189–202.
Lugendo, B. R., A. De Groene, I. Cornelissen, A. Pronker, I. Nagelkerken, G. Van Der Velde & Y. D. Mgaya, 2007. Spatial and temporal variation in fish community structure of a marine embayment in Zanzibar, Tanzania. Hydrobiologia 586: 1–16. https://doi.org/10.1007/S10750-006-0398-3.
Maes, J., M. Stevens & F. Ollevier, 2005. The compostion and community structure of the Ichthyofauna of the upper scheldt estuary: synthesis of a 10-year data collection. Journal of Applied Ichthyology 21: 86–93.
McCawley, J., 2020. Final public hearing—Apalachicola Bay oysters. Florida Fish And Wildlife Conservation Commission.
McClatchie, S., J. Duffy-Anderson, J. C. Field, R. Goericke, D. Griffith, D. S. Hanisko, J. A. Hare, J. Lyczkowski-Shultz, W. T. Peterson, W. Watson, E. D. Weber & G. Zapfe, 2014. Long time series in us fisheries oceanography. Oceanography 27: 48–67.
McNulty, J. K., W. N. Lindall Jr. & J. E. Sykes, 1972. Cooperative Gulf of Mexico estuarine inventory and study. Florida: Phase I, area description. (Vol. 368). US Department Of Commerce, National Oceanographic And Atmospheric Administration, National Marine Fisheries Service.
Menzel, R. W., N. C. Hulings & R. R. Hathaway, 1966. Oyster abundance in Apalachicola Bay, Florida in relation to biotic associations influenced by salinity and other factors. Gulf and Fresh Water Inflow and Oyster Productivity in Apalachicola Bay, Fl Caribbean Research 2: 73–96.
Monteleone, D. M., 1992. Seasonality and abundance of ichthyoplankton in great south bay, New York. Estuaries 15: 230–238.
Muhling, B. A., S.-K. Lee, J. T. Lamkin & Y. Liu, 2011. Predicting the effects of climate change on Bluefin Tuna (Thunnus Thynnus) spawning habitat in the Gulf of Mexico. Ices. Journal of Marine Science 68: 1051–1062. https://doi.org/10.1093/Icesjms/Fsr008.
Newton, A., M. Cañedo-Argüelles, D. March, P. Goela, S. Cristina, M. Zacarias & J. Icely, 2022. Assessing the effectiveness of management measures in the ria formosa coastal lagoon, Frontiers In Ecology And Evolution, Portugal:
Oczkowski, A. J., F. G. Lewis, S. W. Nixon, H. L. Edmiston, R. S. Robinson & J. P. Chanton, 2011. Fresh water inflow and oyster productivity in Apalachicola Bay, Fl (USA). Estuaries and Coasts 34: 993–1005. https://doi.org/10.1007/S12237-011-9383-9.
Petes, L. E., A. J. Brown & C. R. Knight, 2012. Impacts of upstream drought and water withdrawals on the health and survival of downstream estuarine oyster populations. Ecology and Evolution 2: 1712–1724. https://doi.org/10.1002/Ece3.291.
Piazza, B. P. & M. K. La Peyre, 2011. Nekton community response to a large-scale mississippi river discharge: examining spatial and temporal response to river management. Estuarine, Coastal and Shelf Science 91: 379–387. https://doi.org/10.1016/J.Ecss.2010.11.001.
Poff, N. L., M. M. Brinson & J. W. Day, 2002. Aquatic ecosystems and global climate change. Pew Center on Global Climate Change, Arlington, Va 44: 1–36.
Potter, I. C. & P. N. Claridge, 1986. Consistency of seasonal changes in an estuarine fish assemblage. Marine Ecology Progress Series 32: 217–228.
Primer, E., 2021. Primer V7, V7 ed. Primer-E Ltd., Auckland, Nz:
Pusack, T. J., D. L. Kimbro, J. W. White & C. D. Stallings, 2019. Predation on oysters is inhibited by intense or chronically mild, low salinity events. Limnology and Oceanography 64: 81–92. https://doi.org/10.1002/Lno.11020.
Putland, J. N. & R. L. Iverson, 2007. Phytoplankton biomass in a subtropical estuary: distribution, size composition, and carbon: chlorophyll ratios. Estuaries and Coasts 30: 878–885.
Roessig, J. M., C. M. Woodley, J. J. Cech & L. J. Hansen, 2004. Effects of global climate change on marine and estuarine fishes and fisheries. Reviews in Fish Biology and Fisheries 14: 251–275.
Romero, E., J. Garnier, L. Lassaletta, G. Billen, R. Le Gendre, P. Riou & P. Cugier, 2013. Large-scale patterns of river inputs in southwestern Europe: seasonal and interannual variations and potential eutrophication effects at the coastal zone. Biogeochemistry 113: 481–505.
Rozas, L. P. & T. J. Minello, 2011. Variation in penaeid shrimp growth rates along an estuarine salinity gradient: implications for managing river diversions. Journal of Experimental Marine Biology and Ecology 397: 196–207. https://doi.org/10.1016/J.Jembe.2010.12.003.
Ruhl, J. B., 2005. Water wars, eastern style: divvying up the Apalachicola-Chattahoochee-flint river basin. Contemporary Water Research and Education 131: 47–54.
Scavia, D., J. C. Field, D. F. Boesch, R. W. Buddemeier, V. Burkett, D. R. Cayan, M. Fogarty, M. A. Harwell, R. W. Hothward, C. Mason, D. J. Reed, T. C. Royer, A. H. Sallenger & J. G. Titus, 2002. Climate change impacts on U.S. Coastal and Marine Ecosystems. Estuaries 25: 149–164.
Shervette, V. R. & F. Gelwick, 2008. Seasonal and spatial variations in fish and macroinvertebrate communities of oyster and adjacent habitats in a Mississippi estuary. Estuaries and Coasts 31: 584–596.
Solomon, J. A., M. J. Donnelly & L. J. Walters, 2014. Effects of sea level rise on the intertidal oyster Crassostrea Virginica by field experiments. Journal of Coastal Research 68: 57–64. https://doi.org/10.2112/Si68-008.1.
Strzepek, K., G. Yohe, J. Neumann & B. Boehlert, 2010. Characterizing changes in drought risk for the United States from climate change. Environmental Research Letters 5: 044012.
Stunz, G. W., T. J. Minello & L. P. Rozas, 2010. Relative value of oyster reef as habitat for estuarine nekton in Galveston bay, Texas. Marine Ecology Progress Series 406: 147–159.
Surratt, D., J. Cherrier, L. Robinson & J. Cable, 2008. Chronology of sediment nutrient geochemistry in Apalachicola Bay, Florida (U.S.A). Journal of Coastal Research 24: 660–671. https://doi.org/10.2112/06-0717.1.
Syvitski, J. P., C. J. Vorosmarty, A. J. Kettner & P. Green, 2005. Impact of humans on the flux of terrestrial sediment to the global coastal ocean. Science 308: 376–380.
Vasconcelos, R. P., S. Henriques, S. França, S. Pasquaud, I. Cardoso, M. Laborde & H. N. Cabral, 2015. Global patterns and predictors of fish species richness in estuaries. Journal of Animal Ecology 84: 1331–1341.
Vest, R. E., 1993. Water wars in the southeast: Alabama, Florida, and Georgia square off over the Apalachicola-Chattahoochee-flint river basin. Georgia State University Law Review 9: 689.
von Storch, H., K. Fennel, J. Jensen, K.A. Lewis, B. Ratter, T. Schlurmann & W. Zhang, 2021. Climate and coast: Overview and introduction. Oxford Research Encyclopedia of Climate Science. Oxford University Press. https://oxfordre.com/climatescience/view/10.1093/acrefore/9780190228620.001.0001/acrefore-9780190228620-e-816. Accessed 10 April 2023.
Wang, H., W. Huang, M. A. Harwell, L. Edmiston, E. Johnson, P. Hsieh, K. Milla, J. Christensen, J. Stewart & X. Liu, 2008. Modeling oyster growth rate by coupling oyster population and hydrodynamic models for Apalachicola Bay, Florida, USA. Ecological Modelling 211: 77–89. https://doi.org/10.1016/J.Ecolmodel.2007.08.018.
Wang, D., S. C. Hagen & K. Alizad, 2013. Climate change impact and uncertainty analysis of extreme rainfall events in the Apalachicola River Basin, Florida. Journal of Hydrology 480: 125–135. https://doi.org/10.1016/J.Jhydrol.2012.12.015.
Yáñez-Arancibia, A., A. L. Lara-Domínguez & J. W. Day, 1993. Interactions between mangrove and seagrass habitats mediated by estuarine nekton assemblages: coupling of primary and secondary production. Hydrobiologia 264: 1–12.
Zhang, D., G. J. Chen, H. Jin, Z. Wang, W. Feng & Y. Zhang, 2022. Seasonal succession and spatial heterogeneity of the nekton community associated with environmental factors in Hangzhou Bay, China. Regional Studies in Marine Science 49: 102108.
Acknowledgements
We would like to thank fellow Reserve staff especially Lauren Levi for countless hours on a boat in the hot the hot sun counting and identifying samples. Thanks to Graham Lewis for his help in designing the project. We’re grateful to Morgan Whitmer and Katherine Thompson for their assistance in figure and map development, and Samantha Lucas for help with data mining. Our sampling efforts were conducted under Florida Fish and Wildlife Conservation Commission Special Activity License SAL-22-23797-SR. Turtle Excluder Device Exemption (TED) was issued and maintained under the United States National Marine Fisheries Service.
Funding
Thanks to the National Oceanic Atmospheric Administration, National Estuarine Research Reserve System for their generous support with funding (Grant No: NA14NOS4200041 and NA15NOS4200102) and the Florida Department of Environment protection for their support of Reserve staff and equipment.
Author information
Authors and Affiliations
Contributions
JG, KA, ML, KL, and JH contributed to data collection, analysis, draft, and review of the manuscript. LE contributed project design, data collection, and manuscript draft and review. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Erik Jeppesen, Miguel Cañedo-Argüelles, Sally Entrekin, Judit Padisák & S.S.S. Sarma / Effects of induced changes in salinity on inland and coastal water ecosystems
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garwood, J.A., Allen, K., Lamb, M.S. et al. Using long-term ecological monitoring to evaluate how climate and human-induced disturbances impact nekton communities in a Northern Gulf of Mexico estuary. Hydrobiologia 850, 4479–4496 (2023). https://doi.org/10.1007/s10750-023-05206-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05206-6