Abstract
The freshwater pearl mussel Margaritifera margaritifera is an endangered bivalve which is usually regarded as sedentary, although individual movement has been observed both vertically and horizontally. Little is known about the causes and rates of mussel movement. The objective of this study was to test the effect of microhabitat characteristics on the horizontal movement distance and rates of freshwater pearl mussels. A total of 120 mussels (length range 40–59 mm) were marked individually with passive integrated transponder tags, placed in stream microhabitats differing in their sediment composition and monitored biweekly over a period of 10 weeks. Mussels situated in sand-dominated habitats had a significantly higher mean movement rate (3.2 ± 4.2 cm/day, mean ± SD) than mussels situated in gravel-dominated (1.9 ± 2.7 cm/day) or stone-dominated habitats (1.8 ± 3.2 cm/day). The direction of the movements appeared random; however, an emigration from sandy habitats was observed, probably to avoid dislodgment from these hydraulically unstable habitats. This study demonstrates that freshwater pearl mussels can actively emigrate from unsuitable microhabitats. Once suitable streams with respect to physical, chemical, and biological quality were identified, it is therefore only necessary to identify suitable mesohabitats (area of 10–30 m2) when reintroducing or relocating mussels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The freshwater pearl mussel (FPM) Margaritifera margaritifera (Linnaeus, 1758), an extremely long-lived unionid mussel, is decreasing in abundance throughout its Holarctic range (Young et al., 2001; Geist, 2010; Lopes-Lima et al., 2017). The species is globally endangered and classified as critically endangered in Europe (IUCN, 2022). Thus, FPMs have been the focus of numerous captive-rearing activities in Europe for over 30 years (e.g., Buddensiek, 1995; Preston et al., 2007; Gum et al., 2011). In Germany, FPM populations can be found in Bavaria, North Rhine-Westphalia, Lower Saxony, and Saxony where they are subject of several conservation programs (Jecke et al., 2022). Besides habitat optimization, captive rearing is a main action to re-establish a functional, self-reproducing population. Since 2009 reintroduction of reared FPMs in the Saxonian Vogtland has focused on one former pearl stream, which is particularly suitable for habitat restoration since it flows through a NATURA 2000 area (a network of nature protection areas in the European Union). In the last decade long-term studies have been conducted in that area to identify streams with a potential for reintroduction of reared FPMs, focusing on both abiotic (e.g., substrate stability or physical and chemical parameters) and biotic (e.g., growth and survival of FPMs or abundance of host fish) factors and narrowing it down to four suitable streams. Prior to reintroducing high numbers of FPMs to the streams, a method was needed to first determine if the chosen habitats were suitable for mussels and secondly, to evaluate the success of the reintroduction. The method described in this paper was developed for this purpose and furthermore provided the opportunity to study the movement of mussels in the field.
Mussels are traditionally viewed as sessile organisms. However, they can perform both horizontal (across sediment) and vertical (within sediment) movements with the aid of a muscular foot (Trueman, 1983; Saarinen & Taskinen, 2003). Additionally, they may also use passive dispersal methods, which shall be mentioned but are not the focus of this paper: Adult mussels can be dislodged during flood events and transported downstream in lotic systems, referred to as drifting. During the larval stage, natural dispersal vectors (host fishes for glochidia) play a key role in mussel dispersal in both lotic and lentic systems (Kappes & Haase, 2012).
The active horizontal movement rate of adult freshwater bivalves in lotic systems varies drastically between species. While Corbicula fluminea (O.F. Müller, 1774) showed high movement rates of 330 cm/day in field observations (Voelz et al., 1998) and 14 cm/day in laboratory studies (Pernecker et al., 2021), lower movement rates have been observed for Elliptio complanata (Lightfoot, 1786) (0.8 cm/day) (Balfour & Smock, 1995), Unio tumidus Philipsson, 1788, Unio pictorum (Linnaeus, 1758), and Anodonta anatina (Linnaeus, 1758) (2 cm/day) (Schwalb & Pusch, 2007). In all studies the observed movements varied strongly between individuals, e.g., the movement rate in the study of Schwalb and Pusch (2007) varied between 0 and 32 cm/day. The direction of freshwater mussel movement is random and erratic (Bovbjerg, 1957; Balfour & Smock, 1995; Schwalb & Pusch, 2007), but nevertheless several causes for freshwater mussel movement have been discussed in the past, differing between horizontal and vertical movement. Studies have suggested a connection between mussel movement and reproduction resulting in seasonal migration patterns (e.g., Piechocki, 1969; Amyot & Downing, 1997; Melchior, 2021). The horizontal movement pattern of Anodonta sp. was found to vary seasonally with aggregations being most distinct during the spawning seasons (Burla et al., 1974). Watters et al. (2001) identified a seasonal vertical migration pattern in several species of Unionida where mussels surfaced during spawning in spring and reburied in autumn. Amyot and Downing (1998), however, did not find any relation between rate of movement and reproductive output in E. complanata.
The avoidance of adverse conditions is also a significant driver of mussel movement. Movement of mussels has been observed in response to cold winter temperatures (Engel, 1990), exposure to air during low water levels (Gough et al., 2012; Galbraith et al., 2015; Mitchell et al., 2018), or in response to decreasing food supplies (Bovbjerg, 1957). Zieritz et al. (2014) even suggest that mussel distribution patterns and movement behavior are indicative of population health. In lotic systems, where FPMs are found, drifting can transport mussels to unsuitable habitats (Kappes & Haase, 2012; Sansom et al., 2020). Hence, it seems reasonable that mussels could correct their location after dislodgement and move to optimal habitats. In fact, Zajac and Zajac (2011) found that Unio crassus Philipson, 1788 can move to any location in a stream after being disturbed by fluvial processes and that this movement is used for habitat selection. Assuming that these results are applicable to FPMs, they too should be able to select their microhabitat (area of 0–10 m around the mussel) following reintroduction, making the exact position of reintroduction inconsequential as long as the mesohabitat (area of > 10 m around the mussel) is suitable.
Numerous studies have characterized the habitat requirements of FPMs on a microhabitat scale. In Scottish populations Hastie et al. (2000) determined water depths of 0.3–0.4 m and current velocities of 0.25–0.75 m/s to be optimal for FPM based on habitat suitability indices. FPMs were also mostly observed in boulder-stabilized refugia with coarse sand and gravel (Young & Williams, 1983; Hastie et al., 2000; Quinlan et al., 2015). Boulders stabilize the sediment and prevent significant scouring of the river bed during major floods (Vannote & Minshall, 1982), while coarse sediment provides enough interstitial space to oxygenate the hyporheic zone, making it suitable for post-parasitic mussels (Österling et al., 2010). Adult FPMs can tolerate muddy conditions but juvenile mussels are never found in this type of habitat (Hastie et al., 2000). Parameters often used to describe sediment characteristics of FPM habitats are fine sediment ratio and median grain size diameter (D50). Geist and Auerswald (2007) found functional populations in habitats consisting of < 18% particles smaller than 1 mm and a mean particle diameter of 7 mm. These sediment characteristics have been determined as appropriate predictors for presence of FPMs. Hastie et al. (2000) successfully used substratum-based models to predict the occurrence of mussels in a Scottish stream. If sediment characteristics influence the occurrence of FPMs, they can be expected to also have an impact on the movement of FPMs.
The field experiment presented in this paper aimed to investigate the behavior of freshly reintroduced FPMs placed in suitable and adverse microhabitats. By attaching passive integrated transponder (PIT)-tags to the shells it was possible to monitor mussel movements in intervals of 14 days and determine a movement rate for this mussel species. Furthermore, it was investigated if microhabitat characteristics affect the movement of FPMs. It was hypothesized that mussels will move to gravel-dominated habitats since the D50 values in these habitats match the observed values of Geist and Auerswald (2007). We expected that FPMs would move from adverse (sand-dominated or stone-dominated) to optimal (gravel-dominated) habitats. Therefore, FPMs located in adverse habitats should have higher movement rates, resulting in altered sediment characteristics in their independently chosen habitat. Thus, D50 and fine sediment ratios in the independently chosen habitats at the end of the monitoring period were hypothesized to shift toward optimal values (D50: 5–50 mm and fine sediment ratio < 18%) for mussels initially placed in adverse habitats.
Materials and methods
Study area
The experiment was conducted in four similar streams in the Vogtland, Saxony (referred to here as S1, S2, S3, and S4): Three of these locations (S1, S3, and S4) were potential streams for reintroduction of FPMs, while S2 already holds a population of reintroduced FPMs. All streams are upper tributary waters of the Weiße Elster, a 257-km-long river with a catchment area of 5,154 km2. They are small, mountainous streams meandering through forests and meadows. Available river habitats consist of shallow runs, riffles, and small pools (max. 1.5 m deep) and most of the channel area is covered with gravel and stones. The banks of the pool sections have areas of fine sediment deposition while banks of runs or riffles are eroded in some sections of the streams.
During two site inspections in March and June 2020 approx. one kilometer of each stream was examined and searched for sections with suitable habitat conditions for reintroduction of FPMs (following a previously validated protocol (Jecke et al., 2022)) and a diverse sediment composition. Two sections per stream with a maximum length of 10 m were chosen as study sites for the following experiment, resulting in a total of eight study sites. Morphological features of all sites were measured. The position of the bankfull channel was estimated and measured by surveying the top of the bank where the floodplain starts every 1 m along both sides of the stream. The position of the flow channel was estimated and measured by searching for the area of maximum flow velocity within the lower third of the stream using a handheld flow meter (MiniAir2, Schiltknecht Messtechnik AG, Gossau, Switzerland) conducted along 1-m intervals. Areas with sediment dominated by stones or sand were delineated by measuring the outer contours of these areas every 1 m along the stream axis. Channel and flow measurements (Table 1) were taken by generating five cross-sections evenly distributed along the study sections. Flow velocity was recorded at 20-cm intervals in the lower third of the stream. The length of all study sections was measured along the identified flow channel (Table 1).
PIT-tagging
A total of 184 FPMs (120 experimental and 64 control mussels) with a size range of 40–59 mm were selected from cages of the rearing facilities in the Vogtland and measured in length (Table 1) using a digital caliper gauge (accuracy ± 0.2 mm). All FPMs were offspring from wild native mussels and had lived in cages, which were filled with natural sediment and exposed to natural flows, in the study streams for at least 10 years. Mussels were tagged with shellfish glue-on tags (FPN 8 × 4, Hallprint, Hindmarsh Valley, Australia), adhered to the external valve using super glue (Dupla Plantfix, Dohse Aquaristik GmbH, Grafschaft-Gelsdorf, Germany). A PIT-tag (APT12, Biomark, Boise, U.S.A.; 12.5 × 2 mm) was attached on the opposite valve using super glue (Sekundenkleber, UHU, Bühl, Germany) (Hartmann et al., 2016). The PIT-tag was then embedded in dental cement (Fuji I, GC Corporation, Tokyo, Japan). The cement hardened for eight minutes, while the mussel siphons were covered with water, guaranteeing a functional respiration. Tagging took an average of two minutes per individual.
Study design
At the end of July 2020 (calendar week 30), 15 tagged mussels per study site were released across three different habitats (sand-dominated, gravel-dominated, and stone-dominated), resulting in five mussels per habitat. Empty FPM shells were filled with silicone to create mussel dummies which had a similar size and weight (approx. 9–10 g) as the living mussels. One dummy was added to each group of five mussels for a total of 24 dummies and gently pushed into the sediment. In addition to released mussels, a minimum of ten PIT-tagged control mussels were placed in cages in each stream (no more than 1 km away from the study sites). For a detailed overview of all mussels see Table 1. The initial location of the mussels was recorded by measuring the distances to three previously stationed iron rods (one-meter-long rods driven into the soil in a triangular formation around the study site) using a laser rangefinder (GLM 50C, Bosch, Stuttgart, Germany).
The movement of mussels was observed for a duration of ten weeks. Mussels were monitored biweekly using a handheld PIT-tag reader (HPR Plus, Biomark, Boise, U.S.A.) and the microhabitat in which the mussels were found in was characterized. The new location of every detected mussel was recorded by measuring the distances to the three iron rods used to measure initial release location. Afterward, a trilateration was conducted to project all mussel locations from the field into a coordinate system. This made it possible to retrace the direction and the length of mussel movement. After a sixth sampling event at the beginning of October 2020 all mussels were removed from the study sections.
Mussel movement tracking
Each study site was searched for PIT-tagged mussels starting from the downstream end of the respective study site. Once a signal was detected, the area within a 10 cm radius around the signal was examined carefully to determine if other mussels were present. Locations (10 cm radius) of identified mussels were considered as one plot regardless of how many mussels were present within the plot area. After plot sampling (described in the following section), PIT tracking continued in upstream direction. If the end of the study site was reached without detecting all 15 mussels and the three dummies, a second search run was performed using a larger PIT reader antenna to locate missing PIT-tags. In laboratory tests the sediment depth up to which a detection of PIT-tags was possible was determined to be 10 cm for the small antenna and 15 cm for the big antenna regardless of substrate type. Since the big antenna was always used to check for missing mussels, it can be ensured that mussels were detected up to a sediment depth of 15 cm. If mussels were still undetected after two search runs, the search was extended downstream to find mussels that may have drifted downstream. All undetected mussels and dummies were classified as “not found”.
Plot sampling
Directly after detecting a mussel plot, an aquascope was used to visually confirm FPM presence. If a FPM was visible and alone in a plot, location data were collected directly above this mussel. If mussels were not visible or a plot contained multiple FPMs, the center of the plot was used to collect location data. Once located, the vertical position of mussels within the sediment was defined as “on surface” if the mussel was visible and as “under surface” if the mussel was not visible. Exact location data were collected by placing a tripod directly above the mussel. A laser rangefinder on the tripod was used to measure the distances to the three iron rods.
The plot was characterized by the following steps: The water depth above the plot and the flow velocity 2 cm above the substrate were measured using a handheld flow meter. During every survey, the sediment type within the mussel plot was classified into three groups, depending on the dominant sediment type: sandy, gravelly, and stony. During the initial and final sampling event the grain size distribution was assessed first by visual estimation by the operating person and then by sediment coring following the methods described in Geist & Auerswald (2007) but using a slightly smaller coring tube (63 mm diameter). Grain diameters defined sediments as mud (< 0.063 mm), sand (0.063–1 mm), fine gravel (1–10 mm), coarse gravel (10–63 mm), stones (63–200 mm), and boulders (> 200 mm). Finally, water samples were collected above the plot substrate and in 5 cm depth of the interstitial (using a syringe fitted with a semipermeable aluminum pipe) and abiotic parameters (temperature, oxygen concentration, pH, conductivity, and turbidity) were measured using a multiparameter probe (Multi 3630 IDS, WTW, Weilheim, Germany) and a turbidimeter (2100P, Hach, Loveland, U.S.A.).
Data analysis
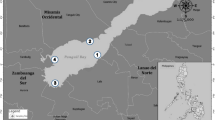
At each study site, the three iron rods (labeled A, B, C) served as fixpoints for a local 2-dimensional coordinate system. Local coordinates were defined such that x = 0, y = 0 at point A and x = 0, y > 0 at point C. In the field, the position P of an object of interest (mussels, structures, etc.) was recorded by measuring the distances P–A, P–B, and P–C. The most likely position of P in x- and y-coordinates was finally determined by least-squares fitting such that the resulting distances P–A, P–B, and P–C resembled the measured distances as close as possible. The calculated data were used to determine the movement distance between surveys and to create maps of each study site, showing the direction of mussel movement (Fig. 1). Movement distance was calculated as the Euclidean distance between a mussel position at time t−1 and t. A daily movement rate was calculated by dividing the movement distance by the number of days between t and t−1.
Map of the study site S4A. Polygons show the dominating sediment classes and black arrow represents the position and direction of the flow channel. Colored lines depict the movement patterns of individual mussels initially placed in a sandy habitat with the triangle being the position at the final survey (after 70 days) and the points in between the positions in biweekly intervals. Only mussels initially placed in the sandy habitat are shown on this map
Since the handheld PIT-tag reader detected mussels in a certain range around the antenna, it was difficult to clearly locate buried mussels. The uncertainty of the exact position of mussels was handled by neglecting movements under 10 cm/2 weeks. This was determined to be the error range of the handheld reader and considered as the maximum distance of nondirectional mussel movement caused by rotary motions; thus, all movement distances of less than 10 cm/2 weeks were set to zero. Since mussel movements were not tracked continuously, it was difficult to classify every observed downstream movement (either voluntary or drifting), while upstream movement clearly resulted from voluntary movement. A classification was carried out by incorporating precipitation data: All downstream movements greater than the maximum observed upstream movement of 2.4 m that occurred in combination with heavy rainfall events were defined as drifting (5.3–36.8 m, n = 7). All other movements were defined as voluntary movements (0.1–3.7 m, n = 400), neglecting that some downstream voluntary movements might also be a result of drift events. While mussels might be able to initiate drift events, they most probably cannot control the length of these events resulting in random destination habitats. Therefore, data of drift events were excluded for most data analysis.
Using data from the sediment estimations and the sediment samples a final grain size distribution was calculated as the ratio among sampled grain sizes. Because stones (63–200 mm) and boulders (> 200 mm) were excluded by the 63 mm diameter of the coring device, their ratio estimations were used to calculate the final grain size distribution. The four sampled grain size categories (mud: < 0.063 mm, sand: 0.063–1 mm, fine gravel: 1–10 mm, coarse gravel: 10–63 mm) were then weighted as the remaining ratio. In plots where sediment cores could not be collected due to grain sizes exceeding the core diameter, the final grain size distribution was determined just through estimation. This applied to 10% of gravelly habitats and 60% of stony habitats across all study sites. The D50 of the sediment and the relative amount of fine material (< 1 mm) were calculated for each plot (i.e., radius of 10 cm around the mussel) and for every plot where sediment samples were collected, respectively.
Data from the four study streams were pooled as we were looking for generalizable conclusions on the behavior of the mussel rather than for specific habitat effects. The hypothesized effect of the start sediment class (defined as the sediment class a mussel was found in during the previous sampling) on movement rate was analyzed by comparing the movement rates between the three possible start sediment types (sandy, gravelly, and stony). To check for differences in sediment characteristics between start and end habitat of moving FPMs, flow-induced sediment alterations were examined by comparing D50 values and fine sediment ratios at three reference sites per study site (covering the sediment types sandy, gravelly, and stony) between start and end of the experiment. Afterward, the differences in sediment characteristics of moving mussels were investigated by comparing D50 values and fine sediment ratios of the start and end habitats of all mussels.
Statistical analyses were performed using R (R Core Team, 2018). The effect of abiotic factors on the vertical position (on surface/under surface) of mussels was analyzed using the Welch two-sample t test for normally distributed abiotic data and the Wilcoxon rank-sum test. A Pearson’s chi-squared test was performed to check for a relationship between vertical position and sediment class. Since the distribution of movement distance data differed very significantly from a normal distribution, with low values dominating, all distance-related data were analyzed using non-parametric tests. Additionally, the data relating to daily movement rates were highly zero inflated since all movements < 10 cm were set to zero. Therefore, effects on daily movement rates (sample date and start sediment class) were analyzed using Pearson’s chi-squared test after transferring the data to a binary scale by setting thresholds of daily movement rates. Thresholds were set to 0 cm, the mean of all mussels and the mean of all moving mussels (excluding sessile mussels) for the considered timeframe. A Fisher’s test was used for group-wise comparison between the investigated test groups. Sediment characteristics (D50 and fine sediment ratio) between start and end habitat of moving mussels were checked for significant differences using the Wilcoxon rank-sum test.
Results
The role of microhabitat characteristics on horizontal FPM movement
Throughout the experiment FPMs appeared to vacate sandy habitats (Fig. 2b). Of the 40 FPMs that were initially released in sandy habitats 12 were found in the same habitat during the final survey. The emigration of mussels from sand-dominated habitats occurred mainly within the first 14 days of the experiment: 55% of mussels placed in sand-dominated habitats left the habitat within this time span. Mussels remaining in sandy habitats stayed there for the duration of the study apart from one or two mussels in every stream moving at a later point in time. Comparing the habitat at the start and end of the experiment 75% of mussels remained in gravelly or stony habitats, while only 30% of mussels remained in sandy habitats (Fig. 3). Approx. 50% of mussels from sandy habitats immigrated to gravelly habitats and 20% to stony habitats. No mussel immigrated to a sandy habitat.
a The relative number of freshwater pearl mussels detected (differed between mussels being visible and not visible on the sediment surface) and not detected during the five surveys. b Ratios of sediment classes where mussels were detected during the experiment. Note Habitat classification during calendar week 32 might be falsified, since data were collected by a different sampler
The daily movement rates differed in relation to the sediment class of the initial habitat (Fig. 4a). This effect was strongest when only considering the first two weeks of the experiment when the greatest departure activity from sandy habitat was observed (Fig. 4b). Within the first two weeks, mussels from sandy habitats moved at a mean rate of 4.6 ± 4 cm/day, while mussels from gravelly and stony habitats had movement rates of 1.9 ± 3.2 cm/day and 1.5 ± 2.8 cm/day, respectively. A Pearson’s chi-squared test revealed a relation between sediment class of start habitats and individuals moving over 0 cm, 2.7 cm (mean of all mussels during first two weeks) and 3.8 cm (mean of moving mussels during first two weeks) per day (Table 2). A Fisher’s test showed that the number of mussels moving over 2.7 and 3.8 cm per day was significantly higher for mussels situated in sandy habitats than it was for mussels situated in stony or gravelly habitats. Furthermore, significantly more mussels stayed sessile in stony and gravelly habitats compared to sandy habitats. A correlation matrix that was computed to determine further predictors for movement rates showed significant correlations for some habitat parameters, such as flow or water temperature, but computed Spearman correlations were all very low (< 0.16). Therefore, no further predictors for movement rates besides the presented predictor sediment characteristics could be identified.
Boxplots of the daily movement rate of freshwater pearl mussels during the whole experiment (a) and during the first two weeks of the experiment (b) in relation to the sediment class at the start habitat; drift events were excluded. Whisker–0.05 and 0.95 percentiles; Box–0.25 quartile, median and 0.75 quartile; Points–outliers exceeding 1.5 times the interquartile range; Cross–mean value
The sediment characteristics differed in the start and end habitats of mussels initially placed in sandy habitats. While alterations of D50 values and fine sediment ratios induced by high flows could be excluded by comparing sediment characteristics at reference sites (Online Resource 1), both values changed significantly between the start and end habitat of mussels initially placed in sandy habitats (Fig. 5a, b). No significant changes of D50 and fine sediment ratio between the start and end habitat were determined for mussels initially placed in gravelly and stony habitats (Fig. 5c, d, e, f).
Boxplots illustrating the altered sediment characteristics (D50–left, ratio of fine sediment–right) between initial and final survey of mussels initially placed in sandy (a, b), gravelly (c, d), or stony (e, f) habitats. Drift events were excluded. Whisker–0.05 and 0.95 percentiles; Box–0.25 quartile, median and 0.75 quartile; Points–outliers exceeding 1.5 times the interquartile range; Cross–mean value; Shared letters indicate a lack of significance using the Wilcoxon rank-sum test
Movement distances and movement patterns
Both upstream and downstream movement of FPMs was observed. While upstream movement can only result from voluntary movement, high water velocity can lead to downstream transport of mussels. In fact, all seven movement distances of over 5 m within 14 days could be linked to discharge-related drift events that occurred after heavy rainfalls (Online Resource 2). These movements only occurred in downstream direction and mussels were always found in areas where debris or current breaks such as large boulders were observed. Data from the seven drift events were excluded for most data analysis. The maximum upstream movement was 2.4 m within 15 days (16 cm/day), while the maximum displacement distance caused by a drift event was 37 m within 14 days. Dummies were also affected by high water velocity during the study and drifted distances ranging from 0 to 9.6 m within 14 days (mean = 0.46 m, SD = 1.22 m). The distances covered by FPMs during the experiment (70 days) differed greatly between individuals but not between rivers. Approx. 50% of mussels remained in a radius of < 1 m, while four individuals moved over 5 m and two mussels did not move at all (Fig. 6). Mussels moving over 2 m were dominated by mussels initially placed in a sandy habitat (Fig. 6). The mean movement rate determined for FPMs across all habitats and streams was 2.1 ± 3.3 cm/day (mean ± SD).
Mussels in all sediment classes did not perform unidirectional movements, rather, the direction of movement appeared to be random with mussels changing their direction during the surveys (Fig. 1).
Detectability
The detectability of PIT-tagged mussels was about 95%, varying between the surveys and across study sites with a trend of increasing detectability as survey weeks progressed (Fig. 2a), possibly resulting from improved searching skill. Tagged FPMs (94%) were located during at least four of the five surveys and only one mussel was just located once. Dummies were detected with 86% frequency throughout the experiment. Four dummies were not found during the final survey and two dummies were found on the shore. During the final survey, a dummy was found with a detached PIT-tag, while the PIT-tag remained functional and was detected in short distance of the dummy. One mussel died during the experiment; the empty shell was found during the final survey.
Mussel visibility on the sediment surface varied between surveys (Fig. 2a). Highly significant abiotic habitat differences were detected between visible and non-visible mussels with water depth, conductivity, and pH values being higher in habitats of non-visible mussels (Table 3). FPMs were more present on the sediment surface (75% of mussels were visible) when the free water pH level was under 7.5, but if the pH exceeded 7.5 most mussels (54%) were buried. A Pearson’s chi-squared test revealed a significant relationship between sediment class and mussel visibility (χ2 (2, N = 683) = 8.3, P = 0.016). Mussels in sandy habitats were visible more often than mussels in gravelly or stony habitats.
Discussion
The role of microhabitat characteristics on horizontal FPM movement
While mussels moved throughout the experiment, an emigration from sandy habitats took place mainly within the first 14 days of the experiment (Fig. 2b). The limited duration of the experiment (10 weeks) was therefore sufficient to observe changes in habitat occupancy and to verify the hypothesis that most mussels released in sandy habitats emigrated to occupy gravel or stone-dominated habitats. This departure was not observed for mussels released in stony habitats (Fig. 3). The maximum D50 value measured in stone-dominated habitats (96 mm) was within the range of D50 values observed by Westberg (2020) in natural FPM habitats (25–210 mm), which might have caused mussels to stay in these habitats. An emigration from stony habitats is therefore only expected for habitats with D50 values beyond 210 mm which may be caused by drifting of mussels not able to burrow in the sediment. However, D50 values in stony habitats might be biased because of the described sampling difficulties in these habitats. Even though the fine sediment ratio in end habitats of mussels departing from sandy habitats was still above the limit of 18% described by Geist and Auerswald (2007), we can verify the hypothesis that active movement enables mussels to resettle in microhabitats with altered sediment characteristics.
One can only speculate about the reasons for emigration of FPMs from sand-dominated habitats with high amounts of fine sediment. One explanation might be a negative impact of deposited fine sediment on FPMs which clog the interstitial spaces in stream substrates with most adverse effects on juvenile FPMs (Geist & Auerswald, 2007; Österling et al., 2010; Denic & Geist, 2015; Hyvärinen et al., 2021). However, the physiological effects of fine sediments on adult and semi-adult freshwater mussels are still not understood. The few studies that have been conducted on freshwater mussels found that increased turbidity due to fine sediments can interfere with filter feeding (Aldridge et al., 1987) or lead to growth reduction (Österling et al., 2007, 2010). A recent study on U. pictorum, however, demonstrated that clearance rates were constant and independent of particle size class or concentration, falsifying the hypothesis that fine particles clog the gills of freshwater mussels (Lummer et al., 2016). We speculate that mussels emigrating from sandy habitats could have searched for better refugia or food supplies. Sandy habitats might be too unstable for FPMs with greater chance of dislodgement to unsuitable habitats and therefore are not considered as potential refugia. Furthermore, sandy habitats are mainly areas with no or low flow velocity and might contain lower concentrations of suspended food particles than areas with high flow velocity. Since we did not test the food quality and availability during this study, it might be possible that this was a proximate cause for mussel movements. In fact, 10 of the 12 mussels that did not emigrate from sand-dominated habitats were observed in sites S1B and S3A where a backflow might have delivered enough food. An emigration from sandy habitats in sites S1B and S3A later in the season seems reasonable due to potential higher water velocities and flooding in autumn. Future experiments will have to determine the role of sediment mixing ratios, bed roughness, or shear stress on the observed emigration behavior from certain habitats.
The number of mussels visible on the sediment surface varied between the surveys (Fig. 2a) and significant abiotic habitat differences were detected between mussels on and below the surface (Table 3). Differences in water depth and sediment class are most likely caused by methodological reasons: Mussels in deeper depths were difficult to survey and it was much harder to distinguish between mussels and stones in gravelly and stony habitats resulting in the risk of missing mussels on the sediment surface under these conditions (Sanchez & Schwalb, 2021). Elevated conductivity and pH levels may have triggered avoidance behavior in FPMs resulting in burrowing below the sediment surface at pH > 7.5. FPMs seem to possess chemoreceptors (Wilson et al., 2012) which might play a role in this behavior.
Movement distances and movement patterns
Sediment characteristics were an appropriate predictor of movement rates. During the first two-week period, significantly more mussels moved over 0, 2.7, and 3.8 cm per day when situated in sandy habitats than in stony or gravelly habitats (Fig. 4, Table 2). Higher movement rates in sand-dominated habitats might result from FPMs seeking a more suitable habitat, as also suggested by Poznańska-Kakareko et al. (2021), or simply because it was easier for mussels to move in habitats with smaller grain sizes. A former study found increased burrowing activity of unionid mussels in sandy habitats in both field and lab experiments (Hernández et al., 2021). In other studies, U. crassus were found to not only prefer fine sediment when moving in a stream but their movement and presence/absence were also dependent on additional habitat features, such as water velocity and depth (Zajac & Zajac, 2011; Stoeckl & Geist, 2016). This was not observed for FPMs and no further predictors of movement rates other than sediment characteristics could be found.
The observed movement rate of 2.1 ± 3.3 cm/day for FPMs is comparable to U. tumidus, U. pictorum, and A. anatina (2 cm/day) in a lowland river (Schwalb & Pusch, 2007). This can be surprising when acknowledging the stonier habitat of FPMs, but it seems that they are well adapted to their habitat and do not necessarily need loose material to perform movements with their foot. In one instance, a tagged FPM was observed moving across flat shale over a distance of approx. 5–10 cm. Former studies suggested that upstream movement of mussels in lotic systems offsets possible downstream dislodgment (Balfour & Smock, 1995). We formed similar conclusions as upstream movement distances were particularly high (2.4 m in 15 days; 16 cm/day) following two of the seven recorded drift events.
The movement distances measured between two-sample dates were considered minimum values as paths taken between start and end positions were not observed and thus a linear path was assumed. True movement distances may have been longer than assumed by this method due to meandering paths. Furthermore, it was impossible to exclude drift events as a cause for measured downstream movements. Flow velocities were occasionally high enough to dislodge mussels and could therefore have also affected the movement distances of mussels whose movements were defined as voluntary movements. Discharge peaks were observed during the experiment (Online Resource 2) but did not lead to a shift of the stream bed properties (Online Resource 1) or to an increased movement of the mussels. However, a few outliers in movement distances were observed at the same time as these discharge peaks.
Direct effects of tagging that may bias FPM behavior have been discussed by Wilson et al. (2011) as a decrease in the burrowing rate of FPM may have been partly due to handling. During this study, negative effects of handling were reduced by minimizing the handling time and not using ethanol to clean shells before tagging. Nevertheless, PIT-tags might still bias animal behavior and this must be reflected in the interpretation of the results. The mussels used in this experiment were offspring from wild native individuals, making a genetic effect on the behavior improbable due to retained genetic integrity of reared FPMs (see Geist et al., 2021). Furthermore, we could document that the design of our cages allowed mussels to perform horizontal and vertical movement and did not lead to a difference in growth rates or survival between the caged and free-living mussels. Therefore, it is not likely that the observed behavior of our investigated mussels is influenced by hatchery or inbreeding effects.
Conclusions
This study contributes to a deeper understanding of how substrate- and river-specific differences affect the movement activities of FPMs. The obtained results can be helpful for designing resettlement plans for this species, particularly when selecting streams or parts of streams with suitable abiotic habitat parameters. The study demonstrated that FPMs can resettle from unsuitable sand-dominated microhabitats and select for gravel- or stone-dominated habitats. Therefore, it is only necessary to identify suitable mesohabitats (areas of approx. 10–30 m2) when reintroducing FPMs from captive-breeding programs into the wild or moving mussels to other rivers or river sections.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aldridge, D. W., B. S. Payne & A. C. Miller, 1987. The effects of intermittent exposure to suspended solids and turbulence on three species of freshwater mussels. Environmental Pollution Elsevier 45: 17–28. https://doi.org/10.1016/0269-7491(87)90013-3.
Amyot, J.-P. & J. Downing, 1997. Seasonal variation in vertical and horizontal movement of the freshwater bivalve Elliptio complanata (Mollusca: Unionidae). Freshwater Biology 37: 345–354. https://doi.org/10.1046/j.1365-2427.1997.00158.x.
Amyot, J.-P. & J. Downing, 1998. Locomotion in Elliptio complanata (Mollusca: Unionidae): a reproductive function? Freshwater Biology 39: 351–358. https://doi.org/10.1046/j.1365-2427.1998.00289.x.
Balfour, D. L. & L. A. Smock, 1995. Distribution, age structure, and movements of the freshwater mussel Elliptio complanata (Mollusca: Unionidae) in a headwater stream. Journal of Freshwater Ecology 10: 255–268. https://doi.org/10.1080/02705060.1995.9663445.
Bovbjerg, R. V., 1957. Feeding related to mussel activity. Proceedings of the Iowa Academy of Science 64: 650–653.
Buddensiek, V., 1995. The culture of juvenile freshwater pearl mussels Margaritifera margaritifera L. in cages: a contribution to conservation programmes and the knowledge of habitat requirements. Biological Conservation Elsevier 74: 33–40.
Burla, H., H.-J. Schenker & W. Stahel, 1974. Das Dispersionsmuster von Teichmuscheln (Anodonta) im Zürichsee. Oecologia 17: 131–140. https://doi.org/10.1007/BF00346276.
Denic, M. & J. Geist, 2015. Linking stream sediment deposition and aquatic habitat quality in pearl mussel streams: Implications for conservation. River Research and Applications 31: 943–952. https://doi.org/10.1002/rra.2794.
Engel, H. H. B. J., 1990. Untersuchungen zur Autökologie von Unio crassus in Norddeutschland. PhD Dissertation, University of Hannover. Hannover.
Galbraith, H. S., C. J. Blakeslee & W. A. Lellis, 2015. Behavioral responses of freshwater mussels to experimental dewatering. Freshwater Science 34: 42–52. https://doi.org/10.1086/679446.
Geist, J., 2010. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): a synthesis of conservation genetics and ecology. Hydrobiologia 644: 69–88. https://doi.org/10.1007/s10750-010-0190-2.
Geist, J. & K. Auerswald, 2007. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshwater Biology 52: 2299–2316. https://doi.org/10.1111/j.1365-2427.2007.01812.x.
Geist, J., H. Bayerl, B. C. Stoeckle & R. Kuehn, 2021. Securing genetic integrity in freshwater pearl mussel propagation and captive breeding. Scientific Reports 11: 16019. https://doi.org/10.1038/s41598-021-95614-2.
Gough, H. M., A. M. Gascho Landis & J. A. Stoeckel, 2012. Behaviour and physiology are linked in the responses of freshwater mussels to drought. Freshwater Biology 57: 2356–2366. https://doi.org/10.1111/fwb.12015.
Gum, B., M. Lange & J. Geist, 2011. A critical reflection on the success of rearing and culturing juvenile freshwater mussels with a focus on the endangered freshwater pearl mussel (Margaritifera margaritifera L.). Aquatic Conservation: Marine and Freshwater Ecosystems 21: 743–751. https://doi.org/10.1002/aqc.1222.
Hartmann, J. T., S. Beggel, K. Auerswald & J. Geist, 2016. Determination of the most suitable adhesive for tagging freshwater mussels and its use in an experimental study of filtration behaviour and biological rhythm. Journal of Molluscan Studies 82: 415–421. https://doi.org/10.1093/mollus/eyw003.
Hastie, L. C., P. J. Boon & M. R. Young, 2000. Physical microhabitat requirements of freshwater pearl mussels, Margaritifera margaritifera. Hydrobiologia 429: 59–71. https://doi.org/10.1023/A:1004068412666.
Hernández, B. A., Z. A. Mitchell, C. R. Robertson & A. N. Schwalb, 2021. Burrowing behaviour of unionid mussels in subtropical rivers: Implications for survey guidelines. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 903–915. https://doi.org/10.1002/aqc.3525.
Hyvärinen, H., M. Saarinen-Valta, E. Mäenpää & J. Taskinen, 2021. Effect of substrate particle size on burrowing of the juvenile freshwater pearl mussel Margaritifera margaritifera. Hydrobiologia 848: 1137–1146. https://doi.org/10.1007/s10750-021-04522-z.
IUCN, 2022. The IUCN Red List of Threatened Species. Version 2022–1. Available on internet at: https://www.iucnredlist.org (Accessed 21 October 2022].
Jecke, F., M. Denic, H. Bayerl, T. Findeis, J. Geist, F. Grunicke, T. Schmidt, A. Wagner, & T. U. Berendonk, 2022. Projekt ArKoNaVera: sechs Jahre Artenschutz für die Flussperlmuschel (Margaritifera margaritifera). Natur und Landschaft 97: 373–380. https://doi.org/10.19217/NuL2022-08-01.
Kappes, H. & P. Haase, 2012. Slow, but steady: dispersal of freshwater molluscs. Aquatic Sciences 74: 1–14. https://doi.org/10.1007/s00027-011-0187-6.
Lopes-Lima, M., R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, K. Douda, E. Froufe, D. Georgiev, C. Gumpinger, A. Karatayev, Ü. Kebapçi, I. Killeen, J. Lajtner, B. M. Larsen, R. Lauceri, A. Legakis, S. Lois, S. Lundberg, E. Moorkens, G. Motte, K.-O. Nagel, P. Ondina, A. Outeiro, M. Paunovic, V. Prié, T. von Proschwitz, N. Riccardi, M. Rudzīte, M. Rudzītis, C. Scheder, M. Seddon, H. Şereflişan, V. Simić, S. Sokolova, K. Stoeckl, J. Taskinen, A. Teixeira, F. Thielen, T. Trichkova, S. Varandas, H. Vicentini, K. Zajac, T. Zajac & S. Zogaris, 2017. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews 92: 572–607. https://doi.org/10.1111/brv.12244.
Lummer, E.-M., K. Auerswald & J. Geist, 2016. Fine sediment as environmental stressor affecting freshwater mussel behavior and ecosystem services. Science of the Total Environment 571: 1340–1348. https://doi.org/10.1016/j.scitotenv.2016.07.027.
Melchior, M., 2021. Partitioning along reproductive niche dimensions in sympatric New Zealand freshwater bivalve species. PhD Thesis, University of Waikato, Hamilton.
Mitchell, Z. A., J. McGuire, J. Abel, B. A. Hernandez & A. N. Schwalb, 2018. Move on or take the heat: Can life history strategies of freshwater mussels predict their physiological and behavioural responses to drought and dewatering? Freshwater Biology 63: 1579–1591. https://doi.org/10.1111/fwb.13187.
Österling, M. E., E. Bergman, L. A. Greenberg, B. S. Baldwin & E. L. Mills, 2007. Turbidity-mediated interactions between invasive filter-feeding mussels and native bioturbating mayflies. Freshwater Biology 52: 1602–1610. https://doi.org/10.1111/j.1365-2427.2007.01791.x.
Österling, M. E., B. L. Arvidsson & L. A. Greenberg, 2010. Habitat degradation and the decline of the threatened mussel Margaritifera margaritifera: influence of turbidity and sedimentation on the mussel and its host. Journal of Applied Ecology 47: 759–768. https://doi.org/10.1111/j.1365-2664.2010.01827.x.
Pernecker, B., A. Czirok, P. Mauchart, P. Boda, A. Móra & Z. Csabai, 2021. No experimental evidence for vector-free, long-range, upstream dispersal of adult Asian clams [Corbicula fluminea (Müller, 1774)]. Biological Invasions 23: 1393–1404. https://doi.org/10.1007/s10530-020-02446-8.
Piechocki, A., 1969. Biologische Beobachtungen von Muscheln aus der Familie Unionidae im Flusse Grabia. Acta Hydrobiologica Sinica 11: 57–67.
Poznańska-Kakareko, M., K. Wiśniewski, D. Szarmach, A. Witkowska, T. Kakareko, Ł. Jermacz, & J. Kobak, 2021. Importance of substratum quality for potential competitive niche overlap between native and invasive unionid mussels in Europe. Science of The Total Environment 799: 149345. https://doi.org/10.1016/j.scitotenv.2021.149345.
Preston, S. J., A. Keys & D. Roberts, 2007. Culturing freshwater pearl mussel Margaritifera margaritifera: a breakthrough in the conservation of an endangered species. Aquatic Conservation: Marine and Freshwater Ecosystems 17: 539–549. https://doi.org/10.1002/aqc.799.
Quinlan, E., C. Gibbins, I. Malcolm, R. Batalla, D. Vericat & L. Hastie, 2015. A review of the physical habitat requirements and research priorities needed to underpin conservation of the endangered freshwater pearl mussel Margaritifera margaritifera. Aquatic Conservation: Marine and Freshwater Ecosystems 25: 107–124. https://doi.org/10.1002/aqc.2484.
R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Saarinen, M. & J. Taskinen, 2003. Burrowing and crawling behaviour of three species of unionidae in Finland. Journal of Molluscan Studies 69: 81–86. https://doi.org/10.1093/mollus/69.1.81.
Sanchez, B. & A. N. Schwalb, 2021. Detectability affects the performance of survey methods: a comparison of sampling methods of freshwater mussels in Central Texas. Hydrobiologia 848: 2919–2929. https://doi.org/10.1007/s10750-019-04017-y.
Sansom, B. J., S. J. Bennett, J. F. Atkinson, & C. C. Vaughn, 2020. Emergent hydrodynamics and skimming flow over mussel covered beds in rivers. Water Resources Research 56: e2019WR026252. https://doi.org/10.1029/2019WR026252.
Schwalb, A. N. & M. T. Pusch, 2007. Horizontal and vertical movements of unionid mussels in a lowland river. Journal of the North American Benthological Society 26: 261–272. https://doi.org/10.1899/0887-3593(2007)26[261:HAVMOU]2.0.CO;2.
Stoeckl, K. & J. Geist, 2016. Hydrological and substrate requirements of the thick-shelled river mussel Unio crassus (Philipsson 1788). Aquatic Conservation: Marine and Freshwater Ecosystems 26: 456–469. https://doi.org/10.1002/aqc.2598.
Trueman, E. R., 1983. Locomotion in molluscs. The Mollusca Volume 4: Physiology. Part 1: 155–198. https://doi.org/10.1016/B978-0-12-751404-8.50012-8.
Vannote, R. L. & G. W. Minshall, 1982. Fluvial processes and local lithology controlling abundance, structure, and composition of mussel beds. Proceedings of the National Academy of Sciences 79: 4103–4107. https://doi.org/10.1073/pnas.79.13.4103.
Voelz, N. J., J. V. McArthur & R. B. Rader, 1998. Upstream mobility of the asiatic clam Corbicula fluminea: Identifying potential dispersal agents. Journal of Freshwater Ecology 13: 39–45. https://doi.org/10.1080/02705060.1998.9663589.
Watters, G. T., S. H. O’Dee & S. Chordas, 2001. Patterns of vertical migration in freshwater mussels (Bivalvia: Unionoida). Journal of Freshwater Ecology 16: 541–549. https://doi.org/10.1080/02705060.2001.9663845.
Westberg, T., 2020. Physical microhabitat requirements for Margaritifera margaritifera and the influence of hydro- and morphodynamics on mussel bed stability. Master thesis, Umeå University, Umeå.
Wilson, C. D., G. Arnott, N. Reid & D. Roberts, 2011. The pitfall with PIT tags: marking freshwater bivalves for translocation induces short-term behavioural costs. Animal Behaviour 81: 341–346. https://doi.org/10.1016/j.anbehav.2010.10.003.
Wilson, C. D., G. Arnott & R. W. Elwood, 2012. Freshwater pearl mussels show plasticity of responses to different predation risks but also show consistent individual differences in responsiveness. Behavioural Processes 89: 299–303. https://doi.org/10.1016/j.beproc.2011.12.006.
Young, M., P. J. Cosgrove, & L. C. Hastie, 2001. The extent of, and causes for, the decline of a highly threatened naiad: Margaritifera margaritifera In Bauer, G. & K. Wächtler (eds), Ecology and evolution of the freshwater mussels Unionoida. Springer, Heidelberg: 337–357.
Young, M. & J. Williams, 1983. Redistribution and local recolonisation by the freshwater pearl mussel Margaritifera margaritifera (L.). Journal of Conchology 31: 225–234.
Zajac, K. & T. Zajac, 2011. The role of active individual movement in habitat selection in the endangered freshwater mussel Unio crassus Philipsson 1788. Journal of Conchology 40: 446–461.
Zieritz, A., J. Geist & B. Gum, 2014. Spatio-temporal distribution patterns of three stream-dwelling freshwater mussel species: towards a strategy for representative surveys. Hydrobiologia 735: 123–136. https://doi.org/10.1007/s10750-014-1897-2.
Acknowledgements
We thank Alexander Erdbeer for his help with the field work and Samuel Andrews for valuable comments on an earlier version of the manuscript. Mussels used in this study originated from a captive-breeding program and were used with the permission of the authority for nature conservation of the federal state of Saxony.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was embedded in the joint project ArKoNaVera and funded by a grant from the Federal Ministry of Education and Research, grant no. 01LC1313B and 01LC1313A.
Author information
Authors and Affiliations
Contributions
FE contributed to conceptualization, methodology, investigation, software, formal analysis, visualization, writing of the original draft, and writing, reviewing, & editing of the manuscript. FG contributed to methodology, formal analysis, supervision, and writing, reviewing, & editing of the manuscript. AW contributed to conceptualization, methodology, formal analysis, supervision, writing, reviewing, & editing of the manuscript, and funding acquisition. DL contributed to investigation and writing, reviewing, & editing of the manuscript. DK contributed to software, formal analysis, visualization, and writing, reviewing, & editing of the manuscript. MW contributed to conceptualization, funding acquisition, and writing, reviewing, & editing of the manuscript. TB contributed to conceptualization, funding acquisition, and writing, reviewing, & editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling editor: María del Mar Sánchez-Montoya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eissenhauer, F., Grunicke, F., Wagner, A. et al. Active movement to coarse grained sediments by globally endangered freshwater pearl mussels (Margaritifera margaritifera). Hydrobiologia 850, 985–999 (2023). https://doi.org/10.1007/s10750-023-05138-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05138-1