Abstract
Water quality guidelines are an important tool for managing environmental pressures on freshwater streams, but guidelines are frequently set using conditions from reference sites that are assumed to be unimpacted. Using biological thresholds potentially provides a better foundation for guidelines. However, guidelines based on field observations alone may be compromised by confounding influences. This study used an outdoor stream mesocosm, an artificial substrate (rope), and six salinity concentrations to assess the veracity of a diatom–salinity threshold determined previously in natural temperate South Australian streams. In addition, shaded treatments assessed the synergistic influences of salinity and reduced sunlight. Salinity had the strongest effect on diatoms, influencing both species and functional compositions. Species diversity and richness, and functional diversity had negative correlations with salinity. Shade strongly reduced diatom concentrations and altered species composition, with no observed interaction between salinity and light. Threshold Indicator Taxa ANalysis indicated a salinity threshold of 1610 μS cm−1 for both shade treatments, lower than the upper limit of the range used in current freshwater guidelines. This study recommends a new candidate guideline of 1600 μS cm−1 for regional freshwater streams and suggests that contemporary methods for deriving water quality guidelines may not adequately protect aquatic health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater environments, including streams, provide substantial benefits for people and biota, particularly via the provision of water supply and habitats that support approximately 6% of the globe’s species (Finlayson & D’Cruz, 2005; 2019; Cazzolla Gatti 2016; Abell et al., 2019). Threats to human water security and/or biodiversity are present in freshwater streams globally, including in temperate Australia (Vörösmarty et al., 2010; Dodds et al., 2013; UNESCO, 2020). Water quality is a strong determinant of freshwater ecosystem health, and land-use change can degrade freshwater ecosystems through chemical stressors such as salinity and nutrients, affecting streams from the cellular to ecosystem level (Cañedo-Argüelles et al., 2013; Feld et al., 2018; Guariento et al., 2011; Hill et al., 2011; Millán et al., 2011), and physical pressures like altered flow, suspended sediment, and changes to light availability which is particularly important for autotrophs (Abell et al., 2019; González del Tánago et al., 2021).
Salinity, in particular, pressures osmoregulation in organisms, decreasing cellular water and nutrient uptake potential (Cañedo-Argüelles et al., 2019; Kefford, 2019). While salt toxicity is highly dependent on the organism (Kefford et al., 2004), tolerance differences between species contribute to sublethal impacts including reduced growth rates and changes to community composition, ultimately impairing biodiversity (Williams, 2001; Herbert et al., 2015). Primary salinisation occurs naturally through weathering and precipitation, groundwater contributions, and direct runoff (Cañedo-Argüelles et al., 2019). However, human activities, particularly land clearance and irrigated agriculture, can intensify this accumulation as salts stored in soils are mobilised by rising groundwaters and are brought to surface and sub-surface soils, leading to landscape and water salinisation (Williams, 2001; Cañedo-Argüelles et al., 2013; Cooper et al., 2013; Herbert et al., 2015). Secondary salinisation has been predicted to worsen in many Australian river catchments during the next centuries, a process likely amplified by climate change (Hart et al., 2003).
Monitoring stream water quality is essential for assessing ecological health and minimising anthropogenic impacts (Bunn et al., 2010; Stevenson & Pan, 2010). Managers use water quality guidelines to identify stressor concentrations that may degrade aquatic health, and determine if water is fit for purpose (e.g. for drinking, irrigation, or aquaculture). The dominant approach used in developed countries (e.g. Australia, European Union, and North America) for setting water quality guidelines is based on monitoring ambient water quality conditions from regional reference sites (Grenier et al., 2010; Birk et al., 2012; van Dam et al., 2019). Regulatory criteria are then set using statistical proportions of these data (e.g. the 80th percentile of salinity), and health is determined as a measure of the distance between a site’s current condition and reference conditions. However, this approach can lack a biological basis, by assuming reference sites are minimally disturbed and have water quality that protects their biota (Chessman, 2021).
Internationally, the reference-condition approach is often applied by defining ecological deterioration as the response of biota, rather than just changes in physical and chemical parameters (Nijboer et al., 2004; Stoddard et al., 2006; Grenier et al., 2010; Birk et al., 2012; Hess et al., 2020). Australia adopted the reference-condition approach in 2000 through a Trans-Tasman framework (ANZECC, 2000) with objectives to set regional guidelines based on local reference data where possible, with regional reference data (termed default in ANZECC, 2000) used in the absence of local information. Physical and chemical default guidelines (based on the 80th percentile) were set using monitoring data collected monthly over two years from minimally impacted regional reference sites (ANZECC, 2000; Davies, 2000; van Dam et al., 2014, 2019). By contrast, toxicant guidelines were set using biological evidence from ecotoxicology studies. Protocols for assessing biological conditions were outlined under the framework for use by monitoring authorities in addition to default guideline values when performing site assessments. However, the default physical and chemical guidelines (widely used in regular monitoring on a national scale) were mostly based on reference monitoring data.
An updated water quality framework, the Australian and New Zealand Guidelines for Fresh and Marine Water Quality, 2018 (herein: the ANZG), provides a new direction for managing aquatic habitats and recommends using physical and chemical reference data only in the absence of biological evidence to set water quality guidelines (van Dam et al., 2019). This approach uses “weight-of-evidence” to balance multiple, and varying, lines of biological evidence to set comprehensive and protective water quality guidelines (USEPA, 2016; Suter et al., 2017; van Dam et al., 2019; Mooney et al., 2020). These lines of evidence should ideally include stressor-response data from various settings, including natural streams, which are naturally variable, and experimental or laboratory evidence that reduce confounding variables (Mooney et al., 2020). In this framework, guideline values from individual studies are characterised as “candidates” to be considered in weight-of-evidence assessments (van Dam et al., 2019).
The ANZG has also revised the aquatic ecoregions where guidelines are applied. Previously, South Australia (including high rainfall temperate areas) only had generic guidelines set for arid and semi-arid waters and lacked default values for upland and temperate streams for many water quality variables including salinity (ANZECC, 2000). Ecoregions are now based on twelve major continental drainage divisions, with South Australia represented by the Murray-Darling, Lake Eyre, Western Plateau, and South Australian Gulf divisions. To this end, this study investigates if previous default salinity values which have been carried over into the ANZG framework are appropriate for the South Australian Gulf Division, and seeks to provide new biological evidence from an experimental setting to underpin water quality guidelines.
Diatoms, a class of single-celled algae, are a widely distributed organism found in almost all freshwaters. Their widespread distribution and ecological sensitivity make them ideal bioindicators of water quality. Diatoms have been widely used for stream monitoring internationally (Zhang et al., 2019) but have been underutilised in managing freshwaters in Australia (Chessman et al., 2007; Bunn et al., 2010; Soininen & Teittinen, 2019; Tibby et al., 2020). Diatom species composition reflects recent water quality conditions due to sensitivity to physical and chemical stressors (most strongly pH, salinity, and nutrients) and the relatively short lifespans of diatoms (four to six weeks) (Reid et al., 1995; Bennion et al., 2010; Julius & Theriot 2010; Stevenson & Pan, 2010; Richards et al., 2020). Diatom community function is also important for aquatic monitoring. Trait-based approaches, which utilise characteristics such as diatom growth strategy, are used to assess the ecological function (and ecological redundancy) in diatom communities (Tapolczai et al., 2016; Stenger-Kovács et al., 2018; Lengyel et al., 2020). The eco-morphological classification (B-Beres et al., 2016) combines diatom guild adaptation strategies (Passy, 2007) and morphological cell sizes (Berthon et al., 2011) to better characterise niche differentiation in analyses (Lengyel et al., 2020).
Salinity strongly influences diatom species composition (Soininen & Teittinen, 2019; Richards et al., 2020; Tibby et al., 2020; Aida Campos et al., 2021). Recent field studies in South Australian streams have demonstrated diatom–salinity thresholds in species composition at 1000 μS cm−1 (Sultana et al., 2020) and 1320 μS cm−1 (Tibby et al., 2020). These concentrations are much lower than the upper range (5000 μS cm−1) which is still used as a default value for low-rainfall, south-central Australian areas in the ANZG framework. Consequently, it has been recommended that new state-wide salinity guidelines be set at 1320 μS cm−1 (Tibby et al., 2020). Salinity may also negatively affect diatom cell size due osmotic pressure, impacting functional composition (Stenger-Kovács et al., 2019).
Light is a fundamental resource for autotrophs and, therefore, freshwater food webs (Lange et al., 2015; Grubisic et al., 2017), and light variability in stream reaches impacts both species composition and concentrations of diatoms (Laviale et al., 2009; Tornés & Sabater 2010; Wang et al., 2017; Liu et al., 2021). Large cell-sized autotrophs may dominate in unshaded streams (Stenger-Kovács et al., 2018), therefore light is an important confounding variable to consider when deriving salinity guidelines in regions where light availability varies markedly between streams and, or reaches.
Most recent freshwater diatom studies investigating water quality impacts have been conducted in the field (Zhang et al., 2019). However, in-field settings may experience substantial covariation between drivers (e.g. salinity and light) that hampers unambiguous assessments of water quality (Hutchins et al., 2010; ; Piggott et al., 2015; Bray et al., 2019; Kefford et al., 2022). Controlled experimental streams (e.g. mesocosms) provide uniform environments that can test specific stressors and limit confounding factors that influence the assessment of water quality variables of interest (Mooney et al., 2020; Sew & Todd, 2020; Kefford et al., 2022). Large outdoor mesocosms have this benefit but provide greater realism than laboratory experiments which are often used in ecotoxicological studies (Ledger et al., 2013; Chapman, 2018; van Dam et al., 2019).
This study aims to answer the following: (1) is 1320 μS cm−1 an appropriate salinity concentration for setting new regional freshwater guidelines; (2) how does shade influence the effect of salinity on diatom communities; and (3) how do diatom–salinity thresholds differ between field and experimental settings? The results contribute to a greater understanding of diatom autecology and have implications for how freshwater guidelines are derived using biological effects data.
Methods
Study area
South Australia is the driest state in Australia (Heneker & Cresswell, 2010), and the Mount Lofty Ranges experience seasonal variability in rainfall resulting in a mix of perennial and nonperennial streams (Van Laarhoven & van der Wielen, 2009). Most permanent streams are fresh throughout the year, with electrical conductivity (EC) generally varying between 200 and 2000 μS cm−1 (Anderson et al., 2019; Tibby et al., 2020). Sodium and chloride ions dominate waters due to proximity to the coast (Blackburn & McLeod 1983; Herczeg et al., 2001; Poulsen et al., 2006). The Mount Lofty Ranges are of immense importance to the state, providing 60% of Adelaide’s drinking water (Frizenschaf et al., 2015; Rashid et al., 2015) and containing a mix of residential, agricultural, and conservation land uses (Daniels & Good 2015).
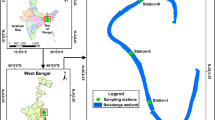
To assess the effects of salinity on freshwater diatoms, an outdoor mesocosm was established at the Waite Arboretum (Adelaide, South Australia; −34°58′13″N, 138°37′49″E) and filled with stream water (1150 μS cm−1) from the Railway Dam (Belair National Park, western Mount Lofty Ranges; −35°0′47″ N, 138°,38’,59″ E) (Fig. 1). The Railway Dam has regular inflows from Minno Creek (a second-order stream) and has similar ionic composition, trace elements, and diatom species to streams in Belair National Park. Both sites are located in the ANZG’s South Australian Gulf Division (Fig. 1).
Experimental design
The mesocosm design consisted of eighteen parallel PVC channels, with six treatments (each with three replicates) that used submersible pumps to recirculate water (see schematic in Supplementary Information), as opposed to other studies that have streamside and flow-through designs (Cañedo-Argüelles et al., 2014, 2016; Nuy et al., 2018). The experiment was run for six weeks over May, June, and July 2021, a sufficient period to allow a representative diatom community to form on either natural or artificial substrates (Kelly et al., 1998; Zidarova et al., 2020).
Salinity treatments had an even concentration distribution around the previously defined diatom–salinity threshold (1320 μS cm−1). This approach increases the accuracy of threshold detection (Sultana et al., 2020). The six treatments used sodium chloride (Sunray pool salt) to achieve average conductivities of 882, 1092, 1307, 1487, 1734, and 1958 μS cm−1. As the source water EC (1150 μS cm−1) was higher than desired in treatments 1 and 2, metropolitan tap water (430 μS cm−1) was used for dilution. This water was left for 24 h in direct sunlight to release any free chlorine (confirmed with pool testing strips) before addition to the mesocosm. Water was circulated in the channels for 48 h before deploying the sampling substrates. In addition to salinity, the effects of reduced light were added as a secondary stressor with green shade cloth covering the end of each channel (shown in Fig. 1c.). The light reaching the shaded channel sections was 91% lower than the unshaded sections, a realistic representation of light penetration in forested riparian zones (Tornés & Sabater, 2010).
Frayed rope was chosen as the artificial sampling substrate in the experiment. Artificial substrates help avoid unobserved influences on diatom communities like nutrient uptake from substrates such as sediment or plants, and rope mimics epiphytic habitats for phytobenthos (Goldsmith, 1996; Elias et al., 2017; Richards et al., 2020). Rope ends were frayed to a length of 5 cm which sat below the water surface, while cable ties ensured no further fraying. Each channel had two ropes, one placed in an unshaded middle section of the channels, and one positioned underneath green shade cloth near the end of the channels (n = 36). The assignment of rope samples to each treatment was fully randomised. Ropes were cut above cable ties to maintain a constant sample surface area. Samples were then placed into a 50 mL centrifuge tube and taken directly to the laboratory for processing on the same day.
Water quality monitoring
EC (specific conductance; μS cm−1) and temperature of each channel were measured weekly with a calibrated YSI ProQuatro multiparameter meter (YSI Incorporated, Ohio, USA). In weeks 1, 3, and 6 of the experiment, we sampled each treatment for other water quality variables, including alkalinity as calcium carbonate, bicarbonate, and carbonate, pH, turbidity, calcium, magnesium, potassium, soluble silicon dioxide, sodium, sulphate, sulphur, ammoniacal nitrogen, chloride, nitrate and nitrite as nitrogen, total nitrogen (TN), and total phosphorus (TP) (see Supplementary Information). Most of these variables were also sampled from the source water. Week 1 water quality testing occurred less than seven days after the dilution of treatments 1 and 2, so these data allow assessment of any potential water quality differences (and therefore impacts on biota) due to the addition of tap water to the two lowest conductivity treatments. Differences in luminous intensity (lux) between the unshaded and shaded treatments were quantified with four DEFI-L series meters (JFE Advantech Co., Hyogo, Japan), placed in pairs in the outside channels of the mesocosm over a ten-day period and averaged hourly.
Laboratory processing
Each centrifuge tube was filled with 35 mL of 25% hydrogen peroxide to cover the rope. Samples were placed into a sonic cleaner for 10 s to dislodge the diatoms and then in a water bath at 80 °C for 4 h to remove organic matter and dislodge any remaining diatoms. Ropes were then rinsed with RO water to remove any remaining diatoms. Samples were centrifuged for four minutes at 1200 RPM and rinsed three times. Microscope slides were prepared using a micropipette and two measured aliquots of 400 µL and 800 µL mounted using the high refractive index resin Naphrax (Brunel Microscopes, Chippenham, UK). One sample (salinity treatment 4; channel 1; shaded treatment) was damaged during the laboratory processing.
Diatom analyses
A Zeiss Axioscope light microscope (Carl Zeiss AG, Oberkochen, Germany) was used at 1000 × magnification to identify 300 diatom valves per sample using Krammer & Lange-Bertalot, (1986, 1988, 1991a; 1991b) and Sonneman et al. (2000). Species counts were expressed as relative abundances to characterise species and functional composition. Diatom concentrations (cm−1 rope) were also calculated using the areal and volumetric proportions of samples (i.e. microscope slides, coverslips, aliquots, and sampling ropes), transect lengths (µm) needed to count 300 valves, and the microscope objective.
Diatom species were assigned to functional traits using relevant literature. Twenty eco-morphological groups (B-Beres et al., 2016), based on combining four ecological guilds (Passy, 2007; Rimet & Bouchez, 2012) and five cell sizes (Berthon et al., 2011), were used to assess diatom functionality across the treatments. The diatom guilds are low-profile, high-profile, motile, and Planktonic, while cell sizes are S1 to S5 (smallest to largest; e.g. MS2, motile size 2). See Supplementary Information for the classification of taxa.
Data analyses
All data were processed in R programming (R Core Team 2021). Two-way permutational analysis of variance (PERMANOVA) (Anderson, 2001; McArdle & Anderson, 2001) and nonmetric multidimensional scaling (NMDS; Bray–Curtis dissimilarity measure) were used to assess the species and functional composition (relative abundances) dissimilarity between diatom communities in each salinity and shade treatment. Homogeneity of multivariate dispersion (Anderson, 2006) was tested to ensure that measured effects were between groups (i.e. treatment effects) rather than due to within-group dispersion (Anderson, 2001; Anderson & Walsh, 2013). These analyses were performed using the packages “vegan” (Oksanen et al., 2020) and “ggplot2” (Wickham et al., 2016).
Species richness and diversity (Shannon-diversity index) were calculated for each sample using “vegan” (Oksanen et al., 2020). Functional diversity was determined with the functional dispersion index using the “FD” package (Laliberté et al., 2022). This distance-based index (using principal coordinate analysis and Gower’s dissimilarity) measures the average distance of each species to the abundance-weighted centroid of all species in a community in functional space (Laliberté & Legendre, 2010). Analogous to multivariate dispersion (Anderson, 2006), functional dispersion helps determine how functionally clustered a community is under given environmental conditions. Importantly, changes to species richness do not influence this index (Laliberté & Legendre, 2010).
Statistical tests were performed on all water quality and community indices. Data were first tested for normality, homogeneity of variance, and then assessed either with one-way analysis of variance (ANOVA; Welch’s or Fisher’s ANOVA) or Kruskal–Wallis test by ranks using the R package “ggstatsplot” (Patil, 2021). P ≤ 0.01 was classified as sufficient evidence in this study. In addition to this “binary” decision-making, recently critiqued (Muff et al., 2022), we report the actual P values (rounded for brevity) and utilise the evidence descriptors recommended in Muff et al. (2022). Correlation between water quality variables, and relationships between community indices and EC, were assessed with Spearman’s rank correlation (rs).
Threshold Indicator Taxa ANalysis (TITAN) (Baker et al., 2020) was used to assess diatom–salinity thresholds with the R package “TITAN2″” (Baker et al., 2020). TITAN uses nonparametric change-point analysis, Indicator Species Analysis (Dufrêne & Legendre, 1997), and standardised z scores to integrate occurrence, abundance, and directionality of relative abundance data along an environmental gradient. TITAN measures positive (z +) and negative (z−) responses independently to quantify tolerant and sensitive species, respectively, and tracks cumulative responses (fsumz) to determine community thresholds at the 5th, 50th, and 95th percentiles (Baker & King, 2010). We determined salinity change points in separate analyses of each shade treatment and in a combined dataset using all samples.
Results
Water quality variation
EC distribution was slightly uneven with a median of 1411 μS cm−1, compared to the desired 1320 μS cm−1 (Fig. 2). There was very strong evidence that EC measurements differed between the treatment systems (Kruskal–Wallis, H5 = 103.37, P < 0.001). There was strong evidence that both potassium (ANOVA, F5,12 = 8.703, P = 0.001) and alkalinity as carbonate (ANOVA, F5,12 = 5.172, P = 0.009) differed between the conductivity treatments (Table 1). Spearman correlation analysis provided little or no evidence that EC, TN, TP, pH and temperature were correlated (see Supplementary Information).
Diatom responses to salinity and shade treatments
Motile Nitzschia species were present in most samples (Fig. 3), and the nine most abundant species, namely Nitzschia paleacea, (Grunow) Grunow 1881; Achnanthidium minutissimum, (Kützing) Czarnecki 1994; Nitzschia palea, (Kützing) W.Smith 1856; Ulnaria acus, (Kützing) Aboal 2003; Navicula veneta, Kützing 1844; Nitzschia incognita, Legler & Krasske 1941; Nitzschia frustulum, (Kützing) Grunow 1880; Rhopalodia gibba, Kützing 1844; and Halamphora veneta, (Kützing) Levkov 2009; respectively, were observed in both shade treatments. More diatom species were found in the unshaded channels, while the relative abundances of dominant taxa, including N. palea, N. paleacea, and A. minutissimum, were much higher in shaded samples. Fragilaria crotonensis, Kitton 1869, was most abundant in treatments 1 and 2, but was largely absent in higher conductivity treatments. Similarly, R. gibba and Hantzschia amphioxys, (Ehrenberg) Grunow 1880, were more abundant in lower salinity treatments, while Halamphora veneta was very rare outside of treatment 1 and treatment 3 samples. U. acus was abundant in several assemblages; however, relative abundance tended towards lower conductivities. Navicula veneta was very abundant in the two highest salinity treatments, despite being common in most other channels. Eolimna minima, (Grunow) Lange-Bertalot 1998, was also most abundant in treatment 5 but was rare outside these channels. Nitzschia palea was most dominant in treatment 6, having a stark, much higher, relative abundance in the highest conductivity streams. N. palacea and A. minutissimum were abundant in all samples and lacked a discernible pattern related to salinity.
Diatom assemblages in all treatments. Dashed horizontal lines represent the divisions of experiment channels and the conductivity treatments. The red dashed line represents the threshold of 1320 μS cm−1 from Tibby et al. (2020)
Two-way PERMANOVA indicated very strong evidence for differences in diatom species composition between the conductivity treatments (Pseudo-F5 = 21.996, P < 0.001). There was also very strong evidence of compositional differences between shaded treatments, although these were less pronounced, compared to within-group variance, than conductivity (Pseudo-F1 = 6.502, P < 0.001). There was moderate evidence of an interaction between conductivity and shade, but little variance between treatments was indicated by PERMANOVA (Pseudo-F5 = 1.77, P = 0.02). Homogeneity of multivariate dispersion tests indicate no evidence for dispersion effects (PERMDISP, Pseudo-F5 = 0.647, P = 0.673) in the diatom data across the conductivity treatments, and marginally weak evidence for the shade treatments (Pseudo-F1 = 4.037, P = 0.051).
NMDS (Fig. 4) indicates that species from conductivity treatments 1, 5, and 6 were the most distinct from each other and the remaining treatments. Treatment 6 had the most distinct species composition, while samples from treatment 5 were the most alike in a given treatment. NMDS clustered samples from treatments 2, 3, and 4 together; however, treatment 2 was more similar to treatment 4 rather than treatment 3. Therefore, species compositions immediately above and below the 1320 μS cm−1 threshold were not notably dissimilar, but rather the greatest dissimilarity was between treatment 4 and treatment 5. NMDS indicated different positioning of shade treatments (within each conductivity-treatment cluster); however, salinity clearly had the greatest influence on diatom species composition.
Diatom concentrations between conductivity treatments in either shade category were not different, while concentrations were substantially higher in the unshaded treatment by an average factor of 2.7:1 (Kruskal–Wallis, H1 = 16.351, P < 0.001). Species richness was highest in treatment 1 channels and lowest in treatment 6 (ANOVA, F5 = 18.388, P < 0.001; Fig. 6), and there was robust evidence of a strong negative correlation between species richness and EC (Spearman’s rank, rs = − 0.84, P < 0.001). The Shannon-diversity index was highest in treatment 1 (Kruskal–Wallis, H5 = 27.003, P < 0.001), and there was a strong negative correlation between species diversity and EC (Spearman’s rank, rs = − 0.87, P < 0.001). There was no evidence that species richness or diversity were different between shade treatments.
Two-way PERMANOVA demonstrates that the strongest influence on trait composition was conductivity (Pseudo-F1 = 34.174, P < 0.001). There was also strong evidence for the effect of shade on trait composition (Pseudo-F1 = 6.979, P = 0.002), but no evidence of an interaction (Pseudo-F5 = 1.749, P = 0.069), comparable to the results of the species PERMANOVA. There was no evidence of multivariate dispersion in the trait compositions between each conductivity and shade treatment. NMDS analysis (Fig. 5) of the functional composition demonstrates some relationships between small motile diatoms and conductivity. Motile small diatoms (MS2) diatoms plotted closely to samples from treatment 5 and treatment 6. Relative abundances of S3, S4, and S5 diatoms from the low, high, and motile guilds tended to plot with the lowest salinity treatments.
NMDS of the eco-morphological composition from each treatment. The first letter of the abbreviations refers to the diatom guild, and the second letter and associated number refers to the size class. Guilds: M, motile; P, Planktonic; H, high-profile; L, low-profile. Size class: S1 to S5 where higher numbers represent larger diatoms. Stress, indicating goodness-of-fit, was 0.09
There was strong evidence of differences in functional dispersion between the conductivity treatments (Kruskal–Wallis, H5 = 29.168, P < 0.001), with a notable decline at, or above, treatment 4 conductivity (1460—1500 μS cm−1) (Fig. 6). Treatments 5 and 6 clearly had the lowest functional dispersion, with the lower treatments having similar mean index values. Based on this measure, there was strong evidence that functional diversity had a moderate negative correlation with EC (Spearman’s rank: rs = − 0.55; P < 0.001). As with species diversity and richness, there was no evidence of functional differences between shade treatments. Functional dispersion had strong evidence of a moderate positive correlation with species richness (Spearman’s rank: rs = 0.439; P = 0.008).
Diatom–conductivity thresholds
Table 2 shows the major change points determined by Threshold Indicator Taxa ANalysis (TITAN) in the unshaded, shaded, and combined datasets, and Fig. 7 shows the differences and range, or narrowness, of responses. In the unshaded treatment, the median of responses was 1492 μS cm−1 for sensitive and 1611 μS cm−1 for tolerant, diatom species. In contrast, the median sensitive response was higher (at 1611 μS cm−1) in the shaded treatment, while tolerant responses occurred lower at 1486 μS cm−1. Thus, the fsumz- and fsumz + scores were alternating between the shade treatments, representing the key differences in TITAN’s analysis of both datasets.
a) The distribution of sensitive (fsumz-) and tolerant (fszum +) diatom community change points from Table 2 (NB: y-axis does not reflect strength of change). b) Key taxa TITAN identified as reliable bioindicators of freshwater salinity, and their relative abundance distribution along the EC gradient. Z-scores represent the magnitude, and emphasise the contribution, of taxon change in relation to the salinity gradient. Black lines are the species distribution median
At the 5th percentile of the change point distributions, the shaded treatment had both sensitive and tolerant responses occurring at a lower EC than in the unshaded treatment. For the 95th percentile, the differences in fsum(z) scores between the unshaded and shaded samples were negligible, indicating that 95% of all diatom communities had either declines in sensitive, or increases in tolerant, species occurring at, or before, 1616 μS cm−1. There was a narrow range of responses in both shade treatments over the EC gradient (880 to 1958 μS cm−1) (Fig. 7). At most, the difference between the 5th and 95th percentiles was 400 μS cm−1 (i.e. between the shaded fsumz- scores), indicating that change, whether in tolerant or sensitive species, occurred over a short EC range. Both the fsumz- and fsumz + responses in the unshaded treatment were slightly left-skewed, while the fsumz- responses in the shaded treatment were highly left-skewed. Approximately 5% of sensitive diatoms responded at a lower EC than tolerant species in the shaded treatment. However, the median of sensitive change occurred higher along the EC gradient.
Discussion
Water quality variation
The value of experimental studies in freshwater ecology is that they minimise the effect of potentially confounding variables on study outcomes. There was not strong evidence of differences in water quality variables (other than conductivity) between the salinity treatments with the exception of alkalinity (as carbonate) and potassium. Statistical tests show that the differences in alkalinity as carbonate likely did not affect pH variation between conductivity treatments. Previous studies suggest that potassium concentrations in this study (3.4 to 6.5 mg/L) were unlikely to affect the diatom assemblages (Jaworski et al., 2003).
Responses of diatoms to salinity and shade
Species assemblages were distinctly different between the source water and the experimental streams. Hence, it is likely that EC was a main driver in establishing the diatom assemblages. This indicates the appropriateness of the six-week experiment length (Kelly et al., 1998; Zidarova et al., 2020), and that diatom species composition is a reliable and valuable tool for monitoring water quality over this timeframe (Reid et al., 1995; Bennion et al., 2010; Stevenson & Pan, 2010).
The salinity treatments had the strongest effect on diatom communities. Salinity increases cellular osmotic pressure in organisms by reducing growth and nutrient uptake potential (Potapova & Donald, 2003; Cañedo-Argüelles et al., 2016, 2019; Kefford 2019). Marked differences in diatom assemblages were observed between the salinity treatments over a relatively short EC gradient (880 – 1960 μS cm−1) typical for permanent streams in the Mount Lofty Ranges (Anderson et al., 2019). Although diatom compositional changes in this study were likely sublethal, diatom responses are important ecological processes as they nevertheless affect the productivity, biodiversity, and trophic interactions, leading to cascading effects in freshwater habitats (Cañedo-Argüelles et al., 2014, 2016; Herbert et al., 2015). Species richness and diversity are negatively affected by increasing salinity (Cañedo-Argüelles et al., 2019), reaffirming the need to monitor water quality in heavily modified and, or agricultural landscapes. These results are commensurate with the strong influence of salinity on freshwater diatoms (Philibert et al., 2006; Pajunen et al., 2017; Passy et al., 2018; Soininen & Teittinen, 2019; Richards et al., 2020; Vélez-Agudelo et al., 2021).
Consideration of diatom autecology is important when using diatoms as bioindicators to manage freshwaters. In relation to increasing salinity, this experiment identified eleven negatively and three positively responding taxa. TITAN indicated a stronger response from sensitive species. N. acicularis, (Kützing) W.Smith 1853, and F. crotonensis were negatively affected between 1100 and 1500 μS cm−1, consistent with their affinity for conductivities below 1500 μS cm−1 (van Dam et al., 1994). N. palea, N. paleacea, and Navicula veneta were positive responders to salinity and are known tolerant species above 1600 μS cm−1 (van Dam et al., 1994; Soininen, 2002; Karacaoğlu & Dalkıran 2017).
Rhopalodia gibba and H. veneta were identified as sensitive species, despite previous TITAN-based studies in South Australia suggesting these species respond positively in freshwaters between 600 and 2500 μS cm−1 (Sultana et al., 2020; Tibby et al., 2020). Nitzschia inconspicua, Grunow 1862, is typically known as a tolerant species (Schröder et al., 2015; Tibby et al., 2020), but was sensitive in this experiment. Tolerance differences between diatom studies may result from antagonistic or synergistic effects caused by covarying chemical stressors, or biological interactions, that are inherent in natural streams (Bray et al., 2019; Piggott et al., 2015). It has been recently suggested that diatom tolerances are determined more by adaptations to local water quality rather than niche conservatism (the retention of ancestral traits) (Soininen & Teittinen, 2019), therefore, responses may vary in streams across the globe. As mesocosms can reduce confounding factors but retain certain biological interactions that occur in natural environments (Ledger et al., 2013; van Dam et al., 2019), they would be useful for future diatom studies to test the role of niche conservatism.
Trait-based approaches are promising aquatic management tools as they assess the relative ecological contribution and redundancy of species. In addition, they enable comparison between studies with different species composition (Tapolczai et al., 2016; Stenger-Kovács et al., 2018; Lengyel et al., 2020). Functional traits may also reduce conflicting findings about the environmental preferences and, or optima of diatoms, and results between trait-based studies will remain relevant despite future taxonomic revisions (Riato et al., 2022).
Increasing salinity in this study resulted in declines in diatom functional diversity and species richness. Functional dispersion in treatment 5 (1730 μS cm−1) and treatment 6 (1960 μS cm−1) was much lower than the less-saline treatments, indicating more functionally clustered diatom communities. The functional dispersion index is not affected by species richness (Laliberté & Legendre 2010), yet there was a clear positive relationship between diatom functional diversity and species richness. It has been postulated that diatom communities with more than 20 species are suitable to account for most functional traits, with greater richness resulting in ecological redundancy and a lower richness rapidly degrading functional diversities (Teittinen & Virta 2021). Our results suggest that a diatom richness above 23 (i.e. treatment 4) leads to redundancy in traits, but richness below this level (associated with conductivities above 1490 μS cm−1) can result in steep declines in diatom functionality. Therefore, future experimental studies should employ longer conductivity gradients and tools such as generalised additive models to assess potential nonlinear declines and, or thresholds in the relationship between salinity, diatom functionality, and species richness.
Functional clustering at higher conductivities was evident in analyses. There was a greater propensity for more diatom traits in treatment 1 and treatment 2 (and to a certain extent treatment 4). Treatments 5 and 6 were strongly characterised by small motile diatoms (MS2), supporting the functional dispersion results. This also demonstrated some relationships between diatom cell size and conductivity as there was a greater prevalence of medium to large-sized diatoms (S3, S4, and S5) from the low, high, and motile guilds in the two lowest salinity treatments. High salinity results in cellular membrane pressure (Cañedo-Argüelles et al., 2019; Kefford, 2019) and small cell size may be a diatom adaptation response to this pressure (Stenger-Kovács et al., 2018). Stenger-Kovács et al., (2018) found that S4 diatoms are more abundant in low salinity sites, and suggested MS1 diatoms may be indicative of high salinity conditions. However, our results were not definitive concerning the relationship between cell size and salinity, and might be due to the smaller conductivity range used in this experiment compared to Stenger-Kovács et al. (2018).
In this study, diatom community concentrations were strongly impacted by shade treatments, but responses in species richness and diversity and functional diversity were not observed, contrasting findings from other studies (Passy, 2007; Liess et al., 2009; Stenger-Kovács et al., 2018; Lange et al., 2011). Shaded treatments (91% lower lux) reduced diatom concentrations by an average of 270%, therefore, lower light inhibited community-wide diatom growth (Tornés & Sabater 2010). Shade also strongly influenced the species composition, as observed elsewhere (Leland et al., 2001; Laviale et al., 2009; Tornés & Sabater 2010; Lange et al., 2011; Liu et al., 2021); however, there was no distinct relationship between diatom functionality and shade. Reduced-light environments are thought to favour small cell-sized and low-profile diatoms like Achnanthidium minutissimum, due to morphological features like higher surface-to-volume ratios (allowing greater resource-utilisation efficiency) than larger diatoms, and adaptations to lower light (Passy, 2007; Laviale et al., 2009; Hill et al., 2011; Lange et al., 2011, 2015; Leira et al., 2015).
No interaction between salinity and shade influenced either diatom taxonomic or functional composition. These were unexpected results considering that reduced light and salinity are covarying stressors for diatoms. Species diversity and richness declines were primarily restricted to salinity rather than shade treatments. Contrary to these observations, TITAN indicated that the median of sensitive diatom species began to decline at a higher EC in the shaded channels than was observed in the unshaded treatment. One reason for the TITAN result may be the increase in relative abundances of smaller size diatom species Luticola mutica (Mann, 1990) and A. minutissimum at higher EC concentrations in the shaded channels. Regardless, these results indicate the predominant influence of EC on species and functional compositions and community metrics, with shade primarily impacting diatom concentration.
Salinity thresholds in the experimental streams
This study aimed to test whether a threshold of 1320 μS cm−1 (Tibby et al., 2020) is appropriate as a default water quality guideline in the South Australian Gulf Division. In this experimental study, TITAN indicated that a median salinity threshold of 1610 μS cm−1 exists for freshwater diatoms, regardless of shade coverage. This finding was supported by NMDS, which indicated that the two highest conductivity treatments (i.e. treatment 5, 1730 μS cm−1; treatment 6, 1960 μS cm−1) had the most distinct diatom assemblages, with the greatest difference between treatments 4 and 5 (1490 and 1730 μS cm−1). Hence, diatom taxonomic compositions in the artificial streams were not notably different immediately above and below the 1320 μS cm−1 threshold proposed by Tibby et al. (2020). The higher median threshold in this study may be due to the lack of multiple pressures on the experimental assemblages. Alternatively, an uneven environmental gradient in Tibby et al. (2020) may have led to an underestimation of the true threshold for diatoms in temperate South Australia.
Recent studies in South Australia have suggested that current default salinity guidelines for freshwater streams are set too high, and our study supports this conclusion. Sultana et al. (2019) showed that change points for stream macroinvertebrates exist at 600 μS cm−1, while a subsequent study in the same region found a diatom–salinity threshold of 1004 μS cm−1 (Sultana et al., 2020). Tibby et al. (2020) found that sensitive diatoms begin to decline at conductivities as low as 280 μS cm−1, while at 1320 μS cm−1 95% of sensitive, and 50% of tolerant, species begin to decline and increase, respectively. While our results suggest that 50% of both sensitive and tolerant species changed significantly at 1610 μS cm−1, these regional studies are all consistent, demonstrating that the upper limit of 5000 μS cm−1, still used as the default guideline value for the South Australian Gulf Division in the ANZG, is set too high.
These findings serve as multiple and varying lines of evidence (from both field and experimental settings) for a framework (ANZG 2018) that is reliant on a weight-of-evidence approach rather than just reference conditions. Here, a candidate salinity value of 1600 μS cm−1 is recommended for the South Australian Gulf Division to conserve freshwater diatom communities. However, the actual value should be derived by assessing relevant evidence (Sultana et al., 2019, 2020; Tibby et al., 2020) based on wider aquatic management objectives (Suter et al., 2017; van Dam et al., 2019; Mooney et al., 2020).
Study limitations
Water quality variables other than salinity may have influenced some diatom species in this study. Treatment 5 had higher TP in week 3 and higher TN in weeks 3 and 6 compared to the other treatments. Treatment 6 had elevated TN in weeks 3 and 6. These treatments had notable relative abundances of Navicula veneta and E. minima, which are known indicators of nutrient enrichment (Cochero et al., 2015; Delgado & Pardo, 2015; Nunes et al., 2019). Moreover, Nitzschia palea is widely known as a tolerant species to conductivity and nutrients (Soininen, 2002; Karacaoğlu & Dalkıran, 2017; Pajunen et al., 2017; Nunes et al., 2019) and was abundant in treatment 6 assemblages. Hence, it is difficult to determine whether the elevated relative abundances of these species are related to salinity alone.
Although sodium and chloride are the dominant ions in freshwaters in the Mount Lofty Ranges (Blackburn & McLeod, 1983; Herczeg et al., 2001; Poulsen et al., 2006), different ions dominate in other parts of the world, hampering direct comparison of diatom–salinity responses between locations (Cañedo-Argüelles et al., 2019). For example, calcium and magnesium are predominant ions in boreal and temperate streams of Europe and North America (Potapova & Donald, 2003; Soininen et al., 2004). Hence, future salinity–mesocosm studies could investigate if different ionic compositions alter conductivity tolerances, which, in turn, can help further assess the nature of diatom niche conservatism (Soininen et al., 2019; Soininen & Teittinen, 2019).
The species pool of the experiment was likely limited by the use of a single inoculum from the source water. Thresholds may change when a greater range of species (both sensitive and tolerant) are included. Future experiments should use more diverse diatom inocula by adding small amounts of a variety of substrates (e.g. vegetation and sediment) or cells concentrated in water samples from regional freshwaters.
Conclusion
Salinity in this experimental study was associated with significant changes in species composition of diatom assemblages at concentrations lower than the regional default water quality guideline value. The greatest dissimilarity between diatom communities existed between 1490 μS cm−1 and 1730 μS cm−1, and Threshold Indicator Taxa ANalysis (TITAN) determined a diatom threshold at 1610 μS cm−1. Moreover, functional diatom diversity had notable declines at conductivities above 1490 μS cm−1. As such, a candidate guideline value of 1600 μS cm−1 (with an uncertainty range of 1500–1750 μS cm−1) is recommended for updated freshwater salinity guidelines applicable to the South Australian Gulf Division. Our threshold value was higher than that proposed by Tibby et al. (2020) likely due to a reduced number of pressures on biota. This demonstrates both the value of mesocosms, precisely testing biotic responses to specific water quality stressors, and their limitation, lacking realistic interactions common in field studies. Therefore, mesocosms should act only as one line of evidence in a weight-of-evidence framework such as the ANZG. Wider experiment gradients with lower conductivities (especially < 500 μS cm−1) would provide more comprehensive experimental evidence for freshwater guidelines. These findings, alongside other regional field-based studies, demonstrate that water quality guideline values derived using reference-condition approaches are potentially hampering ecological management in freshwaters.
Data availability
All data are available at this online repository: https://doi.org/10.6084/m9.figshare.19970708
References
Abell, R., K. Vigerstol, J. Higgins, S. Kang, N. Karres, B. Lehner, A. Sridhar & E. Chapin, 2019. Freshwater biodiversity conservation through source water protection: Quantifying the potential and addressing the challenges. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1022–1038. https://doi.org/10.1002/aqc.3091.
Campos, C.A, M. J. Kennard & J. F. Gonçalves Júnior, 2021. Diatom and Macroinvertebrate assemblages to inform management of Brazilian savanna’s watersheds. Ecological Indicators 128. https://doi.org/10.1016/j.ecolind.2021.107834.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x.
Anderson, M. J., 2006. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 62: 245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x.
Anderson, M. J. & D. C. I. Walsh, 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monographs 83: 557–574. https://doi.org/10.1890/12-2010.1.
Anderson, T. A., E. A. Bestland, I. Wallis & H. D. Guan, 2019. Salinity balance and historical flushing quantified in a high-rainfall catchment (Mount Lofty Ranges, South Australia). Hydrogeology Journal 27: 1229–1244. https://doi.org/10.1007/s10040-018-01916-7.
Australian and New Zealand Environment and Conservation Council & Agriculture and Resource Management Council of Australia and New Zealand (ANZECC), 2000. Australian and New Zealand Guidelines for Fresh and Marine Water Quality.
Australian & New Zealand Guidelines for Fresh and Marine Water Quality (ANZG), 2018. https://www.waterquality.gov.au/anz-guidelines Accessed March 10 2021.
Baker, M., R. King & D. Kahle, 2020. TITAN2: Threshold Indicator Taxa Analysis.
Baker, M. & R. King, 2010. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods in Ecology and Evolution 1: 25–37. https://doi.org/10.1111/j.2041-210X.2009.00007.x.
B-Beres, V., A. Lukacs, P. Torok, Z. Kokai, Z. Novak, E. T-Krasznai, B. Tothmeresz & I. Bacsi, 2016. Combined eco-morphological functional groups are reliable indicators of colonisation processes of benthic diatom assemblages in a lowland stream. Ecological Indicators 64: 31–38. https://doi.org/10.1016/j.ecolind.2015.12.031.
Bennion, H., C. D. Sayer, J. Tibby & H. Carrick, 2010. Diatoms as indicators of environmental change in shallow lakes. In Smol, J. P. & E. F. Stoermer (eds), The Diatoms: Applications for the Environmental and Earth Sciences Cambridge University Press, Second Edition: 152–173.
Berthon, V., A. Bouchez & F. Rimet, 2011. Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south-eastern France. Hydrobiologia 673: 259–271. https://doi.org/10.1007/s10750-011-0786-1.
Birk, S., W. Bonne, A. Borja, S. Brucet, A. Courrat, S. Poikane, S. Angelo, W. van de Bund, N. Zampoukas & D. Hering, 2012. Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the water framework directive. Ecological Indicators 18: 31–41. https://doi.org/10.1016/j.ecolind.2011.10.009.
Blackburn, G. & S. McLeod, 1983. Salinity of atmospheric precipitation in the Murray-Darling drainage division, Australia. Soil Research 21: 411–434. https://doi.org/10.1071/SR9830411.
Bray, J. P., J. Reich, S. J. Nichols, G. Kon Kam King, R. Macally, R. Thompson, A. O’Reilly-Nugent & B. J. Kefford, 2019. Biological interactions mediate context and species-specific sensitivities to salinity. Philosophical Transactions of the Royal Society B. https://doi.org/10.1098/rstb.2018.0020.
Bunn, S., E. Abal, M. Smith, S. Choy, C. Fellows, B. Harch, M. Kennard & F. Sheldon, 2010. Integration of science and monitoring of river ecosystem health to guide investments in catchment protection and rehabilitation. Freshwater Biology 55: 223–240. https://doi.org/10.1111/j.1365-2427.2009.02375.x.
Cañedo-Argüelles, M., B. J. Kefford, C. Piscart, N. Prat, R. B. Schäfer & C.-J. Schulz, 2013. Salinisation of rivers: An urgent ecological issue. Environmental Pollution 173: 157–167. https://doi.org/10.1016/j.envpol.2012.10.011.
Cañedo-Argüelles, M., M. Bundschuh, C. Gutiérrez-Cánovas, B. J. Kefford, N. Prat, R. Trobajo & R. B. Schäfer, 2014. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Science of the Total Environment 476–477: 634–642. https://doi.org/10.1016/j.scitotenv.2013.12.067.
Cañedo-Argüelles, M., M. Sala, G. Peixoto, N. Prat, M. Faria, A. Soares, C. Barata & B. Kefford, 2016. Can salinity trigger cascade effects on streams? A mesocosm approach. Science of the Total Environment 540: 3–10. https://doi.org/10.1016/j.scitotenv.2015.03.039.
Cañedo-Argüelles, M., B. Kefford & R. Schäfer, 2019. Salt in freshwaters: causes, effects and prospects - introduction to the theme issue. Philosophical Transactions of the Royal Society B. https://doi.org/10.1098/rstb.2018.0002.
Cazzolla Gatti, R., 2016. Freshwater biodiversity: a review of local and global threats. International Journal of Environmental Studies 73: 887–904. https://doi.org/10.1080/00207233.2016.1204133.
Chapman, P. M., 2018. Environmental quality benchmarks—the good, the bad, and the ugly. Environmental Science and Pollution Research 25: 3043–3046. https://doi.org/10.1007/s11356-016-7924-2.
Chessman, B. C., 2021. What’s wrong with the Australian River Assessment System (AUSRIVAS)? Marine and Freshwater Research. https://doi.org/10.1071/MF20361.
Chessman, B. C., N. Bate, P. A. Gell & P. Newall, 2007. A diatom species index for bioassessment of Australian rivers. Marine and Freshwater Research 58: 542–557. https://doi.org/10.1071/MF06220.
Cochero, J., M. Licursi & N. Gómez, 2015. Changes in the epipelic diatom assemblage in nutrient rich streams due to the variations of simultaneous stressors. Limnologica 51: 15–23. https://doi.org/10.1016/j.limno.2014.10.004.
Cooper, S. D., P. S. Lake, S. Sabater, J. M. Melack & J. L. Sabo, 2013. The effects of land use changes on streams and rivers in mediterranean climates. Hydrobiologia 719: 383–425. https://doi.org/10.1007/s10750-012-1333-4.
Daniels, C. B. & K. Good, 2015. Building resilience to natural, climate and anthropocentric change in the Adelaide and Mount Lofty Ranges region: a natural resources management board perspective. Transactions of the Royal Society of South Australia 139: 83–96. https://doi.org/10.1080/03721426.2015.1035218.
Davies, P., 2000. Development of a national river bioassessment system (AUSRIVAS) in Australia. In Wright, J., D. Sutcliffe & M. Furse (eds) Assessing the Biological Quality of Fresh Waters: RIVPACS and Other Techniques. Freshwater Biological Association, Ambleside.
Delgado, C. & I. Pardo, 2015. Comparison of benthic diatoms from Mediterranean and Atlantic Spanish streams: Community changes in relation to environmental factors. Aquatic Botany 120: 304–314. https://doi.org/10.1016/j.aquabot.2014.09.010.
Dodds, W. K., J. S. Perkin & J. E. Gerken, 2013. Human Impact on Freshwater Ecosystem Services: A Global Perspective. Environmental Science & Technology 47: 9061–9068. https://doi.org/10.1021/es4021052.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2.
Elias, C. L., R. J. Rocha, M. J. Feio, E. Figueira & S. F. Almeida, 2017. Influence of the colonizing substrate on diatom assemblages and implications for bioassessment: a mesocosm experiment. Aquatic Ecology 51: 145–158. https://doi.org/10.1007/s10452-016-9605-0.
Feld, C. K., M. R. Fernandes, M. T. Ferreira, D. Hering, S. J. Ormerod, M. Venohr & C. Gutiérrez-Cánovas, 2018. Evaluating riparian solutions to multiple stressor problems in river ecosystems — A conceptual study. Water Research 139: 381–394. https://doi.org/10.1016/j.watres.2018.04.014.
Finlayson, M. & R. D’Cruz, 2005. Chapter 20: Inland water systems. In Hassan, R., R. Scholes & N. Ash (eds) Ecosystems and Human Well-Being: Current State and Trends. vol 1. Island Press.
Frizenschaf, J., L. Mosley, R. Daly & S. Kotz, 2015. Securing drinking water supply during extreme drought learnings from South Australia. Paper presented at the Drought: Research and Science-Policy Interfacing, Valencia, Spain.
Goldsmith, B., 1996. A rationale for the use of artificial substrata to enhance diatom-based monitoring of eutrophication in lowland rivers. Paper presented at the Environmental Change Research Centre: Research Papers, London.
González del Tánago, M., V. Martínez-Fernández, F. C. Aguiar, W. Bertoldi, S. Dufour, D. García de Jalón, V. Garófano-Gómez, D. Mandzukovski & P. M. Rodríguez-González, 2021. Improving river hydromorphological assessment through better integration of riparian vegetation: Scientific evidence and guidelines. Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2021.112730.
Grenier, M., I. Lavoie, A. Rousseau & S. Campeau, 2010. Defining ecological thresholds to determine class boundaries in a bioassessment tool: the case of the Eastern Canadian Diatom Index (IDEC). Ecological Indicators 10: 980–989. https://doi.org/10.1016/j.ecolind.2010.03.003.
Grubisic, M., G. Singer, M. C. Bruno, R. H. A. van Grunsven, A. Manfrin, M. T. Monaghan & F. Hölker, 2017. Artificial light at night decreases biomass and alters community composition of benthic primary producers in a sub-alpine stream. Limnology and Oceanography 62: 2799–2810. https://doi.org/10.1002/lno.10607.
Guariento, R. D., L. S. Carneiro, A. Caliman, R. L. Bozelli & F. A. Esteves, 2011. How light and nutrients affect the relationship between autotrophic and heterotrophic biomass in a tropical black water periphyton community. Aquatic Ecology 45: 561–569. https://doi.org/10.1007/s10452-011-9377-5.
Hart, B., P. Laker, A. Webb & M. Grace, 2003. Ecological risk to aquatic systems from salinity increases. Australian Journal of Botany 51: 689–702. https://doi.org/10.1071/BT02111.
Heneker, T. & D. Cresswell, 2010. Potential impact on water resource availability in the Mount Lofty Ranges due to climate change. Department for Water.
Herbert, E., P. Boon, B. Amy, S. Neubauer, R. Franklin, M. Ardón, K. Hopfensperger, L. Lamers & P. Gell, 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere. https://doi.org/10.1890/ES14-00534.1.
Herczeg, A. L., S. S. Dogramaci & F. W. J. Leaney, 2001. Origin of dissolved salts in a large, semi-arid groundwater system: Murray Basin, Australia. Marine and Freshwater Research 52: 41–52. https://doi.org/10.1071/MF00040.
Hess, S., E. Alve, T. Andersen & T. Joranger, 2020. Defining ecological reference conditions in naturally stressed environments - how difficult is it? Marine Environmental Research. https://doi.org/10.1016/j.marenvres.2020.104885.
Hill, W. R., B. J. Roberts, S. N. Francoeur & S. E. Fanta, 2011. Resource synergy in stream periphyton communities. Journal of Ecology 99: 454–463. https://doi.org/10.1111/j.1365-2745.2010.01785.x.
Hutchins, M. G., A. C. Johnson, A. Deflandre-Vlandas, S. Comber, P. Posen & D. Boorman, 2010. Which offers more scope to suppress river phytoplankton blooms: Reducing nutrient pollution or riparian shading? Science of the Total Environment 408: 5065–5077. https://doi.org/10.1016/j.scitotenv.2010.07.033.
Jaworski, G. H. M., J. F. Talling & S. I. Heaney, 2003. Potassium dependence and phytoplankton ecology: an experimental study. Freshwater Biology 48: 833–840. https://doi.org/10.1046/j.1365-2427.2003.01051.x.
Julius, M. & E. Theriot, 2010. The Diatoms: a primer. In Smol, J. P. & E. Stroeermer (eds), The Diatoms: Applications for the Environmental and Earth Sciences 2nd ed. Cambridge University Press, Cambridge: 8–22.
Karacaoğlu, D. & N. Dalkıran, 2017. Epilithic diatom assemblages and their relationships with environmental variables in the Nilüfer Stream Basin, Bursa. Turkey. Environmental Monitoring and Assessment 189: 227. https://doi.org/10.1007/s10661-017-5929-z.
Kefford, B., 2019. Why are mayflies (Ephemeroptera) lost following small increases in salinity? Three conceptual osmophysiological hypotheses. Philosophical Transactions of the Royal Society B. https://doi.org/10.1098/rstb.2018.0021.
Kefford, B., C. Palmer, L. Pakhomova & D. Nugegoda, 2004. Comparing test systems to measure the salinity tolerance of freshwater invertebrates. Water SA 30: 499–506. https://doi.org/10.4314/wsa.v30i4.5102.
Kefford, B. J., J. P. Bray, S. J. Nichols, J. Reich, R. Macally, A. Reillyugent, G. Kon Kam King & R. Thompson, 2022. Understanding salt-tolerance and biota–stressor interactions in freshwater invertebrate communities. Marine and Freshwater Research 73: 140–146. https://doi.org/10.1071/MF21164.
Kelly, M. G., A. Cazaubon, E. Coring, A. Dell’Uomo, L. Ector, B. Goldsmith, H. Guasch, J. Hürlimann, A. Jarlman, B. Kawecka, J. Kwandrans, R. Laugaste, E. A. Lindstrøm, M. Leitao, P. Marvan, J. Padisák, E. Pipp, J. Prygiel, E. Rott, S. Sabater, H. van Dam & J. Vizinet, 1998. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. Journal of Applied Phycology 10: 215. https://doi.org/10.1023/A:1008033201227.
Krammer, K. & H. Lange-Bertalot, 1986. Süßwasserflora von Mitteleuropa. Bacillariophyceae 1: Bacillariaceae, Epithemiacaeae, Surrirellaceae, vol 2. VEB Gustav Fischer Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1988. Süßwasserflora von Mitteleuropa. Bacillariophyceae 2: Bacillariaceae, Epithemiacaeae, Surrirellaceae. VEB Gustav Fischer Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1991a. Süßwasserflora von Mitteleuropa. Bacillariophyceae 3: Centrales, Fragilariaceae, Eunotiaceae. VEB Gustav Fischer Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1991b. Süßwasserflora von Mitteleuropa. Bacillariophyceae 4: Acnanthaceae. VEB Gustav Fischer Verlag, Jena.
Laliberté, E., P. Legendre & B. Shipley, 2022. Measuring Functional Diversity (FD) from Multiple Traits, and ther Tools for Functional Ecology.
Laliberté, E. & P. Legendre, 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91: 299–305. https://doi.org/10.1890/08-2244.1.
Lange, K., A. Liess, J. J. Piggott, C. R. Townsend & C. D. Matthaei, 2011. Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshwater Biology 56: 264–278. https://doi.org/10.1111/j.1365-2427.2010.02492.x.
Lange, K., C. R. Townsend & C. D. Matthaei, 2015. A trait-based framework for stream algal communities. Ecology and Evolution 6: 23–36. https://doi.org/10.1002/ece3.1822.
Laviale, M., J. Prygiel, Y. Lemoine, A. Courseaux & A. Créach, 2009. Stream periphyton photoacclimation response in field conditions: effect of community development and seasonal changes. Journal of Phycology 45: 1072–1082. https://doi.org/10.1111/j.1529-8817.2009.00747.x.
Ledger, M. E., L. E. Brown, F. K. Edwards, L. N. Hudson, A. M. Milner & G. Woodward, 2013. Chapter Six - Extreme Climatic Events Alter Aquatic Food Webs: A Synthesis of Evidence from a Mesocosm Drought Experiment. In Woodward, G. & E. J. O’Gorman (eds), Advances in Ecological Research, Vol. 48. Academic Press: 343–395.
Leira, M., M. L. Filippi & M. Cantonati, 2015. Diatom community response to extreme water-level fluctuations in two Alpine lakes: a core case study. Journal of Paleolimnology 53: 289–307. https://doi.org/10.1007/s10933-015-9825-7.
Leland, H. V., L. R. Brown & D. K. Mueller, 2001. Distribution of algae in the San Joaquin River, California, in relation to nutrient supply, salinity and other environmental factors. Freshwater Biology 46: 1139–1167. https://doi.org/10.1046/j.1365-2427.2001.00740.x.
Lengyel, E., B. Szabó & C. Stenger-Kovács, 2020. Realized ecological niche-based occupancy–abundance patterns of benthic diatom traits. Hydrobiologia 847: 3115–3127. https://doi.org/10.1007/s10750-020-04324-9.
Liess, A., K. Lange, F. Schulz, J. J. Piggott, C. D. Matthaei & C. R. Townsend, 2009. Light, nutrients and grazing interact to determine diatom species richness via changes to productivity, nutrient state and grazer activity. Journal of Ecology 97: 326–336. https://doi.org/10.1111/j.1365-2745.2008.01463.x.
Liu, X., L. Chen, G. Zhang, J. Zhang, Y. Wu & H. Ju, 2021. Spatiotemporal dynamics of succession and growth limitation of phytoplankton for nutrients and light in a large shallow lake. Water Research. https://doi.org/10.1016/j.watres.2021.116910.
McArdle, B. H. & M. J. Anderson, 2001. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 82: 290–297. https://doi.org/10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2.
Millán, A., J. Velasco, C. Gutiérrezánovas, P. Arribas, F. Picazo, Sánchezernández & P. Abellán, 2011. Mediterranean saline streams in southeast Spain: What do we know? Journal of Arid Environments 75: 1352–1359. https://doi.org/10.1016/j.jaridenv.2010.12.010.
Mooney, T. J., C. D. McCullough, A. Jansen, L. Chandler, M. Douglas, A. J. Harford, R. van Dam & C. Humphrey, 2020. Elevated magnesium concentrations altered freshwater assemblage structures in a mesocosm experiment. Environmental Toxicology and Chemistry 39: 1973–1987. https://doi.org/10.1002/etc.4817.
Muff, S., E. B. Nilsen, R. B. O’Hara & C. R. Nater, 2022. Rewriting results sections in the language of evidence. Trends in Ecology & Evolution 37: 203–210. https://doi.org/10.1016/j.tree.2021.10.009.
Nijboer, R., R. Johnson, P. Verdonschot, M. Sommerhauser & A. Buffagni, 2004. Establishing reference connditions for European streams. Hydrobiologia 516: 91–105. https://doi.org/10.1023/B:HYDR.0000025260.30930.f4.
Nunes, M. J., J. Adams & G. Bate, 2019. The use of epilithic diatoms grown on artificial substrata to indicate water quality changes in the lower reaches of the St Lucia Estuary South Africa. Water SA. https://doi.org/10.4314/wsa.v45i1.17.
Nuy, J. K., A. Lange, A. J. Beermann, M. Jensen, V. Elbrecht, O. Röhl, D. Peršoh, D. Begerow, F. Leese & J. Boenigk, 2018. Responses of stream microbes to multiple anthropogenic stressors in a mesocosm study. Science of the Total Environment 633: 1287–1301. https://doi.org/10.1016/j.scitotenv.2018.03.077.
Oksanen, J., G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. Minchin, R. B. O’Hara, G. Simpson, P. Solymos, H. Stevens, E. Szoecs & H. Wagner, 2020. Community Ecology Package: vegan. 2.5–7 edn. CRAN Repository.
Pajunen, V., M. Luoto & J. Soininen, 2017. Unravelling direct and indirect effects of hierarchical factors driving microbial stream communities. Journal of Biogeography 44: 2376–2385. https://doi.org/10.1111/jbi.13046.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany 86: 171–178. https://doi.org/10.1016/j.aquabot.2006.09.018.
Passy, S. I., C. A. Larson, A. Jamoneau, W. Budnick, J. Heino, T. Leboucher, J. Tison-Rosebery & J. Soininen, 2018. Biogeographical patterns of species richness and abundance distribution in stream diatoms are driven by climate and water chemistry. The American Naturalist 192: 605–617. https://doi.org/10.1086/699830.
Patil, I., 2021. ggstatsplot: Visualizatiions with statistical details: The ‘ggstatsplot’ approach. vol 6, Journal of Open Source Software.
Philibert, A., P. Gell, P. Newall, B. Chessman & N. Bate, 2006. Development of diatom-based tools for assessing stream water quality in south-eastern Australia: assessment of environmental transfer functions. Hydrobiologia 572: 103–114. https://doi.org/10.1007/s10750-006-0371-1.
Piggott, J. J., C. R. Townsend & C. D. Matthaei, 2015. Reconceptualizing synergism and antagonism among multiple stressors. Ecology and Evolution 5: 1538–1547. https://doi.org/10.1002/ece3.1465.
Potapova, M. G. & C. Donald, 2003. Distribution of benthic diatoms in U.S. rivers in relation to conductivity and ionic composition. Freshwater Biology 48: 1311–1328. https://doi.org/10.1046/j.1365-2427.2003.01080.x.
Poulsen, D. L., C. T. Simmons, C. Lealleaalle & J. W. Cox, 2006. Assessing catchment-scale spatial and temporal patterns of groundwater and stream salinity. Hydrogeology Journal 14: 1339–1359. https://doi.org/10.1007/s10040-006-0065-9.
R Core Team, 2021. R: A language and environment for statistical computing R Foundation for Statistical Computing, Austria, Vienna:
Rashid, M. M., S. Beecham & R. K. Chowdhury, 2015. Statistical characteristics of rainfall in the Onkaparinga catchment in South Australia. Journal of Water and Climate Change 6: 352–373. https://doi.org/10.2166/wcc.2014.031.
Reid, M. A., J. C. Tibby, D. Penny & P. A. Gell, 1995. The use of diatoms to assess past and present water quality. Australian Journal of Ecology 20: 57–64. https://doi.org/10.1111/j.1442-9993.1995.tb00522.x.
Riato, L., R. A. Hill, A. T. Herlihy, D. V. Peck, P. R. Kaufmann, J. L. Stoddard & S. G. Paulsen, 2022. Genus-level, trait-based multimetric diatom indices for assessing the ecological condition of rivers and streams across the conterminous United States. Ecological Indicators 141: 109131. https://doi.org/10.1016/j.ecolind.2022.109131.
Richards, J., J. Tibby, C. Barr & P. Goonan, 2020. Effect of substrate type on diatom-based water quality assessments in the Mount Lofty Ranges, South Australia. Hydrobiologia 847: 3077–3090. https://doi.org/10.1007/s10750-020-04316-9.
Rimet, F. & A. Bouchez, 2012. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowledge and Managemeent Aquatic Ecosystems. https://doi.org/10.1051/kmae/2012018.
Schröder, M., M. Sondermann, B. Sures & D. Hering, 2015. Effects of salinity gradients on benthic invertebrate and diatom communities in a German lowland river. Ecological Indicators 57: 236–248. https://doi.org/10.1016/j.ecolind.2015.04.038.
Sew, G. & P. Todd, 2020. Effects of salinity and suspended solids on tropical phytoplankton mesocosm communities. Tropical Conservation Science 13: 1–11. https://doi.org/10.1177/1940082920939760.
Soininen, J., 2002. Responses of epilithic diatom communities to environmental gradients in some finnish rivers. International Review of Hydrobiology 87: 11–24. https://doi.org/10.1002/1522-2632(200201)87:1%3c11::AID-IROH11%3e3.0.CO;2-E.
Soininen, J. & A. Teittinen, 2019. Fifteen important questions in the spatial ecology of diatoms. Freshwater Biology 64: 2071–2083. https://doi.org/10.1111/fwb.13384.
Soininen, J., R. Paavola & T. Muotka, 2004. Benthic diatom communities in boreal streams: community structure in relation to environmental and spatial gradients. Ecography 27: 330–342. https://doi.org/10.1111/j.0906-7590.2004.03749.x.
Soininen, J., A. Jamoneau, J. Rosebery, T. Leboucher, J. Wang, M. Kokociński & S. I. Passy, 2019. Stream diatoms exhibit weak niche conservation along global environmental and climatic gradients. Ecography 42: 346–353. https://doi.org/10.1111/ecog.03828.
Sonneman, J., A. Sincock, J. Fluin, M. Reid, P. Newall, J. Tibby & P. Gell, An illustrated guide to common stream diatom species from temperate Australia. In: Hawking, J. (ed) 2nd Australian Algal Workshop, Adelaide University, 2000. Cooperative Research Centre for Freshwater Ecology.
Stenger-Kovács, C., K. Körmendi, E. Lengyel, A. Abonyi, É. Hajnal, B. Szabó, K. Buczkó & J. Padisák, 2018. Expanding the trait-based concept of benthic diatoms: Development of trait- and species-based indices for conductivity as the master variable of ecological status in continental saline lakes. Ecological Indicators 95: 63–74. https://doi.org/10.1016/j.ecolind.2018.07.026.
Stenger-Kovács, C., E. Lengyel, K. Buczkó, J. Padisák & J. Korponai, 2019. Trait-based diatom functional diversity as an appropriate tool for understanding the effects of environmental changes in soda pans. Ecology and Evolution 10: 320–335. https://doi.org/10.1002/ece3.5897.
Stevenson, R. J. & Y. Pan, 2010. Assessing environmental conditions in rivers and streams with diatoms. In Stoermer, E. F. & J. P. Smol (eds), The Diatoms: Applications for the Environmental and Earth Sciences 2nd ed. Cambridge University Press, Cambridge: 57–85.
Stoddard, J., D. Larsen, C. Hawkins, R. Johnson & R. Norris, 2006. Setting expectations for the ecological conditon of streams: the concept of reference conditons. Ecological Applications 16: 1267–1276. https://doi.org/10.1890/1051-0761(2006)016[1267:SEFTEC]2.0.CO;2.
Sultana, J., F. Necknagel, J. Tibby & S. Maxwell, 2019. Comparison of water quality thresholds for macroinvertebrates in two Mediterranean catchments quantified by the inferential techniques TITAN and HEA. Ecological Indicators 101: 867–877. https://doi.org/10.1016/j.ecolind.2019.02.003.
Sultana, J., J. Tibby, F. Recknagel, S. Maxwell & P. Goonan, 2020. Comparison of two commonly used methods for identifying water quality thresholds in freshwater ecosystems using field and synthetic data. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2020.137999.
Suter, G., S. Cormier & M. Barron, 2017. A weight of evidence framework for environmental assessments: Inferring quantities. Integrated Environmental Assessment and Management 13: 1045–1051. https://doi.org/10.1002/ieam.1953.
Tapolczai, K., A. Bouchez, C. Stenger-Kovács, J. Padisák & F. Rimet, 2016. Trait-based ecological classifications for benthic algae: review and perspectives. Hydrobiologia 776: 1–17. https://doi.org/10.1007/s10750-016-2736-4.
Teittinen, A. & L. Virta, 2021. Exploring multiple aspects of taxonomic and functional diversity in microphytobenthic communities: effects of environmental gradients and temporal changes. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2021.668993.
Tibby, J., J. Richards, K. Tyler, C. Barr, J. Fluin & P. Goonan, 2020. Diatom-water quality thresholds in South Australian streams indicate a need for more stringent water quality guidelines. Marine and Freshwater Research 71: 942. https://doi.org/10.1071/MF19065.
Tornés, E. & S. Sabater, 2010. Variable discharge alters habitat suitability for benthic algae and cyanobacteria in a forested Mediterranean stream. Marine and Freshwater Research 61: 441–450. https://doi.org/10.1071/MF09095.
UNESCO, 2020. The United Nations World Water Development Report: Water and Climate Change, UNESCO, Paris:
USEPA, U.S.E.P.A., 2016. Weight of evidence in ecological assessment, EPA/100/R-16/001 Risk Assessment Forum. Washington, DC.
van Dam, H., A. Mertens & J. Sinkeldam, 1994. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherland Journal of Aquatic Ecology 28: 117–133. https://doi.org/10.1007/BF02334251.
van Dam, R., C. Humphrey, A. Harford, A. Sinclair, D. Jones, S. Davies & A. Storey, 2014. Site-specific water quality guidelines: 1. Derivation approaches based on physicochemical, ecotoxicological and ecological data. Environmental Science and Pollution Research 21: 118–130. https://doi.org/10.1007/s11356-013-1780-0.
van Dam, R., A. Hogan, A. Harford & C. Humphrey, 2019. How specific is site-specific? A review and guidance for selecting and evaluating approaches for deriving local water quality benchmarks. Integrated Environmental Assessment and Management 15: 683–702. https://doi.org/10.1002/ieam.4181.
VanLaarhoven, J. & M. van der Wielen, 2009. Environmental water requirements for the Mount Lofty Ranges prescribed water resources areas DWLBC Report 2009/29. Department of Water, Land and Biodiversity Conservation & South Australian Murrary-Darling Basin NRM Board, Adelaide.
Vélez-Agudelo, C., M. A. Espinosa & R. Fayó, 2021. Diatom-based inference model for conductivity reconstructions in dryland river systems from north Patagonia. Argentina. Aquatic Sciences 83: 64. https://doi.org/10.1007/s00027-021-00819-2.
Vörösmarty, C. J., P. B. McIntyre, M. O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S. E. Bunn, C. A. Sullivan, C. R. Liermann & P. M. Davies, 2010. Global threats to human water security and river biodiversity. Nature 467: 555–561. https://doi.org/10.1038/nature09440.
Wang, J., S. Meier, J. Soininen, E. Casamayor, F. Pan, X. Tang, X. Yang, Y. Zhang, Q. Wu, J. Zhou & J. Shen, 2017. Regional and global elevational patterns of microbial species richness and evenness. Ecography 40: 393–402. https://doi.org/10.1111/ecog.02216.
Wickham, H., W. Chang, L. Henry, T. Lin Pedersen, K. Takahashi, C. Wilke, K. Woo, H. Yutani & D. Dunnington, 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag.
Williams, W. D., 2001. Anthropogenic salinisation of inland waters. Hydrobiologia 466: 329–337. https://doi.org/10.1023/A:1014598509028.
Zhang, Y., J. Tao, J. Wang, L. Ding, C. Ding, Y. Li, Q. Zhou, D. Li & H. Zhang, 2019. Trends in diatom research since 1991 based on topic modeling. Microorganisms 7: 213. https://doi.org/10.3390/microorganisms7080213.
Zidarova, R., P. Ivanov & N. Dzhembekova, 2020. Diatom colonization and community development in Antarctic marine waters - a short-term experiment. Polish Polar Research 41: 187–212. https://doi.org/10.24425/ppr.2020.133012.
Acknowledgements
Thank you to the South Australian Environment Protection Authority (EPA) for funding the experimental setup and water quality analysis. Thank you to Dave Palmer (EPA) for providing logistical advice and support. Cameron Barr (the University of Adelaide) assisted with the study design and fieldwork. Many thanks to Eric DeSmit and Leith Barker from the South Australian Department for Environment and Water for access to the dam in Belair National Park and the provision of fire trucks and staff to transport water to the experiment site.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests or conflicts.
Additional information
Handling editor: Judit Padisák
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frost, C., Tibby, J. & Goonan, P. Diatom–salinity thresholds in experimental outdoor streams reinforce the need for stricter water quality guidelines in South Australia. Hydrobiologia 850, 2991–3011 (2023). https://doi.org/10.1007/s10750-023-05163-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05163-0