Abstract
The habitat quality of the littoral zone is of key importance for almost all lentic fish species. In anthropogenically created gravel pit lakes, the littoral zone is often structurally homogenized with limited fish habitats. We supplemented deadwood brush piles in the littoral zone of eight gravel pit lakes and investigated the diurnal and seasonal use of this and other typical microhabitats by six dominant fish species. Shoreline habitats were sampled using point abundance electrofishing during day and night in all four seasons, and patterns of fish abundance were compared amongst unstructured littoral habitats, emerged macrophytes and brush piles. We caught a total of 14,458 specimens from 15 species in the gravel pit lakes. Complex shoreline structures were used by all fish species that we examined, especially during daytime, whilst the use of unstructured habitats was highest during night. The newly added brush piles constituted suitable microhabitats for selected fish species, perch (Perca fluviatilis), roach (Rutilus rutilus) and pike (Esox lucius), particularly during winter. Supplemented deadwood provides suitable fish habitat in gravel pit lakes and may to some degree compensate for the loss of submerged macrophytes in winter by offering refuge and foraging habitat for selected fish species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple threats (e.g. habitat loss, pollution) negatively affect freshwater ecosystems (Arlinghaus et al., 2002; Collen et al., 2014; Reid et al., 2019), resulting in a pertinent biodiversity crisis (Dudgeon et al., 2006; Reid et al., 2019). Conservation and restoration of freshwater ecosystems are key policy goals (Geist, 2011; Geist & Hawkins, 2016; Tickner et al., 2020). In this context, human-created water bodies, such as ponds or quarry lakes, can contribute to biodiversity conservation (Seelen et al., 2021, 2022).
Artificial water bodies created by past mining activities, predominantly to quarry sand and gravel but also peat, clay, and chalk, are common water bodies globally (Blaen et al., 2016; Søndergaard et al., 2018; Nikolaus et al., 2021; Seelen et al., 2021, 2022). In 2019, over 26.000 active excavation sites existed in 24 European countries alone, with Germany being one of the leading sand and gravel producers (European Aggregates Association, 2019). In the Federal State of Lower Saxony (North-Western Germany) more than 37,000 drainable ponds and non-drainable sand and gravel pit lakes (< 20 ha size) currently exist, representing the vast majority (70%) of all stagnant waterbodies (Nikolaus et al., 2020). At such staggering numbers, pit and other quarry lakes are important supplementary habitats for colonization by aquatic species and may serve as areas for biodiversity conservation (Chester & Robson, 2013; Emmrich et al., 2014; Hill et al., 2015; Damnjanović et al., 2018; Oertli, 2018; Søndergaard et al., 2018; Vucic et al., 2019; Reyne et al., 2020; Nikolaus et al., 2021). Moreover, many artificial lakes, especially small ones between 1 and 20 ha, are intensively used for leisure activities and therefore, improving habitat quality for fishes and other wildlife may also enhance recreational quality and ecosystem services, specifically recreational fisheries (Meyerhoff et al., 2019; Seelen et al., 2022; Kaemingk et al., 2022).
The morphology of gravel pit lakes typically differs from natural lakes. For example, gravel pit lakes are on average deeper and have steeper depth gradients than natural lakes (Emmrich et al., 2014; Mollema & Antonellini, 2016; Søndergaard et al., 2018), which results in a reduced littoral zone-to-lake area ratio (Gasith, 1991). Littoral zones play an outstanding ecological role in lake ecosystems (Winfield, 2004; Moss, 2008) and offer important habitats for numerous lake fish species (Hall & Werner, 1977; Crowder & Cooper, 1982; Savino & Stein, 1982; Eklöv, 1997). Many freshwater fish use the littoral zone during particular or all ontogenetic life stages (Grimm & Klinge, 1996; Sammons & Bettoli, 1999; Schulze et al., 2006; Brosse et al., 2007).
Littoral habitat use by fish varies between day and night and amongst seasons caused by factors, such as spawning (Winfield, 2004; Chapman et al., 2011), predation (Lucas & Baras, 2001; Skov et al., 2013), foraging (Thorpe, 1974; Okun & Mehner, 2002), light and turbidity (Utne-Palm, 2002; Pekcan-Hekim & Lappalainen, 2006; Pekcan-Hekim et al., 2010). The habitat use of the littoral zone by fishes, specifically, smaller-bodied individuals, is crucially affected by the availability of microhabitat structures and shallow water zones, which serve as refuge from predation (Tonn & Magnuson, 1982; Hatzenbeler et al., 2000) especially in clear water conditions (Miner & Stein, 1996; Abrahams & Kattenfeld, 1997). Of key importance for many freshwater species is submerged vegetation, which serves as spawning habitat for phytophilic species such as tench (Tinca tinca (Linnaeus, 1758)) or pike (Esox lucius Linnaeus, 1758) and generally as refuge and foraging habitat for many other species subject to predation risk (Savino & Stein, 1989; Bry, 1996; Lewin et al., 2004; Järvalt et al., 2005). The greatest fish species diversity and abundance in the littoral zone of lakes can be observed during the summer months when most fish species have spawned and the larval and juvenile fishes use the warm and productive littoral zone for foraging and as refuge habitat (Hall & Werner, 1977; Fischer & Eckmann, 1997a; Hatzenbeler et al., 2000). However, the use of the littoral zone is species and size specific with some species like pike being present in the littoral zone during the entire year (Rossier, 1995; Hatzenbeler et al., 2000; Brosse et al., 2007; Kobler et al., 2008; Westrelin et al., 2021), whilst others—such as perch—are moving to deeper overwintering habitats as the temperature declines (Imbrock et al., 1996; Vehanen & Lahti, 2003; Westerberg & Sjöberg, 2015).
Abundant underwater vegetation can be a key microhabitat structure that adds resilience to lake ecosystems (Hilt et al., 2006; Scheffer & Jeppesen, 2007). However, cover and biovolume of aquatic vegetation decay when temperature and light intensity decrease in winter (Barko et al., 1982), resulting in a decline of available habitats for structure-dependent fish species (Grimm & Klinge, 1996). Most fish species prefer shallow littoral zones to avoid predation, especially during their early-life stages (Ruiz et al., 1993; Paterson & Whitfield, 2000) and may even overwinter in sheltered habitats in the littoral zone to reduce both their metabolic costs and risk of predation (Jacobsen et al., 2004; Shuter et al., 2012; McMeans et al., 2020). However, studies of habitat use by fish in winter are generally rare (Eklöv, 1997; Hatzenbeler et al., 2000; Jepsen & Berg, 2002; Brosse et al., 2007; Skov et al., 2008, 2013; Brönmark et al., 2010), which has been described as a general void of winter ecology in freshwater studies (Shuter et al., 2012).
Small individuals are particularly susceptible to predation (Mittelbach & Persson, 1998; Gaeta et al., 2018). To avoid predation they are dependent on either turbid conditions that interfere with the success of visual predators (Cook & Bergersen, 1988; Abrahams & Kattenfeld, 1997), shallow zones that limit access to larger-bodied predators (Ruiz et al., 1993; Paterson & Whitfield, 2000) or availability of structurally complex habitats like dense macrophyte stands, which reduce predator success rates, especially in clear waterbodies (Anderson, 1984; Diehl, 1988; Savino & Stein, 1989; Chick & McIvor, 1994; Rossier et al., 1996). In addition to submerged and emerged macrophytes, deadwood structures are important components of littoral zones that enhance the habitat quality for selected species of fish and other aquatic organisms (O’Connor, 1991; Everett & Ruiz, 1993; Lewin et al., 2004; Naimann & Latterell, 2005; Newbrey et al., 2005; Sass et al., 2006; Czarnecka, 2016). Analyses of species-specific use of specific littoral habitat structures repeatedly showed that juvenile pike, tench and rudd (Scardinius erythrophthalmus (Linnaeus, 1758)) strongly associate with emerged and submerged macrophytes, whereas perch (Perca fluviatilis Linnaeus, 1758), roach (Rutilus rutilus (Linnaeus, 1758)) and adult pike have also been reported to regularly use and sometimes prefer woody habitats (Casselman & Lewis, 1996; Lewin et al., 2014; Matern et al., 2021). These findings were obtained from daytime samples, but pronounced diurnal migrations of fish between littoral and pelagic habitats are well documented in lakes (Hall et al., 1979; Bohl, 1980; Gliwicz & Jachner, 1992; Haertel & Eckmann, 2002; Jůza et al., 2014; Nakayama et al., 2018). Larger fish migrate to the banks at night for foraging (Schulz & Berg, 1987; Kubečka, 1993; Wolter & Freyhof, 2004; Říha et al., 2011, 2015), whilst smaller fish often express reverse movements from the structured littoral to the open water column to predate on plankton when visually active predators are less able to hunt (Bohl, 1980; Gliwicz & Jachner, 1992; Gliwicz et al., 2006; Říha et al., 2015). The horizontal diurnal migration of small fish is typically explained by a trade-off between predator avoidance during daytime and resource availability in the form of pelagic dwelling zooplankton during low light conditions at dawn/dusk or during night (Bohl, 1980; Gliwicz & Jachner, 1992; Gliwicz et al., 2006; Říha et al., 2015). For example, Lewin et al. (2004) observed pronounced diel patterns of selection for shallow woody habitats in juvenile fish, specifically roach and perch, in a large German lake. However, much less is known about altered habitat choice of fish after structural enhancement as part of lake restorations and amongst seasons, especially during winter.
Human use and increasing development of lake shorelines have frequently led to a reduction in extent and quality of natural littoral structures (Ostendorp et al., 1995; Christensen et al., 1996; Chhor et al., 2020). Especially, the supply of coarse woody debris (CWD) in lakes is inversely related to human use intensity of lake shorelines and near-lake housing or other infrastructure (e.g. pier) development (Christensen et al., 1996; Jennings et al., 2003; Marburg et al., 2006). For example, research on CWD abundance in young gravel pit lakes with low amounts of large riparian trees, the main source for CWD in natural lakes (Marburg et al., 2006), revealed a lack of complex woody structures compared to natural lakes, which was explained by clean-up actions by recreational anglers removing wood (Matern et al., unpublished data). In gravel pit lakes low quantities of CWD together with limited littoral areas and potentially lower macrophyte abundance due to unstable sandy substrates (Emmrich et al., 2014; Vucic et al., 2019) can reduce the overall structural quality of littoral zones. Therefore, introductions of brush piles as structural habitat enhancement (Cowx & Gerdeaux, 2004; Hickley et al., 2004; Nagayama & Nakamura, 2010; Arlinghaus et al., 2016) may be a promising tool for improving the ecological state of gravel pit shore zones. In Danish lakes research on brush pile installation revealed that these structures may serve as habitats for selected species, such as pike (Skov & Berg, 1999).
The objective of the present study was to identify spatio-temporal patterns of littoral microhabitat use by fish in eight German gravel pit lakes, less than two years after their enhancement with deadwood brush piles. We tested the following hypotheses: I) structured habitats and specifically brush piles are a suitable habitat for various fish species in gravel pit lakes, especially in clear water conditions; II) the use of brush piles is length specific with large individuals preferring brush piles compared to typical young fish habitats (e.g. densely structured macrophytes or shallow water zones); III) use of littoral structures is higher during day compared to night when the use of open habitats increases and IV) microhabitat use of long-lasting brush piles increases in winter when the structural complexity of aquatic vascular plants decay. To test these hypotheses, eight gravel pit lakes that previously received littoral structure enhancement with deadwood brush piles, covering 20% of the shoreline length, were repeatedly electrofished during day and night over all four seasons of the year. This is the first study covering all seasons, day and night, using random point abundance sampling by electrofishing (PASE) in multiple lakes, because most previous studies were focused on individual lakes (e.g. Lewin et al., 2004) or covered only the daytime period (Fischer & Eckmann, 1997a; Brosse et al., 2007).
Methods
Study sites and brush pile implementation
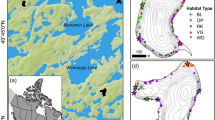
We sampled eight gravel pit lakes (Fig. 1) located in the Federal State of Lower Saxony, North-Western Germany (Matern et al., 2019). All lakes were part of the research project BAGGERSEE (www.baggersee-forschung.de), which investigated the effects of littoral habitat enhancements on fish communities in German gravel pit lakes (Radinger et al., in press). Each lake was enhanced with brush piles between December 2017 and March 2018 (Fig. S1). The brush piles consisted of deadwood in standardized circular bundles each 3 m long and 0.8 m in diameter. The deadwood bundles consisted predominantly of thin branches with mean diameter of 0.5–5.0 cm and included in maximum 2–3 branches with > 15 cm diameter (the overall range in branch diameter was 0.5–21 cm) originating from different, native wood species, mainly European hornbeam (Carpinus betulus L.), birch (Betula spp.) and alder (Alnus glutinosa (L.) Gaertn.). Branches were bundled and tied together with plant-based decomposable sisal ropes using a harvester machine (Pinox 828 forwarder and harvester unit, Pinox Oy, Finland). The bundles (each 300 kg) were transported attached to boats and released in preselected areas manipulating 20% of the lake’s shorelines. The brush piles were placed orthogonally to the shoreline in depths of one to three metres and weighed down using decomposable jute bags filled with gravel. All piles were placed within seven metres maximum distance to shore. In total, 800 brush piles were implemented to cover 20% of the littoral zone in each lake. Accordingly, the number of brush piles per lake varied between 30 in the smallest lake and 190 in the lake with the longest shoreline (Table 1).
Fish sampling
Fish abundance at the microhabitat level was assessed using PASE (Copp & Peňáz, 1988; Copp, 2010) during day and night and in four seasons: autumn (18 October 2018–27 October 2018), winter (10 January 2019–20 January 2019), spring (20 May 2019–31 May 2019) and summer (21 July 2019 –01 August 2019). PASE was conducted from a boat using a generator-powered electrofishing aggregate (8 kW; 150–300 V/300–600 V; EFKO 171 Fischfanggeräte GmbH Leutkirch, www.efko-gmbh.de) with a 4-m-long copper cathode and netted ring anode (ring diameter = 0.45 m, mesh size = 6 mm). Sampling was performed by rapidly immersing the activated anode for ten seconds close to the specific microhabitat. In complex structures such as brush piles, dense submerged and emerged macrophytes, electronic flux between the poles was halted three times to provoke the anodic reaction of fish.
The distance between point samples was kept large enough (at least 5 m) to generate independent samples (Copp, 2010). PASE started after sunrise for daytime fishing and after complete darkness for nighttime fishing. In each lake, day and night fishing were conducted within 24 h. Sampling locations for night fishing were randomly preselected during the day, marked by buoys and left out in day fishing to ensure that different points were always fished to avoid bias by repeated sampling. Sampling points were equally distributed along each lake’s shoreline cardinal direction. All dominant microhabitat structures in the littoral zone of the study lakes were sampled at random. At each point captured fish were determined to species level, counted, measured (total length, TL to the nearest mm) and released. The number per unit effort (NPUE) was calculated for each fish species as individuals per sampling point to enable comparisons of relative abundance within different structures, seasons and daytime.
Microhabitat assessment
The microhabitats in the littoral zone of the study lakes were categorized into six predefined habitat types: (1) open littoral, unvegetated littoral areas with no or low structural complexity, (2) supplemented deadwood, introduced deadwood brush piles, (3) natural deadwood, aggregation of snags, branches or submerged trees, (4) overhanging trees (overhanging branches of shoreline vegetation), often immersed into the waterbody, (5) emerged macrophytes, mainly common reed (Phragmites australis (Cav.) Trin. ex Steud.) stands, cattail (Typha spp.) and water mint (Mentha aquatica L.) and (6) submerged macrophytes, dominated by Elodea spp., Myriophyllum spp. and Stratiotes aloides L.
Abiotic data
We calculated lake-specific mean and maximum depth, total area and depth strata (CEN, 2015) percentages from contour maps (see Matern et al., (2019) for further details). Shoreline length was calculated using QGIS (QGIS Development Team, 2019). At every fishing event, Secchi depth was measured to cover lake turbidity. Conductivity was measured at the surface using a Multi 350i sensor, 164 device (WTW GmbH™, Weilheim, Germany). Total phosphorous (TP) was measured following the molybdenum blue method (Murphy & Riley, 1962; ISO, 2004). Water samples for phosphorous analyses were taken at the surface in the middle of the lake during each fishing event and mean values were estimated from all four samplings to generate robust results for mean annual concentrations.
Statistics
Three microhabitats, natural wood, overhanging trees and submerged macrophytes had to be excluded from further analyses, because of insufficient sample sizes (Table S1). Therefore, we only analysed the three most abundant littoral microhabitats (open littoral, deadwood brush piles and emerged macrophytes) using generalized linear mixed models (GLMM). We defined five model structures a priori. First, we modelled the species-specific NPUE as function of the interaction between the categorical explanatory variables of season (categorical, levels: spring, summer, autumn, winter) and microhabitat (categorical, levels: open littoral, deadwood brush piles, emerged macrophytes) and a random intercept for “lake” (categorical, eight levels) to identify season-specific differences in fish abundance amongst the microhabitats.
-
(1)
Number of Individuals per point (NPUE) ~ Season*Microhabitat + (1|Lake)
Second, to identify possible patterns of turbidity on the microhabitat use of fishes, species NPUE was modelled as a function of an interaction term of microhabitat and mean Secchi depth (continuous variable):
-
(2)
Number of Individuals per point (NPUE) ~ Mean Secchi depth*Microhabitat + (1|Lake)
Third, to identify potential diurnal effects within each season, we modelled species-specific NPUE as function of interactions between daytime (categorical, two levels: day, night) and microhabitat, including lake as a random intercept to account for the dependency of data through multiple measurements within each lake.
-
(3)
Number of Individuals per point (NPUE) ~ Daytime*Microhabitat + (1|Lake)
To identify length differences amongst the three microhabitats amongst seasons, GLMM models were ran in species subsets with fish length (continuous; total length in mm) as numerical-dependent variable against a function of the categorical variable microhabitat and lake as a random effect.
-
(4)
Fish length (mm) ~ Microhabitat + (1|Lake)
Fifth, to identify diurnal effects on fish size distribution in each of the sampled microhabitats amongst the seasons, fish length was modelled against an interaction of daytime and microhabitat and lake as a random effect.
-
(5)
Fish length (mm) ~ Daytime*Microhabitat + (1|Lake)
In total, for each of the six dominant species eel (Anguilla anguilla (Linnaeus, 1758)), perch, pike, roach, rudd and tench one model for patterns amongst seasons, one model for turbidity effects on microhabitat use, four models to identify diurnal patterns within each season, one model for size-specific patterns of microhabitat use amongst seasons and one model for diurnal size-specific patterns amongst microhabitats use were run. Species-specific models were run with data subsets, including only lakes with species occurrence.
GLMM models with negative binominal (NB) distribution with a log link function were estimated using the glmmTMB package (Brooks et al., 2017) and a dispersion parameter allowed for greater variances compared to the mean (Zeileis et al., 2008). The model residuals were tested for overdispersion and heteroscedasticity using the DHARMa package (Hartig, 2020). To account for heteroscedasticity, the dispersion parameter was adjusted using a log link function (Brooks et al., 2017). Zero-inflation was tested using the zero-inflation test implemented in the DHARMa package (Hartig, 2020). In two cases (season*microhabitat interaction model for perch and roach) overdispersion was detected after using a negative binominal distribution. However, as GLMMs are generally robust against violations of assumptions (Schielzeth et al., 2020) and for comparability the model structure was kept.
Pairwise comparisons amongst interactions of estimated marginal means using the emmeans package (Lenth et al., 2018) were applied as post hoc tests, using Tukey (HSD) p-value adjustment (Abdi & Williams, 2010). This procedure allowed direct comparisons amongst contrasts of the respective model to identify differences expressed by incidence rate ratios (IRR) in microhabitat-specific abundance and fish length across season and daytime. The IRR is a comparison of measured rates (e.g. the rate of abundance) between two groups, where an IRR value of one indicates the same rate in both groups, an IRR of 0.5 indicates half the rate in the second group compared to the first, and an IRR of two indicates double the rate in the second group compared to the first. All statistical analyses were performed in R version 3.6.1 (R Core Team, 2021).
Results
Lake environment
All gravel pit lakes were relatively young (mean age ± SD = 42.9 ± 7.96 years, range: 27–53 years; Table 1). The lakes covered a size range from 1.3 to 19.5 ha (mean ± SD = 8.4 ± 6.84 ha), and the shoreline length ranged from 417 to 2752 m with a mean ± SD of 1472.4 ± 802.2 m. Mean lake depth was 5.2 ± 3 m (range = 2.3 to 11.9 m). On average, the sampled lakes were characterized by a mean littoral share (Lake stratum to a depth of 3 m) of 27.6 ± 16%. Conductivity (mean ± SD) ranged from 216.2 ± 3.2 µS cm−1 in Collrunge to 642.5 ± 5.9 µS cm−1 in Meitzer See. The mean Secchi depth estimated for all sampling lakes amongst the sampling seasons was 2.7 ± 1.1 m, the highest mean Secchi depth of 3.9 ± 0.7 m was measured in Meitzer See, whereas the lowest mean Secchi depth was found in Saalsdorf (1.4 ± 0.5 m). Total phosphorous concentrations ± SD varied between 8.5 ± 5.4 µg l−1 in Meitzer See and 29.5 ± 15.8 µg l−1 in Saalsdorf (Table 1). Accordingly, the lakes we sampled were small and mesotrophic with steep depth gradients from the shore. All lakes were actively managed and exploited by recreational fisheries.
Fish sampling, species composition and general habitat preferences
In total, 4097 points were fished with an almost equal effort distribution amongst seasons (Nspring = 1020; 25%, Nsummer = 1158; 28%, Nautumn = 943; 23%, Nwinter = 976; 24%) and between day and night (Nday = 2083; 51%, Nnight = 2014; 49%; Table S1). Brush piles (N = 1206; 29%), open littoral (N = 1091; 27%) and emerged macrophytes (N = 843; 21%) were the most common structures found in the sampling lakes and thus, predominantly sampled through our random sampling design (Table S1). Overhanging trees (N = 349; 8%), natural deadwood (N = 323; 8%) and submerged macrophytes (N = 285; 7%) were scarce and thus, much less sampled, which ultimately did not allow further analyses.
A total of 14,458 specimens from 15 fish species were caught in the eight lakes. Perch and roach were the only species occurring in all eight lakes, whereas eel, pike and tench were each missing in one lake (Tables 2, Fig. S2). Rudd and bream occurred in four and five sampling lakes, respectively, whilst most other species were detected in only one lake (Table 2). Across all lakes, the most abundant species was perch with 8268 sampled fish (57% of the total catch), followed by 2728 roach (19% of the total catch). Perch was dominant in almost all microhabitats, except emerged macrophytes, where cyprinid species (rudd and roach) contributed most to the total catch (Fig. S2). Eel was caught in all microhabitats in low numbers. Pike was mainly caught in submerged and emerged macrophytes, but in overall low abundance (Fig. S2). Tench abundance was highest in the open littoral, under overhanging trees, and in artificial deadwood habitats (Fig. S2).
Microhabitat-specific size differences amongst the seasons
Generally, with eel as an exception, predominantly smaller individuals of each species were caught. The mean total length (meanTL) of fishes differed amongst the microhabitats and amongst the seasons. Overall, the size of eel caught in brush pile habitats was significantly larger (meanTL ± SD = 445.9 ± 142.2 mm) compared to both other microhabitats (open littoral—meanTL ± SD = 281.1 ± 136.4 mm/emerged macrophytes—meanTL ± SD = 359.2 ± 140.3 mm). In spring, summer and autumn, eel within the well-structured habitats were significantly larger than in the open littoral (Fig. 2, Tables 3, Table S3). Only in winter, when eel catches were generally low, no size difference was detected amongst eels in the different habitats (Fig. 2, Table 3, Table S3). Within all seasons perch caught in brush piles (meanTL ± SD = 92.5 ± 30.1 mm) and emerged macrophytes (meanTL ± SD = 93.1 ± 36.1 mm) were significantly larger than in the open littoral (meanTL ± SD = 85.2 ± 27 mm) (Fig. 2, Table 3, Table S3). In summer perch caught in brush piles were significantly larger than in the two other microhabitats (Fig. 2, Table 3, Table S3). Overall, the length of pike caught in brush piles (meanTL ± SD = 323.8 ± 127.4 mm) was on average larger than compared to both other habitats microhabitats (open littoral—meanTL ± SD = 266.7 ± 173.9 mm/emerged macrophytes—meanTL ± SD = 257.2 ± 146.4 mm); however, this difference was not statistically significant (Fig. 2, Tables 3, Table S3). MeanTL of pike did not differ significantly amongst the seasons (Fig. 2; Table 3, Table S3). Overall the roach individuals caught in the unstructured littoral (meanTL ± SD = 92.1 ± 51.2 mm) were significantly larger compared to both structured microhabitats (brush piles—meanTL ± SD = 63.4 ± 38.6 mm/emerged macrophytes—meanTL ± SD = 68.7 ± 25.3 mm) (Fig. 2, Tables 3, Table S3). This pattern was observed in spring, summer and winter. Comparison amongst structured habitats revealed that in spring and winter the size of roach caught within brush piles was significantly larger compared to individuals caught in emerged macrophytes (Fig. 2; Tables 3, Table S3). In all seasons, length of rudd caught in brush piles (meanTL ± SD = 86.7 ± 43.2 mm) and the open littoral (meanTL ± SD = 74.2 ± 34.9 mm) was significantly larger than in the emerged macrophytes (meanTL ± SD = 64.2 ± 34.2 mm), whereas there was no difference in length of rudd within brush piles and the open littoral (Fig. 2; Tables 3, Table S3). Only in autumn rudd caught in emerged macrophytes and the open littoral were significantly smaller than rudd caught in brush piles (Fig. 2; Tables 3, Table S3). Amongst all season, sizes of tench caught in emerged macrophytes (meanTL ± SD = 140.3 ± 104.7 mm) were significantly larger compared to the other main microhabitats (brush piles—meanTL ± SD = 68.9 ± 49.9 mm/open littoral—meanTL ± SD = 74.6 ± 58.2 mm) (Fig. 2; Tables 3, Table S3). In autumn, tench caught in brush piles and emerged macrophytes were significantly larger than tench caught in the open littoral (Fig. 2, Tables 3, Table S3).
Diurnal size differences of fish within the microhabitats
Diurnal size differences in each microhabitat amongst the seasons were found for roach, rudd and eel, whereas no significant diurnal size differences were observed for perch, pike and tench (Table 4). Eel and rudd caught in brush piles and the open littoral did not differ in size amongst day and night catches, but significantly larger individuals were detected during night in emerged macrophytes compared to daytime catches (Table 4). For roach individuals caught during night in the open littoral but also in emerged macrophytes were significantly larger compared to individuals detected during daytime (Table 4).
Seasonal variance in fish distributions amongst littoral microhabitats and effects of turbidity
Abundance patterns of the investigated species within microhabitats varied amongst seasons (Fig. 3; Table S4). In the open littoral winter catches were significantly lower for all fish species, except tench, compared to the other three seasons (Fig. 3; Table S4). In brush piles, relative abundances of perch and pike were highest in winter, and relative abundance of roach was elevated in winter compared to autumn and spring, but highest in summer (Fig. 3; Table S4). No significant differences in catches were detected between the structurally complex habitats emerged macrophytes and brush piles, although in the latter pike and roach catches tended to be higher in winter (Fig. 4; Table S5). Compared to the other two microhabitats, in brush piles perch catches in winter and tench catches in autumn were significantly greater (Fig. 4; Table S5). In emerged macrophytes, relative abundance of eel, pike and rudd were higher compared to open littoral and brush piles in all seasons (Fig. 4; Table S5).
Amongst the seasons two main effects of turbidity on species-specific fish catches were observed. Increasing water clarity generally had a positive effect on catches of eel and pike for all microhabitats (Table S6). By contrast, catches of perch, roach, rudd and tench decreased with increasing water clarity (Table S6). Interacting effects of turbidity and specific microhabitats were observed for some species (e.g. in perch with significantly decreasing predicted catches in emerged macrophytes in clearer water when compared to brush piles), whilst no effect was detected for other species (e.g. tench) (Fig. S3; Table S7).
Diurnal variances in fish distribution amongst littoral microhabitats
Relative abundance as revealed by PASE was usually higher at night compared to daytime, which was especially evident in the open littoral (Fig. 5; Table S8). Diurnal differences in microhabitat catch rates, however, differed according to species (Fig. 5; Table S8). In all microhabitats perch NPUE was always significantly higher during nighttime, except for brush piles in spring (Fig. 5; Table S8). By contrast, relative abundance of roach in the two structurally complex habitats was higher during daytime, especially in summer and winter (Fig. 5; Table S8). No significant differences between day and night samples were found for eel, pike, tench and rudd (Fig. 5; Table S8). In emerged macrophytes, relative abundance of rudd was only significantly higher at night compared to daytime during winter (Fig. 5; Table S8).
Discussion
We studied the spatio—temporal patterns in microhabitat use of fish in eight gravel pit lakes. Our findings provide partial support for our first hypothesis as perch, roach, eel and tench were caught in high proportions in the supplemented deadwood brush piles, particularly during the colder months of the year, but differences to the other littoral habitats were only significant for perch in winter and tench in autumn. Furthermore, we did not observe effects of turbidity on microhabitat use when the unstructured microhabitat was compared to the well-structured microhabitats. Only catches of eel and pike increased with increasing water clarity, whilst catches of the other species decreased with water clarity. Our second hypothesis that larger individual fish prefer brush pile habitats was only supported for eel and perch where we caught larger individuals amongst all seasons in the brush pile habitats. By contrast, amongst seasons smaller cyprinid specimens (roach and rudd) were found in the well-structured brush pile and emerged macrophyte habitats, whereas larger individuals were caught in the open littoral. Our third hypothesis was not supported as we did not detect increased use of structured habitats during the day compared to night for any of the species studied; rather we found species-specific differences in diurnal use of littoral structures. For example, during the day roach abundance was higher in structured habitats compared to unstructured habitats in summer and winter, whilst perch abundance was generally higher during the night in all sampled habitats, with two exceptions in brush piles in spring and summer. In addition to active habitat use, results might also be affected by light-dependent catchability of the electrofishing unit (see below). Supporting our fourth hypothesis, we detected significantly greater species-specific abundance in brush piles compared to unstructured littoral areas in winter. In addition, perch abundance was greater in brush piles than in emerged macrophytes, indicating the relevance of this microhabitat for perch specifically.
Use of littoral structures and implemented brush piles
We focused on six species (eel, perch, pike, roach, rudd, tench) typically occurring in gravel pit lakes and other temperate European lakes (Emmrich et al., 2014; Matern et al., 2019, 2022). All of these species were found to use the newly added brush piles throughout the year, and in some cases (e.g. perch), we detected elevated abundances of fish in brush piles compared to other structures. Also, structured habitat was often hosting larger fish abundances (e.g. eel, pike and rudd) compared to the unstructured open littoral. In particular, eel was strongly associated with structured habitats, mainly emerged macrophytes, supporting previous studies on the habitat choice of this species (Laffaille et al., 2001; Ovidio et al., 2013; Lewin et al., 2014; Matern et al., 2021). Abundances of perch and roach were also strongly associated with structurally complex habitats, including artificially implemented deadwood in some seasons, which was expected based on previous work investigating microhabitats in a natural lake and corresponding natural occurrences of deadwood (Lewin et al., 2004).
Vegetated microhabitats are known to be a key-structured habitat in most lakes and indeed, submerged and emerged macrophyte stands have been identified as key habitat structures for pike (Grimm & Backx, 1990; Eklöv, 1997; Kobler et al., 2008; Matern et al., 2021), rudd (Eklöv & Hamrin, 1989; Lewin et al., 2014; Matern et al., 2021) and tench (Perrow et al., 1996; Lewin et al., 2014). Similarly, in our work, we found emerged macrophytes highly important, especially for rudd, whereas tench and pike were also associated with woody habitats in autumn and winter. In gravel pit lakes, previous research at meso-habitat scales already showed that pike abundance was not associated with the extent of submerged macrophytes, but was positively related to the degree of deadwood habitat (Matern et al., 2021). Accordingly and in line with literature (e.g. Skov & Berg, 1999) supplemented deadwood brush piles offered suitable habitats for pike and other typical lake fish species.
Structurally complex habitats, however, are known to be less important for predation-prone fishes with increasing turbidity, as the hunting success for visual hunting predators is impeded (Cook & Bergersen, 1988; Abrahams & Kattenfeld, 1997; Utne-Palm, 2002; Snickars et al., 2004). Hence, even though the turbidity gradient amongst the lakes was rather small and eutrophic turbid lakes were not included in our study, turbid conditions should have positively impacted the catch rates in the unstructured littoral and in contrast lowered the catches in the structurally complex habitats (Miner & Stein, 1996; Abrahams & Kattenfeld, 1997). Indeed, we found turbidity to positively impact catch rates of perch, roach, rudd and tench in all microhabitats, but did not detect significant differences in fish abundance when the unstructured microhabitat was compared with the structurally complex microhabitats amongst different turbidity levels (Fig. S3, Table S7). The generally positive effect of turbidity on fish abundance was likely related to increased productivity in turbid lakes (Persson et al., 1991; Olin et al., 2002) and/or a generally higher catch efficiency due to lower escape distances of fishes (Korman & Yard, 2017). In contrast to this pattern, abundances of eel and pike increased with decreasing turbidity; however, water clarity affected the abundance in structured and unstructured habitats in the same manner as indicated by a lack of clear interaction effects amongst turbidity and habitat type. As visually hunting predators (Casselman & Lewis, 1996), pike are more effective predators in clearwater conditions where they mostly rely on submerged structures, especially macrophytes (Jacobsen & Engström-Ost, 2018). Hence pike abundances and pike recruitment are described to peak in lakes of intermediate trophic state (which are often quite clear) (Haugen & Vøllestad, 2018), likely explaining the positive effect of increased water clarity on pike abundances in our study. In isolated gravel pit lakes eel abundances depend on stocking (Emmrich et al., 2014; Matern et al., 2021), hence in our sampling lakes eel catches are best explained by stocking intensity, suggesting that clearer waterbodies by chance had higher stocking rates or lower exploitation rates post stocking. Generally, we did not observe the expected shifts in habitat use intensity according to varying turbidity states, most likely because of a relatively narrow turbidity gradient across the mesotrophic sampling lakes.
Size-specific use of littoral microhabitats
We found species-specific variation of fish size distribution in the different studied littoral microhabitats. Amongst the seasons, significantly larger individuals of eel and perch were found in brush piles and emerged macrophytes, but also larger pike were more frequently caught in the brush piles. By contrast, the average size of the cyprinids roach and rudd caught in the structured habitats was lower compared to open habitats. Whereas, juvenile roach and rudd are known to be strongly associated with dense structures such as reed stands where they seek shelter from predation during daytime (Kennedy & Fitzmaurice, 1974; Bohl, 1980; Gliwicz et al., 2006; Nakayama et al., 2018), larger individuals especially of roach are less reliable on structural complexity and are known for inshore movements during night (Wolter & Freyhof, 2004; Říha et al., 2015), which likely explains the greater fish size in the open littoral in the dark. Another reason could simply be reduced fleeing reactions of the larger roach during the night. Tench of all size classes as a cryptic species (e.g. Weatherley, 1959) are known to favour well-structured littoral habitats (Perrow et al., 1996; Herrero et al., 2003; Moreno et al., 2003). Similar to findings reported by Perrow et al. (1996), we found large tench individuals in emerged macrophytes stands, whereas rather small individuals of tench were caught in brush piles (where smaller individuals might have found shelter in the crevices beneath the branches) and in the open littoral (where small tench might have found shelter in benthic coarse organic debris (e.g. accumulations of fallen leaves). The ability of smaller individuals to hide in a vast variety of coarse substrates (e.g. Fischer & Eckmann, 1997b; Christoffersen et al., 2018; Nilsson et al., 2020; Steendam et al., 2020) likely explains why the mean size of eel in the open unstructured littoral was significantly lower compared to individuals caught in the more complex structures, especially in brush piles. In contrast to juveniles, larger eels are known to depend on more complex shelter such as woody structures (e.g. roots) especially during daytime (Baras et al., 1998; Ovidio et al., 2013). Larger individuals of perch and pike were also found in the complex habitats compared to the open littoral, which especially holds true for larger perch individuals in brush piles, which are known to be associated with woody habitats in lakes (e. g. Westrelin et al., 2018; Matern et al., 2021). The brush pile habitats offer accumulations of small sized prey fish such as roach and well-suited hunting conditions for predatory species that rely on structure–open water interfaces where they are able to ambush their prey (Eklöv & Diehl, 1994; Casselman & Lewis, 1996; Eklöv, 1997). Hence, different size classes of typical species in gravel pit lakes benefit from structurally complex microhabitats, with larger individuals especially of predatory species benefitting from improved hunting conditions and smaller specimens, especially of cyprinid species, finding shelter within these habitats.
Diurnal variation in littoral use
In line with other studies, species-specific abundance revealed by electrofishing at night was substantially greater than during daytime (Dumont & Dennis, 1997; Pierce et al., 2001; Ross et al., 2016). This finding was particularly evident in the unstructured littoral microhabitat and might either represent active habitat choice [e.g. foraging in profitable patches of zooplankton or benthos at lower predation risk due to diurnal horizontal migration (Lewin et al., 2004; Gliwicz et al., 2006)] or reflect improved catchability at night (Alabaster & Stott, 1978; Paragamian, 1989). However, the result of greater abundance at night was not general across all six species investigated. Specifically, diurnal differences were identified for perch and roach, moving from the structured habitats to the open littoral at night (Bohl, 1980; Copp & Jurajda, 1993; Lewin et al., 2004; Gliwicz et al., 2006). Juvenile perch are reported to leave their groups and be more broadly distributed in open habitats during nighttime (Copp & Jurajda, 1993; Wang & Eckmann, 1994; Haertel & Eckmann, 2002). Additionally, perch in mesotrophic lakes have been reported to move from the pelagic to the littoral where they remain during night (Jacobsen et al., 2015; Nakayama et al., 2018). Our work thus agrees with previous reports on species-specific diurnal behaviours.
Pattern of diurnal horizontal migrations have been observed for roach and can be explained by higher predation risk during daytime, but also greater zooplankton availability in the open habitats during nighttime (Gliwicz & Jachner, 1992; Okun & Mehner, 2002, 2005; Lewin et al., 2004; Gliwicz et al., 2006; Schulze et al., 2006). Hence, some fish leave the safe-structured habitats during night and swim into open water areas when predation pressure by visually hunting predators (e.g. perch or pike) is reduced (Pitcher & Turner, 1986). This behaviour likely contributed to the observed higher roach abundance during night in the unstructured littoral and higher abundances during daytime in well-structured habitats in summer and winter. In addition, inshore movements by larger individuals (e.g. Říha et al., 2015) during nighttime, as observed in our study, might have caused greater catches in the littoral zone during the night. By contrast, catches of eel, pike, rudd and tench remained generally unaffected by time of day as these species strongly depend on various littoral structures throughout the entire day (Lewin et al., 2014; Matern et al., 2021), rendering diel habitat shifts less relevant and less pronounced compared to perch and roach. Most likely, also elevated catchability in open water during low visibility conditions likely contributed to the roach patterns revealed in our work.
Seasonal variation in littoral use
We detected differences in habitat use intensity amongst seasons, most clearly expressed during winter, when many species were rather structure oriented and far less frequently observed in the unstructured habitats. The use intensities of perch, roach and pike in the structured habitats were especially high during winter. The underlying mechanisms might have differed according to species, but can generally be explained by a seasonal habitat shift into sheltered structures as survival strategy to lower predation risk at reduced foraging and metabolic costs in winter (Shuter et al., 2012). Following the decay of submerged macrophyte stands, structural oriented fish (e.g. pike) are forced to use other available structures during the colder phases of the year (e.g. Grimm & Klinge, 1996; Baade & Fredrich, 1998). Pike as structure-dependent sit-and-wait predator (Grimm & Klinge, 1996) might have found better cover conditions and prey availability around the constantly present woody habitats (e.g. Skov & Berg, 1999). Roach and perch were also found in increasing numbers in the brush pile microhabitats during the colder phases of the year, most likely to reduce their predation risk, which was not only higher due to the presence of pike foraging during winter (Diana & Mackay, 1978) but potentially also due to the higher occurrence of winter migrating piscivorous birds (Orpwood et al., 2010; Lemmens et al., 2016), primarily cormorants (Phalacrocorax carbo (Linnaeus, 1758)), frequently observed on the sampling lakes. As lower temperatures affect the physiology of poikilothermic fish leading to limitation of maximum swimming speed and general activity (e.g. Claireaux et al., 2006), probabilities of evading attacks by piscivores are reduced at low water temperature. Hence, remaining in the persistent structurally complex deadwood structures likely reduced the predation risk and predation-related stress during winter (e.g. Jacobsen et al., 2004). Only eel abundance was low within artificial deadwood structures during winter, likely because of avoidance of shallow zones and dormancy behaviour expressed by low activity rates when temperatures decrease (Walsh et al., 1983; Westerberg & Sjöberg, 2015).
Fish aggregations in seasonally robust structures, such as woody structures, as a response to changing conditions in winter observed here are in agreement with previous research, which showed that fish using supplemented woody habitats had reduced predation risk and higher survival chance (Russell et al., 2008; Orpwood et al., 2010; Lemmens et al., 2016). However, when both predator (e.g. perch) and prey share the same habitat during winter, it can create an ecological trap for the prey (Robertson & Hutto, 2006). Hence, an increase of long-lasting complex deadwood structures in gravel pit lakes, that otherwise lack structural complexity, certainly increases the availability of shelter to predation-prone fish, potentially leading to an increased winter survival and generally better conditioned fish. Alternatively, aggregations of piscivorous fish together with their prey might lead to higher predation rates, reversing the positive outcomes of shelter for the prey fish. Answering the latter question, however, needs before-after-control-impact study designs. Our work only examined the habitat use and distribution of fish and did not study how artificial brush piles might have affected total abundance of fish.
Limitations
We used electrofishing to identify fish distributional patterns, which has different efficiencies according to species and size classes (Dolan & Miranda, 2003; Menezes et al., 2013; Rümmler, 2015). Hence, certain species and size classes might be underrepresented in the present dataset; however, our results are based on intraspecific comparisons across habitat types and should, thus, not be affected by gear selectivity. Additionally, electrofishing is less effective in deeper and unstructured habitats (Bohlin et al., 1989), which could have resulted in lower and, hence, biased catches in open water habitat. Specifically, daytime catches might be underestimated when fish detect the approaching boat and escape earlier, whilst at night escape distance is less (e.g. Paragamian, 1989). However, electrofishing is widely used and results are robust concerning species diversity and abundance, especially when applied in complex habitats where other methods are not applicable (Jurajda et al., 2009; Copp, 2010; Mueller et al., 2017). Further studies of microhabitat use in deeper littoral areas of especially cautious, larger fish that were underrepresented here might use scuba diving (e.g. Brosse et al., 2001) or camera-based observations (Ellender et al., 2012) to avoid this sampling bias.
Longevity of brush pile structures and effects on water quality
Decomposition rates of our brush piles and thus longevity of management measures remain unknown. Hardwood as used in our study is known to decompose slower in aquatic compared to terrestrial environments (Bilby et al., 1999) and mass loss can be very slow (France et al., 1997). As a consequence, brush piles made from hardwood can potentially last for decades under water (Bilby et al., 1999). In agreement with this assumption we did not observe visible reductions of brush piles within the first years after application. Leaching of nutrients from the deadwood was not measured, but no changes in pH values and nutrient compositions were observed on the lake level before and after brush pile addition (Arlinghaus et al., in press), indicating no significant changes in water chemistry as a consequence of deadwood addition to mesotrophic lakes.
Conclusion and implications
The present study showed the generally high relevance of structurally heterogeneous microhabitats for common fish species in the littoral zones of gravel pit lakes throughout all seasons. The efficiency of deadwood brush piles immediately after supplementation was indicated by its attraction of selected fish species, especially during the colder phase of the year. Hence, habitat enhancement by adding deadwood structures increases the overall habitat availability for structurally oriented fish and might lead to higher fish abundance of some species, especially in artificial water bodies (Radinger et al., in press). It is recommended that fisheries managers consider deadwood applications, especially in shallow areas, to support fish populations by improving the structural complexity of littoral zones rather than solely relying on stocking or harvest regulations (e.g. Sass et al., 2017). Authorities could support such deadwood applications by keeping bureaucratic hurdles low. However, this study identified spatio-temporal dynamics, i.e. effects of deadwood provision on fish distribution rather than fish productivity or abundance. Further research is needed to differentiate distributional effects of habitat placement from additive effects on abundance. Alternatives to deadwood implementations, such as creation of shallow water zones where underwater vegetation can develop, should also be investigated in terms of effects on habitat use and abundance, because deadwood installation in deeper water might also serve as an ecological trap for prey fish by attracting both predators and prey. By contrast, shallow water zones might be less accessible to larger predators and thus more effective in raising fish abundance than deadwood placements (Radinger et al., in press).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdi, H. & L. J. Williams, 2010. Tukey’s honestly significant difference (HSD) test. In Salkind, N. (ed), Encyclopedia of Research Design Sage, Thousand Oaks, CA: 1–5.
Abrahams, M. & M. Kattenfeld, 1997. The role of turbidity as a constraint on predator-prey interactions in aquatic environments. Behavioral Ecology and Sociobiology 40: 169–174.
Alabaster, J. S. & B. Stott, 1978. Swimming activity of perch, Perca fluviatilis L. Fish Biology 12: 587–591.
Anderson, O., 1984. Optimal foraging by largemouth bass in structured environments. Ecology 65: 851–861.
Arlinghaus, R., M. Emmrich, D. Hühn, S. Schälicke, W.-C. Lewin, T. Pagel, T. Klefoth & T. Rapp, 2016. Ufergebundene Fischartenvielfalt fischereilich gehegter Baggerseen im Vergleich zu eiszeitlich entstandenen Naturseen in Norddeutschland. Fischer & Teichwirt 8: 288–290.
Arlinghaus, R., T. Mehner & I. G. Cowx, 2002. Reconciling traditional inland fisheries management and sustainability in industrialized countries, with emphasis on Europe. Fish and Fisheries 3: 261–316.
Arlinghaus, R., Klefoth, T., Matern, S., Radinger, J., Nikolaus, N., Meyerhoff, J., Schafft, M., Cyrus, E.-M., Emmrich, M., Hering, D. & Wolter, C. (in press). Biodiversität, Angeln und Gesellschaft: Wissensbasierte Empfehlungen für ein nachhaltiges Fischereimanagement an Baggerseen. Berichte des IGB, Band 32.
Baade, U. & F. Fredrich, 1998. Movement and pattern of activity of the roach in the River Spree, Germany. Journal of Fish Biology 52: 1165–1174.
Baras, E., D. Jeandrain, B. Serouge & J. C. Philippart, 1998. Seasonal variations in time and space utilization by radio-tagged yellow eels Anguilla anguilla (L.) in a small stream. Hydrobiologia 371–372: 187–198.
Barko, J. W., D. G. Hardin & M. S. Matthews, 1982. Growth and morphology of submersed freshwater macrophytes in relation to light and temperature. Canadian Journal of Botany 60: 877–887.
Bilby, R. E., J. T. Heffner, B. R. Fransen, J. W. Ward & P. A. Bisson, 1999. Effects of immersion in water on deterioration of wood from five species of trees used for habitat enhancement projects. North American Journal of Fisheries Management 19: 687–695.
Blaen, P. J., M. A. Macdonald & R. B. Bradbury, 2016. Ecosystem services provided by a former gravel extraction site in the UK under two contrasting restoration states. Conservation & Society 14: 48–56.
Bohl, E., 1980. Diel pattern of pelagic distribution and feeding in planktivorous fish. Oecologia 44: 368–375.
Bohlin, T., S. Hamrin, T. G. Heggberget, G. Rasmussen & S. J. Saltveit, 1989. Electrofishing – theory and practice with special emphasis on salmonids. Hydrobiologia 173: 9–43.
Brönmark, C., J. Brodersen, B. B. Chapman, A. Nicolle, P. A. Nilsson, C. Skov & L. A. Hansson, 2010. Regime shifts in shallow lakes: the importance of seasonal fish migration. Hydrobiologia 646: 91–100.
Brooks, M. E., K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Mächler & B. M. Bolker, 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal 9: 378–400.
Brosse, S., G. D. Grossman & S. Lek, 2007. Fish assemblage patterns in the littoral zone of a European reservoir. Freshwater Biology 52: 448–458.
Brosse, S., P. Laffaille, S. Gabas & S. Lek, 2001. Is scuba sampling a relevant method to study fish microhabitat in lakes? Examples and comparisons for three European species. Ecology of Freshwater Fish 10: 138–146.
Bry, C., 1996. Role of vegetation in the life cycle of pike. In Craig, J. F. (ed), Pike: Biology and Exploitation Springer, Dodrecht: 45–67.
Casselman, J. M. & C. A. Lewis, 1996. Habitat requirements of northern pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 53: 161–174.
CEN, 2015. Water quality—Sampling of fish with multi-mesh gillnets. Brussels, Germany, https://standards.globalspec.com/std/9929986/EN 14757.
Chapman, B., C. Brönmark, J. Nilsson & L. Hansson, 2011. The ecology and evolution of partial migration. Oikos 120: 1764–1775.
Chester, E. T. & B. J. Robson, 2013. Anthropogenic refuges for freshwater biodiversity: their ecological characteristics and management. Biological Conservation 166: 64–75.
Chhor, A. D., D. M. Glassman, J. P. Smol, J. C. Vermaire & S. J. Cooke, 2020. Ecological consequences of shoreline armoring on littoral fish and benthic macroinvertebrate communities in an Eastern Ontario lake. Aquatic Sciences 82: 1–13.
Chick, J. H. & C. C. McIvor, 1994. Patterns in the abundance and composition of fishes among beds of different macrophytes: viewing a littoral zone as a landscape. Canadian Journal of Fisheries and Aquatic Science 51: 2973–2882.
Christensen, D. L., B. R. Herwig, D. E. Schindler & S. R. Carpenter, 1996. Impacts of lakeshore residential development on coarse woody debris in north temperate lakes. Ecological Applications 6: 1143–9.
Christoffersen, M., J. C. Svendsen, J. A. Kuhn, A. Nielsen, A. Martjanova & J. G. Støttrup, 2018. Benthic habitat selection in juvenile European eel Anguilla anguilla: implications for coastal habitat management and restoration. Journal of Fish Biology 93: 996–999.
Claireaux, G., C. Couturier & A. L. Groison, 2006. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). Journal of Experimental Biology 209: 3420–3428.
Collen, B., F. Whitton, E. E. Dyer, J. E. M. Baillie, N. Cumberlidge, W. R. T. Darwall, C. Pollock, N. I. Richman, A. Soulsby & M. Böhm, 2014. Global patterns of freshwater species diversity, threat and endemism. Global Ecology and Biography 23: 40–51.
Cook, M. F. & E. P. Bergersen, 1988. Movements, habitat selection, and activity periods of northern pike in eleven mile reservoir, Colorado. Transactions of the American Fisheries Society 117: 495–502.
Copp, G. H., 2010. Patterns of diel activity and species richness in young and small fishes of European streams: a review of 20 years of point abundance sampling by electrofishing. Fish and Fisheries 11: 439–460.
Copp, G. H. & P. Jurajda, 1993. Do small riverine fish move inshore at night? Journal of Fish Biology 43: 229–241.
Copp, G. H. & M. Peňáz, 1988. Ecology of fish spawning and nursery zones in the flood plain, using a new sampling approach. Hydrobiologia 169: 209–224.
Cowx, I. G. & D. Gerdeaux, 2004. The effects of fisheries management practises on freshwater ecosystems. Fisheries Management and Ecology 11: 145–151.
Crowder, L. B. & W. E. Cooper, 1982. Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813.
Czarnecka, M., 2016. Coarse woody debris in temperate littoral zones: implications for biodiversity, food webs and lake management. Hydrobiologia 767: 13–25.
Damnjanović, B., M. Novković, A. Vesić, M. Živković, S. Radulović, D. Vukov, A. Anđelković & D. Cvijanović, 2018. Biodiversity-friendly designs for gravel pit lakes along the Drina River floodplain (the Middle Danube Basin, Serbia). Wetlands Ecology and Management 27: 0–20.
Diana, J. S. & W. C. Mackay, 1978. Timing and magnitude of energy deposition and loss in the body, liver, and gonads of northern pike (Esox lucius). Journal of the Fisheries Board of Canada 36: 481–487.
Diehl, S., 1988. Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53: 207–214.
Dolan, C. R. & L. E. Miranda, 2003. Immobilization thresholds of electrofishing relative to fish size. Transactions of the American Fisheries Society 132: 969–976.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. Kawabata, R. J. Naiman, D. J. Knowler & C. Le, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Dumont, S. C. & J. A. Dennis, 1997. Comparison of day and night electrofishing in Texas reservoirs. North American Journal of Fisheries Management 17: 939–946.
Eklöv, P., 1997. Effects of habitat complexity and prey abundance on the spatial and temporal distributions of perch (Perca fluviatilis) and pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 54: 1520–1531.
Eklöv, P. & S. Diehl, 1994. Piscivore efficiency and refuging prey: the importance of predator search mode. Oecologia 98: 344–353.
Eklöv, P. & S. Hamrin, 1989. Predatory efficiency and prey selection: Interactions between pike Esox lucius, perch Perca fluviatilis and rudd Scardinus erythrophthalmus. Oikos 56: 149–156.
Ellender, B. R., A. Becker, O. L. F. Weyl & E. R. Swartz, 2012. Underwater video analysis as a non-destructive alternative to electro fishing for sampling imperilled headwater stream fishes. Aquatic Conservation: Marine and Freshwater Ecosystems 22: 58–65.
Emmrich, M., S. Schälicke, D. Hühn, C. Lewin & R. Arlinghaus, 2014. No differences between littoral fish community structure of small natural and gravel pit lakes in the northern German lowlands. Limnologica 46: 84–93.
European Aggregates Association, 2019. Annual review 2018–2019. European aggregates association. https://uepg.eu/mediatheque/media/UEPG-Annual-Review-2018-2019.pdf.
Everett, R. A. & G. M. Ruiz, 1993. Coarse woody debris as a refuge from predation in aquatic communities. Oecologia 93: 475–486.
Fischer, P. & R. Eckmann, 1997a. Seasonal changes in fish abundance, biomass and species richness in the littoral zone of a large European lake, Lake Constance, Germany. Archiv für Hydrobiologie 139: 433–448.
Fischer, P. & R. Eckmann, 1997b. Spatial distribution of littoral fish species in a large European lake, Lake Constance, Germany. Archiv für Hydrobiologie 140: 91–116.
France, R., H. Culbert, C. Freeborough & R. Peters, 1997. Leaching and early mass loss of boreal leaves and wood in oligotrophic water. Hydrobiologia 345: 209–214.
Gaeta, J. W., T. D. Ahrenstorff, J. S. Diana, W. W. Fetzer, S. Jones, Z. J. Lawson, M. C. Mcinerny, V. J. Santucci & M. J. Vander, 2018. Go big or... don’ t? A field-based diet evaluation of freshwater piscivore and prey fish size relationships. PLoS ONE 13: 1–20.
Gasith, A., 1991. Can littoral resources influence ecosystem processes in large, deep lakes? Internationale Vereinigung für Theoretische und Angewandte Limnologie: Verhandlungen 24: 1073–1076.
Geist, J., 2011. Integrative freshwater ecology and biodiversity conservation. Ecological Indicators 11: 1507–1516.
Geist, J. & S. J. Hawkins, 2016. Habitat recovery and restoration in aquatic ecosystems: current progress and future challenges. Aquatic Conservation: Marine and Freshwater Ecosystems 26: 942–962.
Gliwicz, Z. M. & A. Jachner, 1992. Diel migrations of juvenile fish: a ghost of predation past or present? Archiv für Hydrobiologie 124: 285–410.
Gliwicz, Z. M., J. Slon & I. Szynkarczyk, 2006. Trading safety for food: evidence from gut contents in roach and bleak captured at different distances offshore from their daytime littoral refuge. Freshwater Biology 51: 823–839.
Grimm, M. P. & J. J. G. M. Backx, 1990. Pike, aquatic vegetation and nutrient concentration. Hydrobiologia 200: 557–566.
Grimm, M. P. & M. Klinge, 1996. Pike and some aspects of its dependence on vegetation. In Craig, J. F. (ed), Pike: Biology and Exploitation Springer, Amsterdam: 125–156.
Haertel, S. & R. Eckmann, 2002. Diel diet shift of roach and its implications for the estimation of daily rations. Journal of Fish Biology 60: 876–892.
Hall, D. J. & E. E. Werner, 1977. Seasonal distribution and abundance of fishes in the littoral zone of a Michigan lake. Transactions of the American Fisheries Society 106: 545–555.
Hall, D. J., E. E. Werner, J. F. Gilliam, G. G. Mittelbach, D. Howard, C. G. Doner, J. A. Dickerman & A. J. Stewart, 1979. Diel foraging behavior and prey selection in the golden shiner (Notemigonus crysoleucas). Journal of the Fisheries Research Board of Canada 36: 1029–1039.
Hartig, F., 2020. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. http://florianhartig.github.io/DHARMa/.
Hatzenbeler, G. R., M. A. Bozek, M. J. Jennings & E. E. Emmons, 2000. Seasonal variation in fish assemblage structure and habitat structure in the nearshore littoral zone of Wisconsin lakes. North American Journal of Fisheries Management 20: 360–368.
Haugen, T. O. & L. A. Vøllestad, 2018. Pike population size and structure: influence of density-dependent and density-independent factors. In Skov, C. & P. A. Nilsson (eds), Biology and Ecology of Pike CRC Press, Boca Raton: 123–163.
Herrero, M. J., J. A. Madrid & F. J. Sánchez-Vázquez, 2003. Entrainment to light of circadian activity rhythms in tench (Tinca tinca). Chronobiology International 20: 1001–1017.
Hickley, P., R. Arlinghaus, R. Tyner, M. Aprahamian, K. Parry & M. Carter, 2004. Rehabilitation of urban lake fisheries for angling by managing habitat: general overview and case studies from England and Wales. Ecohydrology & Hydrobiology 4: 365–378.
Hill, M. J., K. L. Mathers & P. J. Wood, 2015. The aquatic macroinvertebrate biodiversity of urban ponds in a medium-sized European town (Loughborough, UK). Hydrobiologia 760: 225–238.
Hilt, S., E. M. Gross, M. Hupfer, H. Morscheid, J. Mählmann, A. Melzer, J. Poltz, S. Sandrock, E. M. Scharf, S. Schneider & K. van de Weyer, 2006. Restoration of submerged vegetation in shallow eutrophic lakes—a guideline and state of the art in Germany. Limnologica 36: 155–171.
Imbrock, F., A. Appenzeller & R. Eckmann, 1996. Diel and seasonal distribution of perch in Lake Constance: a hydroacoustic study and in situ observations. Journal of Fish Biology 49: 1–13.
ISO, 2004. ISO 6878: Water quality - Determination of phosphorus - Ammonium molybdat spectrometric method. Geneva, Switzerland, https://www.sis.se/api/document/preview/904914/.
Jacobsen, L., S. Berg, H. Baktoft & C. Skov, 2015. Behavioural strategy of large perch Perca fluviatilis varies between a mesotrophic and a hypereutrophic lake. Journal of Fish Biology 86: 1016–1029.
Jacobsen, L., S. Berg, N. Jepsen & C. Skov, 2004. Does roach behaviour differ between shallow lakes of different environmental state? Journal of Fish Biology 65: 135–147.
Jacobsen, L., & J. Engström-Ost, 2018. Coping with environments: vegetation, turbidity and abiotics In Skov, C., & P. A. Nilsson (eds), Biology and ecology of pike. CRC Press, Boca Raton, pp 32–61.
Järvalt, A., T. Krause & A. Palm, 2005. Diel migration and spatial distribution of fish in a small stratified lake. Hydrobiologia 547: 197–203.
Jennings, M. J., E. E. Emmons, G. R. Hatzenbeler, C. Edwards & M. A. Bozek, 2003. Is littoral habitat affected by residential development and land use in watersheds of Wisconsin lakes? Lake and Reservoir Management 19: 272–279.
Jepsen, N. & S. Berg, 2002. The use of winter refuges by roach tagged with miniature radio transmitters Lake Stigsholm. Hydrobiologia 483: 161–165.
Jurajda, P., M. Janáč, S. M. White & M. Ondračková, 2009. Small – but not easy: evaluation of sampling methods in floodplain lakes including whole-lake sampling. Fisheries Research 96: 102–108.
Jůza, T., M. Vašek, M. Kratochvíl, P. Blabolil, P. Blabolil, M. Čech, V. Draštík, J. Frouzová, M. Muška, J. Peterka, M. Prchalová, M. Říha, M. Tušer & J. Kubečka, 2014. Chaos and stability of age-0 fish assemblages in a temperate deep reservoir: unpredictable success and stable habitat use. Hydrobiologia 724: 217–234.
Kaemingk, M. A., R. Arlinghaus, M. H. Birdsong, C. J. Chizinski, R. Lyach, K. L. Wilson & K. L. Pope, 2022. Matching of resource use and investment according to waterbody size in recreational fisheries. Fisheries Research 254: 1–6.
Kennedy, M. & P. Fitzmaurice, 1974. Biology of the rudd Scardinius erythrophthalmus (L.) in Irish Waters. Proceedings of the Royal Irish Academy Section B: Biological, Geological, and Chemical Science 74: 245–303.
Kobler, A., T. Klefoth, C. Wolter, F. Fredrich & R. Arlinghaus, 2008. Contrasting pike (Esox lucius L.) movement and habitat choice between summer and winter in a small lake. Hydrobiologia 601: 17–27.
Korman, J. & M. D. Yard, 2017. Effects of environmental covariates and density on the catchability of fish populations and interpretation of catch per unit effort trends. Fisheries Research 189: 18–34.
Kubečka, J., 1993. Night inshore migration and capture of adult fish by shore seining. Aquaculture Research 24: 685–689.
Laffaille, P., S. Brosse & S. Gabas, 2001. Fish spatial distribution in the littoral zone of Lake Pareloup (France) during summer. Archiv für Hydrobiologie 153: 129–144.
Lemmens, P., L. De Meester & S. A. J. Declerck, 2016. Can underwater refuges protect fish populations against cormorant predation? Evidence from a large-scale multiple pond experiment. Fisheries Management and Ecology 23: 89–98.
Lenth, R., H. Singmann, & J. Love, 2018. Emmeans: Estimated marginal means, aka least-squares means. https://github.com/rvlenth/emmeans.
Lewin, W. C., T. Mehner, D. Ritterbusch & U. Brämick, 2014. The influence of anthropogenic shoreline changes on the littoral abundance of fish species in German lowland lakes varying in depth as determined by boosted regression trees. Hydrobiologia 724: 293–306.
Lewin, W. C., N. Okun & T. Mehner, 2004. Determinants of the distribution of juvenile fish in the littoral area of a shallow lake. Freshwater Biology 49: 410–424.
Lucas, M. C. & E. Baras, 2001. Migration of freshwater fishes, Blackwell Science ltd, Oxford, UK.
Marburg, A. E., M. G. Turner & T. K. Kratz, 2006. Natural and anthropogenic variation in coarse wood among and within lakes. Journal of Ecology 94: 558–568.
Matern, S., M. Emmrich, T. Klefoth, C. Wolter, R. Nikolaus, N. Wegener & R. Arlinghaus, 2019. Effect of recreational-fisheries management on fish biodiversity in gravel pit lakes, with contrasts to unmanaged lakes. Journal of Fish Biology 94: 865–881.
Matern, S., T. Klefoth, C. Wolter & R. Arlinghaus, 2021. Environmental determinants of fish abundance in the littoral zone of gravel pit lakes. Hydrobiologia 848: 2449–2471.
Matern, S., T. Klefoth, C. Wolter, A. Hussner, J. Simon, & R. Arlinghaus, 2022. Fish community composition in small lakes: the impact of lake genesis and fisheries management. Freshwater Biology 67: 2130–2147.
McMeans, B. C., K. S. McCann, M. M. Guzzo, T. J. Bartley, C. Bieg, P. J. Blanchfield, T. Fernandes, H. C. Giacomini, T. Middel, M. D. Rennie, M. S. Ridgway & B. J. Shuter, 2020. Winter in water: differential responses and the maintenance of biodiversity. Ecology Letters 23: 922–938.
Menezes, R. F., F. Brochsenius, J.-C. Svenning, M. Søndergaard, T. L. Laruidsen, F. Landkildehus & E. Jeppesen, 2013. Variation in fish community structure, richness, and diversity in 56 Danish lakes with contrasting depth, size, and trophic state: does the method matter? Hydrobiologia 710: 47–59.
Meyerhoff, J., T. Klefoth & R. Arlinghaus, 2019. The value artificial lake ecosystems provide to recreational anglers: implications for management of biodiversity and outdoor recreation. Journal of Environmental Management 252: 1–12.
Miner, J. G. & R. A. Stein, 1996. Detection of predators and habitat choice by small bluegills: effects of turbidity and alternative prey. Transactions of the American Fisheries Society 125: 97–103.
Mittelbach, G. & L. Persson, 1998. The ontogeny of piscivory and its ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences 55: 1454–1465.
Mollema, P. N. & M. Antonellini, 2016. Water and (bio)chemical cycling in gravel pit lakes: a review and outlook. Earth-Science Reviews 159: 247–270.
Moreno, P. R., J. M. Gallardo, E. García Ceballos, J. J. P. Regadera & J. C. E. García, 2003. Determination of substrate preferences of tench, Tinca tinca (L.), under controlled experimental conditions. Journal of Applied Ichthyology 19: 138–141.
Moss, B., 2008. The kingdom of the shore: achievement of good ecological potential in reservoirs. Freshwater Reviews 1: 29–42.
Mueller, M., J. Pander, J. Knott & J. Geist, 2017. Comparison of nine different methods to assess fish communities in lentic flood-plain habitats. Journal of Fish Biology 91: 144–174.
Murphy, J. & J. P. Riley, 1962. A modified songle solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36.
Nagayama, S. & F. Nakamura, 2010. Fish habitat rehabilitation using wood in the world. Landscape and Ecological Engeneering 6: 289–305.
Naimann, R. J. & J. J. Latterell, 2005. Principles for linking fish habitat to fisheries management and conservation. Journal of Fish Biology 67: 166–185.
Nakayama, S., P. Doering-arjes, S. Linzmaier, J. Briege, T. Klefoth, T. Pieterek & R. Arlinghaus, 2018. Fine-scale movement ecology of a freshwater top predator, Eurasian perch (Perca fluviatilis), in response to the abiotic environment over the course of a year. Ecology of Freshwater Fish 27: 798–812.
Newbrey, M. G., M. A. Bozek, M. J. Jennings & J. E. Cook, 2005. Branching complexity and morphological characteristics of coarse woody structure as lacustrine fish habitat. Canadian Journal of Fisheries and Aquatic Sciences 62: 2110–2123.
Nikolaus, R., S. Matern, M. Schafft, T. Klefoth, A. Maday, C. Wolter, A. Manfrin, J. U. Lemm & R. Arlinghaus, 2020. Einfluss anglerischer Bewirtschaftung auf die Biodiversität von Baggerseen: Eine vergleichende Studie verschiedener gewässergebundener Organismengruppen. Lauterbornia 87: 153–181.
Nikolaus, R., M. Schafft, A. Maday, T. Klefoth, C. Wolter & R. Arlinghaus, 2021. Status of aquatic and riparian biodiversity in artificial lake ecosystems with and without management for recreational fisheries: Implications for conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 153–172.
Nilsson, P. A., I. J. Pettersson, C. Tamario, E. Degerman, J. Elghagen, J. Watz & O. Calles, 2020. Substrate-size choice in European eel (Anguilla anguilla) elvers is not altered by piscivore chemical cues. Journal of Fish Biology 96: 1534–1537.
O’Connor, N. A., 1991. The effects of habitat complexity on the macroinvertebrates colonising wood substrates in a lowland stream. Oecologia 85: 504–512.
Oertli, B., 2018. Editorial: freshwater biodiversity conservation: the role of artificial ponds in the 21st century. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 264–269.
Okun, N. & T. Mehner, 2002. Reed as an alternative habitat for young fish in a shallow eutrophic lake. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Verhandlungen 28: 1669–1672.
Okun, N. & T. Mehner, 2005. Distribution and feeding of juvenile fish on invertebrates in littoral reed (Phragmites) stands. Ecology of Freshwater Fish 14: 139–149.
Olin, M., M. Rask, J. Ruuhijärvi, M. Kurkilahti, P. Ala-Opas & O. Ylönen, 2002. Fish community structure in mesotrophic and eutrophic lakes of southern Finland: the relative abundances of percids and cyprinids along a trophic gradient. Journal of Fish Biology 60: 593–612.
Orpwood, J. E., M. S. Miles, I. C. Russell & J. D. Armstrong, 2010. Efficacy of artificial shelters for roach, Rutilus rutilus, against predators in the presence of reeds. Fisheries Management and Ecology 17: 356–365.
Ostendorp, W., C. Iseli, M. Krauss, P. Krumscheid-Plankert, J. Moret, M. Rollier & F. Schanz, 1995. Lake shore deterioration, reed management and bank restoration in some Central European lakes. Ecological Engineering 5: 51–75.
Ovidio, M., A. L. Seredynski, J. C. Philippart & B. Nzau Matondo, 2013. A bit of quiet between the migrations: the resting life of the European eel during their freshwater growth phase in a small stream. Aquatic Ecology 47: 291–301.
Paragamian, V. L., 1989. Comparison of day and night electrofishing: size structure and catch per unit effort for smallmouth bass. North American Journal of Fisheries Management 9: 37–41.
Paterson, A. W. & A. K. Whitfield, 2000. Do shallow-water habitats function as refugia for juvenile fishes? Esturaine, Coastal and Shelf Sience 51: 359–364.
Pekcan-Hekim, Z. & J. Lappalainen, 2006. Effects of clay turbidity and density of pikeperch (Sander lucioperca) larvae on predation by perch (Perca fluviatilis). Naturwissenschaften 93: 356–359.
Pekcan-Hekim, Z., L. Nurminen, T. Ojala, M. Olin, J. Ruuhijärvi & J. Horppila, 2010. Reversed diel horizontal migration of fish: turbidity versus plant structural complexity as refuge. Journal of Freshwater Ecology 25: 649–656.
Perrow, M. R., A. J. D. Jowitt & S. R. Johnson, 1996. Factors affecting the habitat selection of tench in a shallow eutrophic lake. Journal of Fish Biology 48: 859–870.
Persson, L., S. Diehl, L. Johansson, L. Andersson & S. F. Hamrin, 1991. Shifts in fish communities along the productivity gradient of temperate lakes—patterns and the importance of size-structured interactions. Journal of Fish Biology 38: 281–293.
Pierce, C. L., A. M. Corcoran, A. N. Gronbach, S. Hsia, B. J. Mullarkey & A. J. Schwartzhoff, 2001. Influence of diel period on electrofishing and beach seining assessments of littoral fish assemblages. North American Journal of Fisheries Management 21: 918–926.
Pitcher, T. J. & J. R. Turner, 1986. Danger at dawn: experimental support for the twilight hypothesis in shoaling minnows. Journal of Fish Biology 29: 59–70.
QGIS Development Team, 2019. QGIS Geographic Information System. Open Source Geospatial Foundation, https://qgis.org/de/site/.
R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.r-project.org.
Radinger, R., Matern, S., Klefoth T., Wolter C., Feldhege F., Monk C. T. & Arlinghaus R. (in press). Ecosystem-based management outperforms species-focused stocking for enhancing fish populations, Science.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. Maccormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
Reyne, M., M. Nolan, H. Mcguiggan, A. Aubry, M. Emmerson, F. Marnell & N. Reid, 2020. Artificial agri-environment scheme ponds do not replicate natural environments despite higher aquatic and terrestrial invertebrate richness and abundance. Journal of Applied Ecology 00: 1–12.
Říha, M., J. Kubečka, M. Prchalová, T. Mrkvička, M. Čech, V. Draštík, J. Frouzová, E. Hohausová, T. Jůza, M. Kratochvíl, J. Peterka, M. Tušer & M. Vašek, 2011. The influence of diel period on fish assemblage in the unstructured littoral of reservoirs. Fisheries Management and Ecology 18: 339–347.
Říha, M., D. Ricard, M. Vašek, M. Prchalová, T. Mrkvička, T. Jůza, M. Čech, V. Draštík, M. Muška, M. Kratochvíl, J. Peterka, M. Tušer, J. Seďa, P. Blabolil, M. Bláha, J. Wanzenböck, J. Kubečka & J. Kubečka, 2015. Patterns in diel habitat use of fish covering the littoral and pelagic zones in a reservoir. Hydrobiologia 747: 111–131.
Robertson, B. A. & R. L. Hutto, 2006. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87: 1075–1085.
Ross, J. E., C. M. Mayer, J. T. Tyson, E. J. Weimer, C. M. Mayer, J. T. Tyson & E. J. W. Comparison, 2016. Comparison of electrofishing techniques and effort allocation across diel time periods, seasons, sites, and habitat in the Ohio coastal waters of Western Lake Erie. North American Journal of Fisheries Management 36: 85–95.
Rossier, O., 1995. Spatial and temporal separation of littoral zone fishes of Lake Geneva. Hydrobiologia 300: 321–327.
Rossier, O., E. Castella & J. B. Lachavanne, 1996. Influence of submerged aquatic vegetation on size class distribution of perch (Perca fluviatilis) and roach (Rutilus rutilus) in the littoral zone of Lake Geneva (Switzerland). Aquatic Sciences 58: 1–14.
Ruiz, G. M., A. H. Hines & M. H. Posey, 1993. Shallow water as a refuge habitat for fish and crustaceans in non-vegetated estuaries: an example from Chesapeake Bay. Marine Ecology Progress Series 99: 1–16.
Rümmler, F., 2015. Elektrotechnische Grundlagen der Elektrofischerei. Schriften des Instituts für Binnenfischerei e.V. Potsdam-Sacrow.
Russell, I., D. Parrott, M. Ives, D. Goldsmith, S. Fox, D. Clifton-Dey, A. Prickett & T. Drew, 2008. Reducing fish losses to cormorants using artificial fish refuges: an experimental study. Fisheries Management and Ecology 15: 189–198.
Sammons, S. M. & P. W. Bettoli, 1999. Spatial and temporal variation in electrofishing catch rates of three species of black bass (Micropterus spp.) from Normandy Reservoir, Tennessee. North American Journal of Fisheries Management 19: 454–461.
Sass, G. G., C. M. Gille, J. T. Hinke & J. F. Kitchell, 2006. Whole-lake influences of littoral structural complexity and prey body morphology on fish predator-prey interactions. Ecology of Freshwater Fish 15: 301–308.
Sass, G. G., A. L. Rypel & J. D. Stafford, 2017. Inland fisheries habitat management: lessons learned from wildlife ecology and a proposal for change. Fisheries 42: 197–209.
Savino, J. F. & R. A. Stein, 1982. Predator-prey interaction between largemouth bass and bluegills as influenced by simulated, submersed vegetation. Transactions of the American Fisheries Society 111: 255–266.
Savino, J. F. & R. A. Stein, 1989. Behavioural interactions between fish predators and their prey: effects of plant density. Animal Behaviour 37: 311–321.
Scheffer, M. & E. Jeppesen, 2007. Regime shifts in shallow lakes. Ecosystems 10: 1–3.
Schielzeth, H., N. J. Dingemanse, S. Nakagawa, D. F. Westneat, H. Allegue, C. Teplitsky, D. Réale, N. A. Dochtermann, L. Zsolt & Y. G. Araya-ajoy, 2020. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods in Ecology and Evolution 11: 1–12.
Schulz, U. & R. Berg, 1987. The migration of ultrasonic-tagged bream, Abramis brama (L), in Lake Constance (Bodensee-Untersee). Journal of Fsh Biology 31: 409–414.
Schulze, T., H. Dörner, F. Hölker & T. Mehner, 2006. Determinants of habitat use in large roach. Journal of Fish Biology 69: 1136–1150.
Seelen, L. M. S., S. Teurlincx, M. R. Armstrong, M. Lürling, E. van Donk & L. N. de Senerpont Domis, 2022. Serving many masters at once: a framework for assessing ecosystem services delivered by quarry lakes. Inland Waters 12: 121–137.
Seelen, L. M. S., S. Teurlincx, J. Bruinsma, T. M. F. Huijsmans, E. van Donk, M. Lürling & L. N. de Senerpont Domis, 2021. The value of novel ecosystems: disclosing the ecological quality of quarry lakes. Science of the Total Environment 769: 144294.
Shuter, B. J., A. G. Finstad, I. P. Helland, I. Zweimüller & F. Hölker, 2012. The role of winter phenology in shaping the ecology of freshwater fish and their sensitivities to climate change. Aquatic Sciences 74: 637–657.
Skov, C. & S. Berg, 1999. Utilization of natural and artificial habitats by YOY pike in a biomanipulated lake. Hydrobiologia 408–409: 115–122.
Skov, C., J. Brodersen, P. Nilsson & L. Hansson, 2008. Seasonal migration determined by a trade-off between predator avoidance and growth. PLoS ONE 3: 1–6.
Skov, C., B. B. Chapman, H. Baktoft, J. Brodersen, C. Brönmark, H. Lars-Anders, K. Hulthén & P. A. Nilsson, 2013. Migration confers survival benefits against avian predators for partially migratory freshwater fish. Biology Letters 9: 1–4.
Snickars, M., A. Sandström & J. Mattila, 2004. Antipredator behaviour of 0+ year Perca fluviatilis: effect of vegetation density and turbidity. Journal of Fish Biology 65: 1604–1613.
Søndergaard, M., T. L. Lauridsen, L. S. Johansson & E. Jeppesen, 2018. Gravel pit lakes in Denmark: chemical and biological state. Science of the Total Environment 612: 9–17.
Steendam, C., V. Pieterjan, V. Wassenbergh & J. De Meyer, 2020. Burrowing behaviour of the European eel (Anguilla anguilla): effects of life stage. Journal of Fish Biology 97: 1332–1342.
Thorpe, E., 1974. The movements of brown trout, Salmo trutta (L.) in Loch Leven, Kinross, Scotland. Journal of Fish Biology 6: 153–180.
Tickner, D., J. J. Opperman, R. Abell, M. Acreman, A. H. Arthington, S. E. Bunn, S. J. Cooke, J. Dalton, W. Darwall, G. Edwards, I. Harrison, K. Hughes, T. Jones, D. Leclère, A. J. Lynch, P. Leonard, M. E. McClain, D. Muruven, J. D. Olden, S. J. Ormerod, J. Robinson, R. E. Tharme, M. Thieme, K. Tockner, M. Wright & L. Young, 2020. Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. BioScience 70: 330–342.
Tonn, W. M. & J. J. Magnuson, 1982. Patterns in the species composition and richness of fish assemblages in Northern Wisconsin lakes. Ecology 63: 1149–1166.
Utne-Palm, A. C., 2002. Visual feeding of fish in a turbid environment: physical and behavioural aspects. Marine and Freshwater Behaviour and Physiology 35: 111–128.
Vehanen, T. & M. Lahti, 2003. Movements and habitat use by pikeperch (Stizostedion lucioperca (L.)) in a hydropeaking reservoir. Ecology of Freshwater Fish 12: 203–215.
Vucic, J. M., R. S. Cohen, D. K. Gray, A. D. Murdoch, A. Shuvo & S. Sharma, 2019. Young gravel-pit lakes along Canada’s Dempster Highway: how do they compare with natural lakes? Arctic, Antarctic, and Alpine Research Taylor & Francis 51: 25–39.
Walsh, P. J., G. D. Foster & T. W. Moon, 1983. The effects of temperature on metabolism of the American Eel Anguilla rostrata (LeSueur): compensation in the summer and torpor in the winter. Physiological Zoology 56: 532–540.
Wang, N. & R. Eckmann, 1994. Distribution of perch (Perca fluviatilis L.) during their first year of life in Lake Constance. Hydrobiologia 277: 135–143.
Weatherley, A. H., 1959. Some features of the biology of the tench Tinca tinca (Linnaeus) in Tasmania. Journal of Animal Ecology 28: 73–87.
Westerberg, H. & N. Sjöberg, 2015. Overwintering dormancy behaviour of the European eel (Anguilla anguilla L.) in a large lake. Ecology of Freshwater Fish 24: 532–543.
Westrelin, S., J. Cucherousset, R. Roy, L. Tissot, F. Santoul & C. Argillier, 2021. Habitat partitioning among three predatory fish in a temperate reservoir. Ecology of Freshwater Fish 31: 1–14.
Westrelin, S., R. Roy, L. T. Laurent & C. Argillier, 2018. Habitat use and preference of adult perch (Perca fluviatilis L.) in a deep reservoir: variations with seasons, water levels and individuals. Hydrobiologia 809: 121–139.
Winfield, I. J., 2004. Fish in the littoral zone: Ecology, threats and management. Limnologica 34: 124–131.
Wolter, C. & J. Freyhof, 2004. Diel distribution patterns of fishes in a temperate large lowland river. Journal of Fish Biology 64: 632–642.
Zeileis, A., C. Kleiber & S. Jackman, 2008. Regression models for count data in R. Journal of Statistical Software 27: 1–25.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was jointly financed by the German Federal Ministry of Education and Research (BMBF) and the German Federal Agency for Nature Conservation (BfN) with funds granted by the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU; grant number: 16LC1320A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The fish sampling complied with Lower Saxonian fisheries law and included permission for electrofishing (# 34.2–65434–IV, # 34.2–65434–II). The authors have no conflict of interest.
Additional information
Handling editor: Antti P. Eloranta
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
Deadwood brush piles placed in the open littoral of a gravel pit lake nearly one year after the introduction in autumn 2018 (Picture: Möllers, F.; Anglerverband Niedersachsen e. V.) (EPS 29097 kb)
Fig. S2
Microhabitat-specific seasonal and diurnal contribution (in %) of the most common fish species to the total catch. Numbers above the bars show total number of fishes caught (D = day, N = night) (EPS 46589 kb)
Fig. S3
Predicted catches by GLMM estimated using marginal means amongst the three different microhabitats with increasing Secchi depth (in m) (EPS 5223 kb)
Table S1
Summary of PASE points within each habitat, daytime and season. Numbers display the fished points with percentage of fished points per season in brackets (XLSX 10 kb)
Table S2
Species-specific catch characteristics: total abundance, presence among sampling lakes, average NPUE ± SD within all sampling lakes with species present, lake specific total numbers and NPUE (total N / lake NPUE). Lakes are abbreviated (COL = Collrunge, DK = Donner Kiesgrube, KTB = Kiesteich Brelingen, KOL = Kolshorner Teich, LIN = Linner See, MEI = Meitzer See, SAA = Saalsdorf, WEK = Weidekampsee) (XLSX 15 kb)
Table S3
Pairwise comparisons, averaged over daytime, of microhabitat specific fish length among all seasons and within each season estimated by GLMM using estimated marginal means and Tukey HSD p-value adjustment. Values present the incidence rate ratio (IRR). Bold characters indicate significant differences (p < 0.05) (XLSX 13 kb)
Table S4
Pairwise comparisons of microhabitat specific catches (NPUE, diurnal means) among seasons, estimated by GLMM using estimated marginal means and Tukey HSD p-value adjustment. Values represent the incidence rate ratio (IRR). Bold characters indicate significant differences (p < 0.05) (XLSX 13 kb)
Table S5
Pairwise comparisons of microhabitat specific catches (NPUE) within each season estimated by GLMM using estimated marginal means and Tukey HSD p-value adjustment. Values present the incidence rate ratio (IRR). Bold characters indicate significant differences (p < 0.05) (XLSX 13 kb)
Table S6
Microhabitat specific effect on NPUE with increasing Secchi depth estimated by GLMM using estimated marginal means (XLSX 12 kb)
Table S7
GLMM results for each of the investigated species modelling NPUE as an interaction of microhabitat and mean Secchi depth. The intercept contains the interaction of the brush pile microhabitat and mean Secchi depth. Bold characters indicate significant differences (p < 0.05) (XLSX 13 kb)
Table S8
Pairwise comparisons of diurnal catches (NPUE) within each microhabitat and season estimated by GLMM using estimated marginal means and Tukey HSD p-value adjustment. Values present the incidence rate ratio (IRR) indicating lower NPUE during daytime compared to nighttime at IRR < 1. Bold characters indicate significant differences (p < 0.05) (XLSX 13 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maday, A., Matern, S., Monk, C.T. et al. Seasonal and diurnal patterns of littoral microhabitat use by fish in gravel pit lakes, with special reference to supplemented deadwood brush piles. Hydrobiologia 850, 1557–1581 (2023). https://doi.org/10.1007/s10750-023-05152-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05152-3