Abstract
Diamondback terrapins (Malaclemys terrapin) are sexually dimorphic generalist turtles that inhabit salt marshes and estuaries along the Atlantic and Gulf coasts of the United States. On October 29th, 2012, Hurricane Sandy made landfall in New Jersey, USA, directly impacting terrapin populations inhabiting central and southern Barnegat Bay. To examine potential food web mediated impacts to the terrapin population and their foraging dynamics we examined carbon and nitrogen stable isotope values collected from terrapin tissues (2011, 2015, 2019) and resource taxa (2015, 2019) within Barnegat Bay. Isotopic analysis revealed that mature females had lower carbon and higher nitrogen values than immature females and males with almost no isotopic niche overlap, whereas males and immature females had statistically similar values with overlapping niches. Terrapins and resources collected from island habitats contained higher carbon and nitrogen values than those from mainland habitats, with little overlap in niche between habitats. There were no significant temporal variations detected in either carbon or nitrogen values from terrapins between years, or within each habitat pre- and post-Hurricane Sandy. These findings suggest long-term terrapin foraging dynamics have remained relatively stable, signifying resilience to disturbance events within the study site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal salt marshes and estuaries are critically important biological habitats that serve to maintain biodiversity in aquatic systems. Tidal marshes sequester excess nutrients, provide vital nursery habitat for several recreational and commercial fishery species, and absorb hurricane energy (MacKenzie & Dionne, 2008; Day Jr. et al., 2012; Velinsky et al., 2017). However, environmental stressors directly threaten these coastal ecosystems and the critical services they provide. Urbanization of coastlines impacts eutrophication of nearshore waters through increases in wastewater and stormwater inputs (McClelland et al., 1997; Ulseth & Hershey, 2005), and systems can become highly eutrophic if there is relatively low freshwater inflow and poor tidal flushing (Kennish et al., 2007; Velinsky et al., 2017). In addition to the steady pressure of human influence on the coast, hurricanes can cause sudden and extensive changes to coastal ecosystems. Hurricanes can be significant community-structuring events, altering benthic communities through the physical effects of the storm (Mallin & Corbett, 2006; Engle et al., 2009; Longenecker et al., 2018). The increased rainfall typical of hurricanes can lower salinity levels below the threshold tolerance of benthic communities or cause stratification in the water column resulting in hypoxia in bottom waters (Boesch et al., 1976; Mallin et al., 1999; Cho et al., 2012). Thus, it is reasonable to hypothesize that storm driven changes in community structure may have the potential to create shifts in the estuarine food webs.
Stable isotope analysis is commonly used to investigate trophic interactions between species and habitats, as carbon and nitrogen isotope tracers can integrate both consumer habitat and dietary resources (Deegan & Garritt, 1997; Sanzone et al., 2003; Fry, 2006; Michener & Kaufman, 2008). Carbon isotope values can discriminate between marine versus terrestrial habitats, while nitrogen isotopes inform on the trophic position. Because animal tissues reflect the isotopic composition of their diet in a predictable manner, simultaneous analysis of carbon and nitrogen isotope tracers (i.e., isotopic niche) can describe the trophic ecology of organisms over space and time (Layman et al., 2007; Newsome et al., 2007). Consumers that feed on the same prey have similar δ13C values with only a slight enrichment < 1‰ (DeNiro & Epstein, 1978), whereas nitrogen undergoes an increase of ~ (2–4‰) with each trophic level, but these discrimination factors can vary between species and tissue type (Hobson & Wassenaar, 2008). Isotopic analysis has also been used to study the response of species to habitat change and environmental stressors. For example, habitat conversion and fragmentation led to shifts in isotopic niche resulting from shifts in both carbon and nitrogen values of skinks (Resasco et al., 2018), and there were contrasting food web responses of the Seaside Sparrow, Ammodramus maritimus (Wilson, 1811), following impacts to the composition of terrestrial and saltmarsh communities in Louisiana following the Deepwater Horizon oil spill (Olin et al., 2017).
Diamondback terrapins, Malaclemys terrapin (Schoepff, 1793), occur exclusively in estuaries and salt marshes along the Atlantic and Gulf coasts. They are long-lived turtles that exhibit high site fidelity and are sexually dimorphic generalist predators that forage on benthic invertebrates including crabs, snails, bivalves, and barnacles with species varying by location [Tucker et al., 1995; Erickson et al., 2011; Denton et al., 2016; Kays et al., 2019; Weimer and Wnek (MATES Project Terrapin), oral communication, (August 18th, 2020)]. Resource partitioning between sexes was identified by Petrochic (2009) and Tucker et al. (2018) and Alleman and Guillen (2017), while Denton et al. (2019) identified partitioning between size classes with the diets of larger mature females likely different from males and immature females due to larger head and stronger bite force.
Barnegat Bay is the largest body of water in New Jersey, along the coast of Ocean County. It is a lagoonal-type estuary that encompasses a unique estuary ecosystem with a mosaic of coastal habitats including barrier islands, eelgrass beds, salt marshes, and tidal flats. On October 29th, 2012, Barnegat Bay was directly impacted by Hurricane Sandy, an extraordinarily large storm that affected most of the eastern coast of the USA. Hurricane Sandy’s path tracked over the Edwin B. Forsythe National Wildlife Refuge with winds > 160 km/h but the strongest impacts were caused by storm surge and tides (Hall & Sobel, 2013). Subsequent research examining impacts of Hurricane Sandy suggested no widespread long-term impact on the salt marshes (Elsey-Quirk, 2016; Longenecker et al., 2018), and the benthic invertebrate species composition were still diverse and abundant with minimal changes across 45 years (Taghon et al., 2017a, b; Watson & Gannon, 2020). Northern Diamondback terrapin, M. terrapin terrapin (Schoepff, 1793), populations within central and southern Barnegat Bay have been studied since 2005, during which biological samples were collected (i.e., 2011, 2015, and 2019).
We aimed to characterize the isotopic niche of terrapins within southern Barnegat Bay to investigate potential food web mediated impacts to the local terrapin population after Hurricane Sandy. We assessed temporal and spatial variance in terrapins’ isotopic niche within mainland and island habitats. We also sampled potential resource taxa to establish baseline values to assess if terrapin isotopic values were reflective of those within the capture locations. The objectives were to (1) examine intraspecific niche partitioning between turtles of different sexes, size classes, and those occupying different habitats and (2) determine if there were long-term temporal variations in terrapin isotopic niche within this study site.
Methods
Study sites
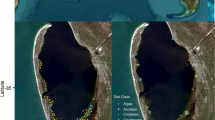
We selected sampling sites within the central and southern portions of Barnegat Bay, NJ, based on known terrapin nesting and foraging areas (Sheridan et al., 2010; Wnek, 2010; Basile et al., 2011; Winters et al., 2015). We categorized sites as either “mainland” (Conklin, Double Trouble, Osprey Cove, Cedar Run) or “island” (Sedge Islands, Sloop Sedge Minor) habitats (Fig. 1, created in QGIS Desktop 3.16.0 with GRASS 7.8.4). Mainland study sites were within the Edwin B. Forsythe National Wildlife Refuge (EBFNWR), located in southern NJ on the inland side of Barnegat Bay and Cedar Run, located on the mainland side of the Bay, in Stafford Township just south of the EBFNWR Barnegat Division (Fig. 1). Island sites were within EBFNWR and north in the Sedge Island Wildlife Management area, managed by the NJ Division of Fish and Wildlife within the Sedge Island Marine Conservation Zone (SIMCZ). Both the mainland and island sites are primarily comprised of salt marsh habitat with open bay access. The northern portion of Barnegat Bay is more developed, has a greater salinity gradient and more shoreline structures than the southern Bay, which has a higher percentage of salt marsh habitat along the mainland site (Lathrop et al., 2001; Kennish et al., 2007).
Isotope sampling
Terrapins
During 2015 and 2019 we captured terrapins using hoop nets, fyke nets, dip nets, and by hand as part of ongoing long-term population studies (Sheridan et al., 2010; Wnek, 2010; Lester et al., 2013). Female terrapins reach sexual maturity at approximately 13.2–17.6 cm straight plastron lengths (SPL), and males around 9 cm (Lovich & Gibbons, 1990; Wnek, 2010), thus, we considered all females ≥ 13.5 cm mature and all females < 13.5 cm immature. In 2015, we sampled 49 terrapins (17 mature and 11 immature female, and 21 mature male) from two mainland sites (Double Trouble, Osprey Cove) and 10 (1 immature and 2 mature females, and 7 mature males) from an island site (Sloop Sedge Minor). During 2019, we sampled an additional 23 (3 immature and 16 mature females, and 4 mature males) terrapins at a mainland site (Cedar Run) and 4 (3 mature females, 1 mature male) from an island site (Sedge Islands). We recorded standard morphometric measurements and capture information for each terrapin and collected whole blood samples (2 ml) through either dorsal coccygeal or subcarapacial venipuncture, and immediately stored on ice until frozen in laboratory at − 20 °C. After all measurements and samples were collected, terrapins were released back at their capture location within 24 h. To enable comparison of terrapins’ isotopic values from southern Barnegat Bay prior to Hurricane Sandy we utilized red-blood cell (RBC) fractions from nesting females sampled in 2011. For this study all whole blood and RBC fractions were dried in the laboratory at 60 °C for up to 48 h and ground into a homogeneous powder. As part of an endocrine study, Winters et al. (2016) sampled nesting female terrapins from island (Sedge Islands; 12) and mainland (Conklin; 18) sites during which they collected heparinized blood samples (2 ml) stored on ice 3–6 h prior to centrifugation, with the resulting RBC fractions having been stored frozen since collection (see Winters et al. 2016 for details on terrapin capture and blood handling/storage). The use of heparinized anticoagulants did not show a significant difference in δ13C or δ15N values from non-heparinized samples of red blood cells from green sea turtles, Chelonia mydas (Linnaeus, 1758), or leopard sharks, Triakis semifasciata, Girard, 1855, (Kim & Koch, 2012; Lemons et al., 2012), and only marginal differences with minimal effects (< 0.2019‰) on the nitrogen values in blacktip reef shark, Carcharhinus melanopterus (Quoy and Gaimard, 1824), tissues (Weideli et al., 2019). Therefore, it is reasonable to assume there was little to no influence of the preservation method on the terrapins’ RBC isotopic values.
It is also important to note the possibility that any observed differences in isotopic values and niche prior to Hurricane Sandy (2011) to those collected after (2015, 2019) could be attributed to the different tissue types used. Animal tissues assimilate the diet-derived stable isotopic signatures into consumer body tissues at varying rates (discrimination factors) based primarily on tissue-specific metabolism (Gannes et al., 1997), which results in dietary information integrated over different time scales due to the varying turnover rates (Seminoff et al., 2007). For example, discrimination factors for nitrogen (Δdt15N) are slightly larger for whole blood than RBC for green (C. mydas) and leatherback sea turtles, Dermochelys coriacea (Vandeli, 1761), (Seminoff et al., 2006, 2009), and pond sliders, Trachemys scripta (Thunberg in Schoepff, 1792), (Seminoff et al., 2007), while carbon (Δdt13C) in RBC was slightly more depleted than whole blood of green sea turtles (Seminoff et al., 2006). However, the values were similar with whole blood having only slightly higher carbon and nitrogen values. Seminoff et al. (2006, 2007, 2009) also determined turnover rates for turtle tissues were of the long-term nature reflecting diet histories of 5–7 months. Because RBC integrates diet over a longer time span than whole blood, our data may reflect foraging and diet assimilation across different time scales that could affect niche size. For example, the 2011 samples could be reflective of foraging over 7 months or longer, while the whole blood samples may only be reflective of 5 months, potentially spanning different seasons. However, for our purposes we consider the 4 years between each of our sample collections reflective of three individual time steps.
Resource taxa
To determine if terrapin values were reflective of the basal resources within each site, during 2015 terrapin sampling we opportunistically sampled prey species that we knew were part of terrapin diets at Cedar Run and Sedge Island (MATES Project Terrapin), oral communication (August 18th, 2020); Callinectes sapidus Rathbun, 1896, Palaemonetes pugio, Holthuis, 1949, Ilyanassa obsoleta (Say, 1822), Geukensia demissa (Dillwyn, 1817) which were collected within the hoop traps or within close proximity of terrapin capture site. We stored samples on ice in labeled plastic bags or 50 ml centrifuge tubes until freezing. We also collected estuarine vegetation within close proximity to terrapin capture sites, Codium fragile (Suringar) Hariot, 1889, Ulva lactuca, Linnaeus, 1753, Ulva intestinalis, Linnaeus, 1753, Zostera marina, Linnaeus, 1753, Spartina alterniflora, Loisel, Spartina patens, (Aiton) Muhl., 1813, Salicornia virginica L., 1753. During the 2019 season, logistic constraints limited our resource samples to opportunistically collected I. obsoleta, and C. sapidus from close proximity of terrapin captures. In the laboratory, we thawed and rinsed vegetation samples with distilled water after which a dissecting scope was used to remove all non-plant material before a final rinse and placing in a sterile labeled vial to be dried. We then rinsed all prey specimens using distilled water, and muscle tissue was dissected. The tissue was again rinsed using distilled water and placed in individually labeled vials to be dried. All vegetative and prey samples were dried at 60 °C for up to 48 h and ground into a homogenous powder.
Once homogenized, we weighed samples (turtles/prey: 0.4–0.8 mg; vegetation: 1.4–1.6 mg) into 5 × 9 mm pressed tin capsules, then sealed them and sent for analysis; 2019 samples were sent to the UC Davis Stable Isotope Facility to be analyzed for 13C and 15N isotopes using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). Delta values are expressed relative to international standards VPDB (Vienna Pee Dee Belemnite) and Air for carbon and nitrogen, respectively. All prior samples were sent to Washington State University’s Bioanalytical Laboratory and analyzed using an elemental analyzer interfaced to a Finnegan MAT Delta-S stable isotope ratio mass spectrometer via a Finnigan MAT ConFlo II interface. Results are presented in δ notation (where δ13C or δ15N = [{Rsample/Rstandard − 1}] × 1,000, with R = 13C/12C or 15N/14N). The SD of internal reference materials from Washington State were (bovine liver, δ13C = ± 0.43‰, δ15N = ± 0.30‰; apple leaves, δ13C = ± 0.12‰, δ15N = ± 0.08‰), and (± 0.04‰ for δ13C and ± 0.04‰ for δ15N) from UC Davis. We compared δ13C and δ15N values of internal references of bovine liver from both labs and detected no statistical difference between labs (P > 0.05). Duplicates of every 10th sample were run to test efficiency of homogenization. In aquatic animals with low C:N ratios (< 3.5), lipid removal has little effect on δ13C signatures (Post et al., 2007). Mean C:N ratios for animal tissues in this study were 3.6 ± 0.3 SD, and there was a significant correlation between C:N and 13C among turtles (P < 0.0001, t = − 5.5399, df = 114) and potential prey items (P < 0.0001, t = 7.363, df = 75). Additionally, some of the vegetation samples contained more than 40% carbon, therefore, we accounted for lipids mathematically using the equations described by Post et al. (2007) to normalize the effect of lipid concentration on the δ13C values. Aquatic animals: δ13Cnormalized = δ13Cuntreated − 3.32 + 0.99 * C:N; Vegetation samples with carbon > 40%: δ13Cnormalized = δ13Cuntreated − 5.83 + 0.14 * % Carbon.
Statistical analyses
We ran a 3-way MANOVA design using Type IV sum of squares to adjust for missing treatment combinations. The bivariate response was δ13C and log(δ15N + 1), and the three predictors were year = (2011, 2015, 2019), sex (Female-immature, Female-mature, Male) and habitat (Island, Mainland). However, to obtain bivariate normality on the residuals, we dropped year = 2011 from the MANOVA and analyzed year = 2011 separately. Note that year = 2011 had only one sex, hence a 1-way ANOVA was run on the δ13C and an Kruskal–Wallis test (nonparametric) was run on δ15N to compare the habitats. All models except for the nonparametric model were tested for homogeneity and normality of the residuals, (α = 0.05 for all tests). PROC GLM and PROC NPAR1WAY using SAS 9.4 (SAS Institute, Inc., 2018) was used for the analyses. Tukey’s test was used to compare means. To investigate temporal variation, we assumed normality so we could include year 2011 in the MANOVA (δ13C was normal and δ15N is somewhat unimodal and symmetric) with a somewhat linear χ2 plot for the bi-variate normal assumption (Johnson & Wichern, 2007).
We used R Statistical Software (v.4.1.2; R Core Team, 2021) via the R packages: SIBER (v2.1.6; Jackson et al., 2011), ggplot2 (v3.3.5; Wickham, 2016) and ggpubr (v0.4.0; Kassambara, 2020). Isotopic diversity indices were calculated using SIBER—Stable Isotope Bayesian Ellipses in R (Jackson et al., 2011). All samples prior to the hurricane were from nesting females (2011), thus we only included samples collected from mature females sampled in 2015 and 2019 when comparing niches between years. We calculated the standard ellipse area (SEA) using the variance and covariance of bivariate isotope data to contain approximately 40% of data to represent a core isotopic niche for each population. Values were then corrected (SEAC) to minimize bias due to small sample size by using an (n − 2) correction on the denominator (Jackson et al., 2011, 2012). The SEAC was then used to calculate the degree of isotopic niche overlap, representing a quantitative measure of dietary similarity between populations. We then used a Bayesian approach (SEAB) as described in Jackson et al. (2011) to obtain highest density intervals for isotopic niche areas. These intervals allow for statistical comparisons of the sizes of isotope niche among populations. All data analyzed during this study are included as a U.S. Geological Survey data release (Denton et al., 2022).
Results
Terrapins
Mean SPLs were greater for females captured at the island sites (16.9 cm) than for those captured at the mainland sites (14.4 cm; t = 2.4473, df = 50, P < 0.0085) while there was no difference in the SPLs of males between habitats (island: 9.7 cm, mainland: 9.6 cm; t = 0.2273, df = 31, P = 0.8217). Terrapins (both sexes) captured within island habitats had higher mean δ13C (P < 0.0042) and δ15N (P < 0.0001) values compared to those captured within mainland habitats (island: δ13C = −16.03‰, δ15N = 11.59‰; mainland: δ13C = −16.75‰, δ15N = 9.96‰). Within each habitat isotopic values differed between sexes/size classes (Table 1). Terrapin δ13C values from 2015 and 2019 were negatively correlated with SPL for females within the mainland habitats (R= −0.56, P < 0.0001), while there was no correlation within the island habitat nor for males within either habitat (P > 0.5, Fig. 2). Female terrapin δ15N values from 2015 and 2019 were positively correlated with SPL within both habitats (island: R = 0.89, P = 0.033; mainland: R = 0.65, P < 0.0001), however there was no correlation between nitrogen values and SPL for males in either habitat (Fig. 2).
Using AICc comparisons we found that the best MANOVA model kept only the main effects of sex and habitat. The MANOVA model had sex (Wilks’ λ = 12.76, ndf = 4, ddf = 162, P-value < 0.0001) and habitat (Wilks’ λ = 26.62, ndf = 2, ddf = 81, P-value < 0.0001) significant. The mean comparisons showed that for δ13C, Female_m < Female_i, Male and Mainland < Island. Likewise for log(δ15N + 1), Female_i, Male < Female_m and Mainland < Island. For year = 2011, the ANOVA for δ13C was significant [P-value = 0.0015, F(1, 28) = 12.45], and mean comparison showed that for δ13C, Mainland < Island. The nonparametric ANOVA for δ15N was significant (P-value = 0.0081, 1, χ2 = 7.0036) and mean comparison showed that Mainland < Island. When normality was assumed and year 2011 was included, MANOVA results were similar. Sex * Habitat was significant (Wilks’ λ = 3.15, ndf = 4, ddf = 214, P-value = 0.0152) but Year was not significant (Wilks’ λ = 0.9, ndf = 4, ddf = 214, P-value = 0.4662). Mean comparisons showed that for δ13C means comparisons using a Bonferroni correction (α = 0.05/9), Mainland: Female_m < Female_i, Male, Female_i and Male: not significant, Female_m: Mainland < Island. For δ15N means comparisons Mainland: Male, Female_i < Female_m, Island: not significant, Male: Mainland < Island, Female_i and Female_m: not significant.
SIBER analyses indicate the niche of mature females was smaller than both immature females and males, with little overlap between female size classes (mature, 18%; immature, 14%) or mature females and males (m-F, 9%; M, 5%), while there was significantly more overlap between immature females and males (i-F, 81%; M, 61%, Fig. 3a). The SEAC of mainland terrapins were larger than those from the island for both sexes and female size classes. Terrapins within the mainland habitat followed a similar pattern as they did overall with the greatest overlap being between immature females and males (i-F, 77%; M, 82%), while there was little overlap between mature females with immature females (m-F, 25%; i-F, 14%) and effectively no overlap with males (m-F, 0.9%; M, 0.5%, Fig. 4a). There was significant overlap in the niche of mature female amongst years, with a relatively small shift in carbon or nitrogen values between years (Fig. 5a). Bayesian models suggests niche size varied between sexes (M > F, i-F), habitats (F: mainland < island; M: island > mainland), and years (F: 2011 < 2019 < 2015, Figs. 3b, 4b, 5b).
Intraspecific isotopic niches of M. terrapin terrapin within central and southern Barnegat Bay, NJ (a) and their associated Bayesian standard ellipse area (SEAB) estimated posterior distributions (b). Red circles represent mature females, green squares represent immature females, and blue triangles represent male terrapins. Isotopic niches of males and females (both mature and immature) within the Bay. Bayesian estimates show 50% (light), 75% (middle), and 95% (dark grey) credible intervals. The black dots indicate mode, with the red x indicating estimated mean from SEAC
Intraspecific isotopic niches of M. terrapin terrapin within island and mainland habitats in central and southern Barnegat Bay, NJ (a), and their associated Bayesian standard ellipse area (SEAB) estimated posterior distributions (b). Red circles represent mature females, green squares represent immature females, and blue triangles represent male terrapins. Isotopic niches of male and female terrapins (both mature and immature) within each habitat, solid line ellipses represent mainland niches, dashed line ellipses represent island niches. Bayesian estimates show 50% (light), 75% (middle), and 95% (dark grey) credible intervals. The black dots indicate mode, with the red x indicating estimated mean from SEAC for males and females (both mature and immature) within each habitat
Isotopic niches of mature female M. terrapin terrapin within Barnegat Bay, NJ during each of the three sampling years (a), and their associated Bayesian standard ellipse area (SEAB) estimated posterior distributions (b). The orange open triangles and solid line ellipse represent 2011, gray plus signs and dashed line ellipse represent 2015, and purple “x”s and dotted line ellipse represent 2019 samples; ellipses represent 40% core niche area within each year. Bayesian estimated posterior distributions show 50% (light), 75% (middle), and 95% (dark grey) credible intervals. The black dots indicate mode, with the red x indicating estimated mean from SEAC
Resource taxa
Resource Taxa sampled from the island habitats had higher δ13C and δ15N values compared to those sampled from the mainland (Table 2; Fig. 6). Within the mainland sites, terrapin carbon values were more similar to marine vegetation than the relatively 13C-depleted S. virginica or slightly 13C-enriched Spartina sp. Nitrogen values for marine vegetation were more than twice that of S. virginica and Spartina sp. which were similar to each other (Fig. 6). Snails and crabs sampled from the mainland habitats exhibited a wider range in nitrogen values (snails: 6.8 to 12.1‰; crabs: 5.9 to 11.7‰) than those from the islands (snails: 10.8 to 12.1‰; crabs: 8.2 to 11.7‰), whereas the range in carbon values were similar in both habitats (Fig. 6).
Bivariate isotope plots of the lipid corrected isotopic niches of M. terrapin terrapin and mean (± 1 SD) δ13C and δ15N values for primary producers and potential prey collected in each habitat during 2015 and 2019. Open triangles and dotted line ellipse represent males, open square and dashed ellipse represent immature females, and open circles and solid ellipse represent mature female terrapins. Small solid circles represent mean isotopic values for potential resource species, and large solid circles indicate mean values by resource taxa
Discussion
Terrapin niche among habitats
Within both habitats the terrapins’ niche was 13C-enriched relative to terrestrial C3 vegetation, with values more similar to those of marine vegetation (Fig. 6) and slightly 13C-depleted relative to terrestrial C4 vegetation within the mainland habitat. There is a greater density of submerged aquatic vegetation, Z. marina, and macroalgae along the Barnegat Bay’s barrier island shoreline than along the mainland shoreline. This is especially true within the Sedge Island Marine Conservation Zone where Z. marina is the predominant benthic vegetation along with submerged macroalgae including Ulva sp., C. fragile, and Desmarestia aculeata, (Linnaeus) J.V. Lamouroux, 1813. Within the Sedge Island Marine Conservation Zone, this mixed vegetation provides critical habitat for blue crabs and other invertebrate species (Wilson et al., 1990; Jivoff et al., 2017). The carbon results of our study were similar to those from Watson & Gannon (2020), with mud snails having carbon values reflecting C4 grasses, blue crabs and grass shrimp reflected a mix of macroalgae and Spartina, and ribbed muscles reflecting Ulva sp., all of which are key prey items for terrapins.
The higher nitrogen values of terrapins from island habitats could be due to differences in proportions of food resource use, terrapin movements, or differences in baseline values that correspond to differences in the dominant pathways of primary production, hydrology, and nutrient cycling (Post, 2002). Thus, we compared inferences drawn from the resources to determine whether isotopic variations in the prey items themselves were driving isotopic variations in the terrapins. Our results indicated that carbon and nitrogen isotopic values varied at each site with the resources available in the island habitats having higher nitrogen values than those in the mainland (Table 2; Fig. 6). Conversely, in the mainland sites, there are likely many other terrestrial resources available, generally, with lower nitrogen values than marine resources. One possible cause could be the closer proximity of Sedge Island, our northernmost site, to the northern Barnegat Bay watershed where there is higher nutrient concentration and loading occurring. This is attributed to greater land area, as well as the greater density development in the northern part of the watershed (Pang et al., 2017). Because Barnegat Inlet serves as the greatest exchange in tidal flow for northern Barnegat Bay, the proximity of North Sedge Island to the Inlet could account for the higher nitrogen values from Sedge Islands samples. Also contributing to the higher nitrogen values around North Sedge Island is the lack of flow within the SIMCZ (Baker et al., 2014). A study by Demetriades (2020) found higher nitrogen values in Tritia obsoleta (I. obsoleta) at IBSP (Sedge) which had poorer sediment and water quality than further south at Long Beach Island. Because the terrapins’ isotopic values mirrored these differences between sites, it is reasonable to conclude that the spatial variation in terrapins’ isotopic values are reflective of the different baselines at each site.
Niche partitioning
Unlike male terrapins whose body size was not correlated with either isotopic value, female terrapin body size was negatively correlated with carbon within mainland habitats, and positively correlated with nitrogen in both habitats. This indicates as females mature, they may begin foraging in different locations and or undergo a shift in prey species, or the proportions of prey consumed not experienced by the males. The larger mature female terrapins had higher δ15N values relative to smaller immature females and males, possibly due to the ability to ingest a wider diversity of prey from higher trophic levels due to their larger jaw size (Tucker et al., 1995; Petrochic, 2009; Butler et al., 2012; Tulipani, 2013). Our results suggest that male terrapins have a larger niche than females and mature female terrapins’ core isotopic niche has little to no overlap with those of immature females or males. This further suggests that mature females may be exploiting a different proportion of resources or foraging in different habitats. Our results are similar to Denton et al. (2019) who found body size (mature female versus male) was correlated with both carbon and nitrogen in mainland mangrove forests, while only nitrogen values differed between sexes within an island habitat. In that study the authors suggested mature females within mainland mangrove forests increased consumption of relatively 15N-depleted mangrove periwinkle, Littorina angulifera (Lamarck, 1822), while male diets consisted more of relatively 13C and 15N-enriched barnacles and crabs which resulted in a reduced niche overlap between the sexes. Those results were consistent with fecal analysis which indicated consumption of similar prey species but in varying proportions (Denton et al., 2016). Species exhibiting sexual size dimorphism may utilize different resources due to difference in nutritional requirements (Wearmouth & Sims, 2008). In addition, the increase in body size, mouth gape, and crushing force throughout the ontogenetic development is also important in allowing the exploitation of new resources or foraging microhabitats (Werner & Gilliam, 1984) and reduces competition for resources once sexually mature. The shift in carbon and nitrogen values indicate an ontogenetic shift as females get larger and begin to consume different prey, or ratios of prey with varying isotopic values.
When accounting for sex/size class, estimated niche areas were larger for terrapins sampled from the mainland than the island sites. However, this could be an artifact of the number of sampling sites, and thus potentially a wider range of isotopic values of the available resources included in mainland (four) than island (two) sites. Consistent species collection of potential resources at all sampling sites in future studies could help to provide a more robust analysis of the potential available niches. Mature females sampled on the mainland had the smallest niche with males and immature females having larger niches more similar in size, while on the island it was reversed with the mature females having the larger niche than males (no immature females were sampled). The smaller niche of mature females on the mainland with almost no overlap with immature females or males may reflect their ability to focus on preferred resources potentially unavailable to smaller terrapins, with the larger overlapping niche of immature females and males suggesting generalist strategies incorporating less favorable resources to meet metabolic demands. However, the larger niche for mature females from the island sites compared to males may simply be attributed to variation in capture numbers and sampling sites. Females were sampled from Sedge Islands (2011, 2019) and Sloop Sedge Minor (2015), while all but one male (Sedge 2019) was sampled from Sloop Sedge Minor in 2015. With the limited capture events of male terrapins within the islands compared to mature females it is not surprising to find less variation and thus a smaller niche. There was no overlap in core isotopic niche between mature females and males on the mainland suggesting that while they consumed similar resources, their core resource use consisted of dissimilar diets, while those from the islands were more similar with ~ 35% overlap in niche between sexes (Fig. 4a). The overlap between sexes within the islands (Fig. 4a) may be due to less variation in their prey’s isotopic values or less available resources relative to those sampled from the mainland sites.
Similar to Denton et al. (2019), our results suggest an ontogenetic shift in female terrapin foraging locations or strategies, while male terrapins do not exhibit the same shift, thus potentially competing for food resources with smaller or similar sized immature females. Tucker et al. (1995) detected resource partitioning related to ontogenetic niche of terrapins, similarly the relatively low niche overlap between sexes/size class overall (Fig. 3a) and amongst habitats (Fig. 4a) suggests little overlap in resource use compared high overlap and thus similarity of resource use between males and immature females. Yet in recent years, Kayes et al. (2019) found no dietary partitioning between terrapin size classes in two New Jersey populations using fecal and stomach contents. This illustrates the importance of using multiple approaches to investigate diets. In the Kays et al. (2019) study the authors used Schoener’s formula to determine overlap using frequency of occurrence, a method that does not account for partitioning of resources by prey size or foraging location. For example, Denton et al. (2016) only detected operculum from large adult tree snails in the fecal remains of mature females, and Tucker et al. (1995) also found larger terrapins ingested significantly larger periwinkles with the males only consuming smaller littorinids as their small head size and subsequent bite force likely prevented them from consuming the larger mature snails. Additionally, the same prey may inhabit multiple microhabitats within a study site each with its own distinct isotopic baselines which would not be revealed through fecal analysis.
Temporal variation
Analogous to previous studies on Barnegat Bay’s benthic community, we did not detect a significant temporal difference in either carbon or nitrogen values from terrapin tissues between years or pre–post-hurricane. If the hurricane had caused a major impact, we would have expected to see shifts in the carbon and or nitrogen values due to changes in the available resources, with a subsequent shift in niche size and/or position in whichever habitat was more impacted. Given we found relatively consistent niches and overlap between years and amongst habitats, we propose that a relatively stable food web exists not only within the Bay, but within each habitat. While a slightly larger niche was measured for terrapins before Hurricane Sandy compared to after the event (Fig. 5), it was not significantly different, and this variation may be a result of a difference in the season or tissue sample collected. Pre-hurricane tissue samples were RBC which have longer turnover rates and were collected from nesting females which exhibit natal philopatry and often travel from foraging grounds to nesting beaches (Burger & Garber, 1995; Sheridan et al., 2010) and thus may be reflective of multiple resource pools. In contrast, post-hurricane tissue samples were whole blood with a relatively faster turnover rate and sampled from terrapins in late summer after females completed nesting and have returned to their home foraging grounds.
Hurricane Sandy’s impacts on Barnegat Bay’s benthic community were undetectable within 30–60 days after the storm event, possibly because the passage of the hurricane had no detectable effects on salinity or dissolved oxygen concentrations throughout the bay, and the dissipation of storm surge and wave energy prevented substantial sediment disturbance (Elsey-Quirk, 2016; Taghon et al., 2017a). Thus, our observation of consistency in the terrapins’ isotopic niche may be due to the sampling time frame post-hurricane. Given the short duration the benthic community was impacted, it is possible we were not able to sample soon enough post-hurricane to detect any potential changes. Future work sampling terrapins and resources immediately after the storm and every three to four months thereafter may allow for detection of short-lived variations in isotopic signatures due to disruptions of the benthic food web that are directly due to hurricane impacts. Future studies investigating impacts from disturbance events may also benefit from analyzing blood plasma instead of whole blood, due to the more rapid turnover of plasma than whole blood.
Conclusion
Terrapins’ core isotopic niche remained relatively stable exhibiting little overlap between habitats or size class between years. Our results indicate that despite the direct passage of a major hurricane over our study sites, there were no temporal changes to the terrapins’ foraging dynamics or isotopic values. These findings are consistent with previous research that detected few long-term impacts from Hurricane Sandy on the salt marsh and benthic community (Elsey-Quirk, 2016; Taghon et al., 2017b; Watson & Gannon, 2020). This may further suggest terrapin resilience to short-term disruptions in their food web, or potential shifts in diet were not sufficient to affect stable isotope levels, potentially due to overlap in isotopic signatures among potential prey. These findings shed light on the foraging dynamics of this long-lived reptile, and how potential impacts could affect this population. The lessons learned from this study can help to improve study design of future projects interested in investigating impacts from disturbance events, and help managers understand the complex dynamics of estuarine food webs.
Data availability
Data is available in a USGS Data Release, Denton et al. 2023
References
Alleman, B. J. & G. J. Guillen, 2017. Prey availability and diet analysis of Texas diamond-backed terrapin (Malaclemys terrapin littoralis). Chelonian Conservation and Biology 16: 52–61. https://doi.org/10.2744/CCB-1228.1.
Baker, R. J., C. M. Wieben, R. G. Lathrop, & R. S. Nicholson, 2014. Concentrations, loads, and yields of total nitrogen and total phosphorus in the barnegat bay-little egg harbor watershed, New Jersey, 1989–2011, at Multiple Spatial Scales. Scientific Investigations Report USGS 5072: 1989–2011. https://doi.org/10.7282/T3M0475B
Basile, E. R., H. W. Avery, W. F. Bien & J. M. Keller, 2011. Diamondback terrapins as indicator species of persistent organic pollutants: using Barnegat Bay, New Jersey as a case study. Chemosphere 82: 137–144.
Boesch, D. F., R. J. Diaz & R. W. Virnstein, 1976. Effects of tropical storm Agnes on soft-bottom macrobenthic communities of the James and York Estuaries and the Lower Chesapeake Bay. Chesapeake Science 17: 246–259.
Burger, J. & S. D. Garber, 1995. Risk assessment, life history strategies, and turtles: could declines be prevented or predicted? Journal of Toxicology and Environmental Health 46: 483–500.
Butler, J. A., G. L. Heinrich & M. L. Mitchell, 2012. Diet of the Carolina diamondback terrapin (Malaclemys terrapin centrata) in Northeastern Florida. Chelonian Conservation and Biology 11: 124–128.
Cho, K., H. V. Wang, J. Shen, A. Valle-Levinson & Y. Teng, 2012. A modeling study on the response of Chesapeake Bay to hurricane events of Floyd and Isabel. Ocean Modelling 49–50: 22–46. https://doi.org/10.1016/j.ocemod.2012.02.005.
Day, J. W., Jr., A. Yàñez-Arancibia, W. M. Kemp & B. C. Crump, 2012. Introduction to estuarine ecology. Estuarine Ecology. https://doi.org/10.1002/9781118412787.ch1.
Deegan, L. A. & R. H. Garritt, 1997. Evidence for spatial variability in estuarine food webs. Marine Ecology Progress Series 147: 31–47.
Demetriades, P., 2020. Prevalence of infection with Pleurogonius malaclemys in Tritia obsoleta in relation to environment quality. Folia Malacologica 28: 242–252.
Denton, M. J., K. M. Hart, A. W. J. Demopoulos, A. Oleinik & J. D. Baldwin, 2016. Diet of diamondback terrapins (Malaclemys terrapin) in subtropical mangrove habitats in south Florida. Chelonian Conservation and Biology 15: 54–61. https://doi.org/10.2744/CCB-1187.1.
Denton, M. J., A. W. J. Demopoulos, J. D. Baldwin, B. Smith & K. M. Hart, 2019. Stable isotope analysis enhances our understanding of diamondback terrapin (Malaclemys terrapin) foraging ecology. Estuaries and Coasts 42: 596–611. https://doi.org/10.1007/s12237-018-0476-6.
Denton, M. J., K. M. Hart, J. Wnek, S. A. Moss & H. W. Avery, 2022. Carbon and Nitrogen Isotopic Analysis of Diamondback Terrapin Tissues, Vegetative, and Benthic Invertebrate Resources Within Barnegat Bay, New Jersey During 3 Sampling Sessions Between 2011–2019. U.S. Geological Survey Data Release. https://doi.org/10.5066/P9I2C3MR.
Elsey-Quirk, T., 2016. Impact of Hurricane Sandy on salt marshes of New Jersey. Estuarine, Coastal and Shelf Science 183: 235–248.
Engle, V. D., J. L. Hyland & C. Cooksey, 2009. Effects of Hurricane Katrina on benthic macroinvertebrate communities along the northern Gulf of Mexico coast. Environmental Monitoring and Assessment 150: 193–209.
Erickson, M. R., A. Grosse & J. C. Maerz, 2011. Dietary analysis of the diamondback terrapin (Malaclemys terrapin) along the Georgia coast. In The Society for Integrative and Comparative Biology (SICB) Annual Meeting: Abs. P3.66, 2011.
Fry, B., 2006. Stable Isotope Ecology. Springer, New York [available on internet at: http://tocs.ulb.tu-darmstadt.de/178042439.pdf]. Accessed 19 Mar 2014.
Gannes, L. Z., D. M. O’Brien & C. Martinez del Rio, 1997. Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78: 1271–1276.
Hall, T. M. & A. H. Sobel, 2013. On the impact angle of Hurricane Sandy’s New Jersey landfall. Geophysical Research Letters 40: 2312–2315.
Hobson, K. A., & L. I. Wassenaar, 2008. Tracking animal migration with stable isotopes. Terrestrial Ecology Series 2.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER – Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80: 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x.
Jackson, M. C., I. Donohue, A. L. Jackson, J. R. Britton, D. M. Harper & J. Grey, 2012. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 7: 1–12.
Jivoff, P. R., L. Moritzen, J. Kels, J. McCarthy, A. Young, A. Barton, P. Ferdinando, F. Pandolfo & C. Tighe, 2017. The relative importance of the Sedge Island Marine Conservation Zone for adult blue crabs in Barnegat Bay, New Jersey. Journal of Coastal Research 78: 269–276.
Johnson, R. A. & D. W. Wichern, 2007. Applied Multivariate Statistical Analysis, 6th ed. Pearson Prentice Hall, Upper Saddle River:
Kassambara, A., 2020. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.4.0 [available on internet at: https://CRAN.R-project.org/package=ggpubr]. Accessed 19 Feb 2021.
Kays, D. M., J. Fingerut & S. P. McRobert, 2019. Dietary analysis of northern diamondback terrapins (Malaclemys terrapin terrapin) in two New Jersey coastal saltmarshes. Russian Journal of Herpetology 26: 1–7.
Kennish, M. J., S. B. Bricker, W. C. Dennison, P. M. Glibert, R. J. Livingston, K. A. Moore, R. T. Noble, H. W. Paerl, J. M. Ramstack, S. Seitzinger, D. A. Tomasko & I. Valiela, 2007. Barnegat Bay-Little Egg Harbor Estuary: case study of a highly eutrophic coastal bay system. Ecological Applications 17: 3–16.
Kim, S. L. & P. L. Koch, 2012. Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environmental Biology of Fishes 95: 53–63.
Lathrop, R. G., J. A. Bognar, W. P. Beach, R. G. Lathrop & J. A. Bognar, 2001. Barnegat Bay–Little Egg Harbor, New Jersey: estuary and watershed assessment. Habitat loss and alteration in the Barnegat Bay Region. Journal of Coastal Research 212–228 [available on internet at: https://www.jstor.org/stable/25736235]. Accessed 24 Feb 2021.
Layman, C. A., D. A. Arrington, C. G. Montaña & D. M. Post, 2007. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88: 42–48.
Lemons, G. E., T. Eguchi, B. N. Lyon, R. LeRoux & J. A. Seminoff, 2012. Effects of blood anticoagulants on stable isotope values of sea turtle blood tissue. Aquatic Biology 14: 201–206.
Lester, L. A., H. W. Avery, A. S. Harrison & E. A. Standora, 2013. Recreational boats and turtles: behavioral mismatches result in high rates of injury. PLoS ONE 8(12): e82370.
Longenecker, R. A., J. L. Bowman, B. J. Olsen, S. G. Roberts, C. S. Elphick, P. M. Castelli & W. Gregory Shriver, 2018. Short-term resilience of New Jersey tidal marshes to Hurricane Sandy. Wetlands 38: 565–575.
Lovich, J. E. & J. W. Gibbons, 1990. Age at maturity influences adult sex ratio in the turtle Malaclemys terrapin. Oikos 59: 126–134.
MacKenzie, R. A. & M. Dionne, 2008. Habitat heterogeneity: importance of salt marsh pools and high marsh surfaces to fish production in two Gulf of Maine salt marshes. Marine Ecology Progress Series 368: 217–230.
Mallin, M. A. & C. A. Corbett, 2006. How hurricane attributes determine the extent of environmental effects: multiple hurricanes and different coastal systems. Estuaries and Coasts 29: 1046–1061.
Mallin, M. A., M. H. Posey, G. C. Shank, M. R. McIver, S. H. Ensign & T. D. Alphin, 1999. Hurricane effects on water quality and benthos in the cape fear watershed: natural and anthropogenic impacts. Ecological Applications 9: 350–362.
McClelland, J. W., I. Valiela & R. H. Michener, 1997. Nitrogen-stable isotope signatures in estuarine food webs: a record of increasing urbanization in coastal watersheds. Limnology and Oceanography 42: 930–937.
Michener, R. H. & L. Kaufman, 2008. Stable isotope ratios as tracers in marine food webs: an update. Stable Isotopes in Ecology and Environmental Science. https://doi.org/10.1002/9780470691854.ch9.
Newsome, S. D., C. Martinez del Rio, S. Bearhop & D. L. Phillips, 2007. A niche for isotopic ecology. Frontiers in Ecology and the Environment 5: 429–436.
Olin, J. A., C. M. Bergeon Burns, S. Woltmann, S. S. Taylor, P. C. Stouffer, W. Bam, L. Hooper-Bui & R. E. Turner, 2017. Seaside Sparrows reveal contrasting food web responses to large-scale stressors in coastal Louisiana saltmarshes. Ecosphere 8: 1–18.
Pang, H. H., P. Ingelido, B. Hirst, J. Pflaumer, A. Witt, A. Zaman & J. Aiello, 2017. Water quality condition and assessment within the Barnegat Bay Watershed between 2011 and 2015. Journal of Coastal Research 78(10078): 7–21.
Petrochic, S. L., 2009. Feeding ecology of the northern diamondback terrapin, Malaclemys terrapin terrapin. Thesis, Long Island University.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2.
Post, D. M., C. A. Layman, D. A, Arrington, G. Takimoto, J. Quattrochi & C. G. Montaña, 2007. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152(1): 179–189. https://doi.org/10.1007/s00442-006-0630-x.
R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [available on internet at: https://www.R-project.org/]. Accessed 31 Mar 2021.
Resasco, J., K. T. Tuff, S. A. Cunningham, B. A. Melbourne, A. L. Hicks, S. D. Newsome & K. F. Davies, 2018. Generalist predator’s niche shifts reveal ecosystem changes in an experimentally fragmented landscape. Ecography 41: 1209–1219.
Sanzone, D. M., J. L. Meyer, E. Marti, E. P. Gardiner, J. L. Tank & N. B. Grimm, 2003. Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 134: 238–250.
SAS Institute, Inc., 2018. SAS/STAT®15.1 User’s Guide, SAS Institute, Inc., Cary:
Seminoff, J. A., T. T. Jones, T. Eguchi, D. R. Jones & P. H. Dutton, 2006. Stable isotope discrimination (δ13C and δ15N) between soft tissues of the green sea turtle Chelonia mydas and its diet. Marine Ecology Progress Series 308: 271–278.
Seminoff, J. A., K. A. Bjorndal & A. B. Bolten, 2007. Stable carbon and nitrogen isotope discrimination and turnover in pond sliders Trachemys scripta: insights for trophic study of freshwater turtles. Copeia 2007: 534–542. https://doi.org/10.1643/0045-8511(2007)2007%5B534:SCANID%5D2.0.CO%3B2.
Seminoff, J. A., T. T. Jones, T. Eguchi, M. Hastings & D. R. Jones, 2009. Stable carbon and nitrogen isotope discrimination in soft tissues of the leatherback turtle (Dermochelys coriacea): insights for trophic studies of marine turtles. Journal of Experimental Marine Biology and Ecology 381: 33–41 [available on internet at: http://www.sciencedirect.com/science/article/pii/S0022098109003633]. Accessed 04 Feb 2014.
Sheridan, C. M., J. R. Spotila, W. F. Bien & H. W. Avery, 2010. Sex-biased dispersal and natal philopatry in the diamondback terrapin, Malaclemys terrapin. Molecular Ecology 19: 5497–5510 [available on internet at: http://www.ncbi.nlm.nih.gov/pubmed/21091556]. Accessed 22 Mar 2012.
Taghon, G. L., P. A. Ramey, C. M. Fuller, R. F. Petrecca & J. P. Grassle, 2017a. Benthic community structure and sediment properties in Barnegat Bay, New Jersey, before and after Hurricane Sandy. Estuaries and Coasts 40: 160–172. https://doi.org/10.1007/s12237-016-0133-x.
Taghon, G. L., P. A. Ramey, C. M. Fuller, R. F. Petrecca, J. P. Grassle & T. J. Belton, 2017b. Benthic invertebrate community composition and sediment properties in Barnegat Bay, New Jersey, 1965–2014. Journal of Coastal Research 78: 169–183.
Tucker, A. D., N. N. Fitzsimmons & J. W. Gibbons, 1995. Resource partitioning by the estuarine turtle Malaclemys terrapin: trophic, spatial, and temporal foraging constraints. Herpetologica 51: 167–181.
Tucker, A. D., R. L. Burke & D. C. Tulipani, 2018. Foraging ecology and habitat choice. In Ecology and Conservation of the Diamond-Backed Terrapin. Johns Hopkins University Press, Baltimore: 147–156.
Tulipani, D. C., 2013. Foraging Ecology and Habitat Use of the Northern Diamondback Terrapin (Malaclemys terrapin terrapin) in Southern Chesapeake Bay, The College of William and Mary in Virginia, Williamsburg:
Ulseth, A. J. & A. E. Hershey, 2005. Natural abundances of stable isotopes trace anthropogenic N and C in an urban stream. Journal of the North American Benthological Society 24: 270–289. https://doi.org/10.1899/03-080.1.
Velinsky, D. J., B. Paudel, T. J. Belton & C. K. Sommerfield, 2017. Tidal marsh record of nutrient loadings in Barnegat Bay, New Jersey. Journal of Coastal Research 78: 79–88.
Watson, E. B. & M. E. Gannon, 2020. Stable Isotope (CNS) Variability in Flora and Fauna of Barnegat Bay, New Jersey. Report submitted to the Partnership for the Delaware Estuary, Wilmington.
Wearmouth, V. J. & D. W. Sims, 2008. Chapter 2: sexual segregation in marine fish, reptiles, birds and mammals. Behaviour patterns, mechanisms and conservation implications. Advances in Marine Biology 54: 107–170.
Weideli, O. C., J. J. Kiszka, P. Matich & M. R. Heithaus, 2019. Effects of anticoagulants on stable-isotope values (δ13C and δ15N) of shark blood components. Journal of Fish Biology 95: 1535–1539.
Werner, E. E. & J. F. Gilliam, 1984. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics 15: 393–425.
Wickham, H., 2016. ggplot2: Elegant Graphics for Data Analysis, Springer, New York:
Wilson, K. A., K. W. Able & K. L. Heck, 1990. Habitat use by juvenile blue crabs: a comparison among habitats in southern New Jersey. Bulletin of Marine Science 46: 105–114.
Winters, J. M., H. W. Avery, E. A. Standora & J. R. Spotila, 2015. Between the bay and a hard place: altered diamondback terrapin nesting movements demonstrate the effects of coastal barriers upon estuarine wildlife. Journal of Wildlife Management 79: 682–688.
Winters, J. M., W. C. Carruth, J. R. Spotila, D. C. Rostal & H. W. Avery, 2016. Endocrine indicators of a stress response in nesting diamondback terrapins to shoreline barriers in Barnegat Bay, NJ. General and Comparative Endocrinology 235(2016): 136–141.
Wnek, J. P., 2010. Anthropogenic Impacts on the Reproductive Ecology of the Diamondback Terrapin, Malaclemys terrapin, Drexel University, Philadelphia:
Acknowledgements
We thank Loreena Avery, Kate Doherty, Paul Fidel, Michael Oden, and Laura Versaggi for assisting with data collection and field research. We are indebted to Fred Kallmuenzer, Pete Poulas, Marine Academy of Technology and Environmental Science (MATES) Project Terrapin (Manahawkin NJ), and Barnegat Township (Barnegat, NJ) for local support of project logistics. We thank the Edwin B. Forsythe National Wildlife Refuge (U.S. Fish and Wildlife Service), and the New Jersey Department Environmental Protection Fish and Wildlife Exotic and Nongame Permit Unit for issuing research permits, and Julianne Winters for the 2011 terrapin samples. We also thank Maggie Connor, Jamie Casteel, Melissa Gonzalez, Aslyn Mattson, and the many student volunteers who assisted in the laboratory processing of samples, and Darren Johnson for technical assistance. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Funding
Funding for this research was from the U.S. Geological Survey Hurricane Sandy Supplemental Funding with additional support from U.S. Geological Survey Priority Ecosystems Science Program. We also thank the Earthwatch Institute (Boston, MA) and Betz Chair of Environmental Science, Drexel University (Philadelphia, PA) for past financial support of field research, and The Leatherback Trust (Fort Wayne, IN) for personnel and grant management for this project.
Author information
Authors and Affiliations
Contributions
KH, HA, JW, and MD contributed to the study conception and design. Material preparation, data collection and analysis were performed by MD, HA, JW, and SM. The first draft of the manuscript was written by MD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
All animals were captured and sampled in accordance with New Jersey Fish and Wildlife guidelines for humane capture, handling, and testing, and USGS-SESC-IACUC 2011-05 and USGS-SESC-2014-02.
Additional information
Handling Editor: Daniele Nizzoli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Denton, M.J., Hart, K.M., Wnek, J. et al. Isotopic niche of New Jersey terrapins suggests intraspecific resource partitioning, and little variability following a major hurricane. Hydrobiologia 850, 2975–2990 (2023). https://doi.org/10.1007/s10750-023-05148-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05148-z