Abstract
In the western US, freshwater mussels (Order Unionida) contribute valuable ecosystem functions to riverine systems, yet have declined across their range following widespread degradation of freshwater habitat and parallel declines in salmonids, host fish for larval western pearlshell mussels (Margaritifera falcata). The status of M. falcata populations is of particular conservation interest in isolated coastal watersheds given unique freshwater mussel-host fish relationships. To understand M. falcata population ecology in Oregon’s coastal watersheds, we analyzed stream survey data on presence/absence of mussels collected over a recent eleven-year period, explored co-varying habitat characteristics, and summarized mussel distribution and host fish co-occurrence. We also collected M. falcata and compared condition indices among eight locations. Naïve occupancy in surveyed areas was 12.3%, about half of predicted occupancy (ψ = 0.24, CI 0.19–0.31) based on modeling repeated visits over a ten year assumed closed period. Mussel occupancy was correlated with reach-scale habitat variables, and the probability of mussel observations was positively correlated with presence of coho (Oncorhynchus kisutch) salmon. Condition varied significantly among locations. Spatial relationships between existing mussel distribution, host species, and habitat variables answer questions about coastal freshwater mussel populations, as well as serve to identify priorities for further research and population assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater mussels (Bivalvia:Unionida) are among the most imperiled species groups worldwide, with many species and populations remaining unassessed throughout their current ranges (“IUCN Red List of Threatened Species”, 2021; Lydeard et al., 2004). Despite an exceptional diversity of species native to North America, and a high rate of decline with > 90 species currently listed under the US federal Endangered Species Act, freshwater mussels remain understudied and may otherwise lack basic information, such as habitat needs and associations. This is especially true in the western US, where freshwater mussels have been of increasing interest for freshwater aquatic conservation and restoration groups across the region, yet considerable population-specific information is still needed to facilitate comprehensive management and conservation.
Three extant taxonomic freshwater mussel groups are native to Oregon, USA, including the western pearlshell [Margaritifera falcata (A. Gould, 1850)], the western ridged mussel [Gonidea angulata (I.Lea, 1838)], and multiple species of floaters (genus Anodonta; currently under taxonomic review and revision). Each of these taxa are thought to be declining or face some risk of extinction based on comparisons of historical and recent distributions across western US states and Canadian provinces (Blevins et al., 2017). Margaritifera falcata, which has been assessed as Near Threatened due to declining occurrence and abundance across its range, is found west of the Rocky Mountains to the Pacific Coast, from northern California to Alaska, and with some small isolated populations persisting east of the continental divide in the headwaters of the Missouri River (Blevins et al., 2017). It is particularly known from coastal watersheds spanning the Pacific Coast of North America, including every coastal watershed in Oregon and Washington and nearly every coastal watershed from Monterey Bay northward in California (Xerces Society and CTUIR, 2021). With respect to Oregon’s mussel community, M. falcata is by far the dominant, and often only, species of mussel present in coastal watersheds, particularly those originating from the mountainous Coast Range Ecoregion.
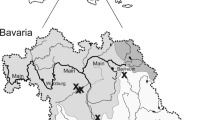
In Oregon, the Coast Range Ecoregion is defined by a series of biogeographically similar coastal watersheds draining from a low coastal mountain range, commonly referred to as the Coast Range, to the Pacific Ocean (Fig. 1). The region is characterized by steep slopes, a wet and mild climate, and high forest productivity (Wimberly & Ohmann, 2004). Steep slopes and high rainfall in forested watersheds in the Coast Range affect debris flow and sediment transport in low order streams, resulting in highly dynamic channel morphology (May & Gresswell, 2003). The majority of these watersheds drain comparatively small areas relative to large regional drainage systems like the Puget Sound, or Columbia, Willamette, Klamath, or Sacramento rivers (Fig. 2).
The Coast Range ecoregion (orange) extends from the southern portion of Oregon north to the Columbia River, and comprises watersheds originating in the Coast Range Mountains (Omernik, 1987). Collection locations and upstream watersheds for condition analysis comparisons are outlined in green. The occupancy/distribution survey area (patterned) is comprised of the coastal watersheds in the Coast Range of Oregon, and divided into regions based on salmonid biogeographic strata designations
Pacific coastal watersheds in Oregon drain a smaller area on average than other river basins in the region with resident M. falcata. Not shown is the Columbia River system (of which the Willamette is a subbasin), which drains nearly 194 million acres before reaching the Pacific Ocean. Of the 26 coastal drainages in Oregon included in this study (excluding the lower Columbia), only 2 consist of more than one subbasin (HUC8), while the Willamette consists of 11, the Klamath of 12, Puget Sound of 21, and the Sacramento of 28
Regional population investigations comparing genetic variability between and among western freshwater mussel species (Mock et al., 2013) or their distribution within larger watersheds (Brim-Box et al., 2003; Davis et al., 2013) have highlighted the importance of life history traits and habitat associations in shaping distribution of M. falcata within other watershed contexts. As such, M. falcata distribution, persistence, abundance, and condition across Coast Range watersheds is also likely responsive to a suite of habitat associations and factors associated with watershed context that differ from populations elsewhere in the species’ range. For example, populations located in smaller and more relatively isolated coastal watersheds could be influenced by decreased interconnectivity, a smaller gene pool, smaller or more distant patches of appropriate habitat, or greater stochasticity associated with the more dynamic stream conditions resulting from the underlying geology and hydrology of the Coast Range.
Additionally, reproductive ecology adaptations may play a role in understanding M. falcata distribution within watersheds. The species displays a unique obligate species relationship with salmonids: M. falcata release glochidia in conglutinate clusters into the water column where they can make contact with the host species (Haag, 2012). Once contact is made, glochidia attach themselves to the fish’s gills and encyst, persisting in this temporary parasitic stage for up to several weeks (Haag, 2012). This relationship, though adaptive for dispersal of mussels in stream networks, does limit the species’ reproductive potential within its range if host fish are no longer present or abundant. In the Pacific Northwest, multiple threatened or endangered salmonid species that serve as obligate hosts for M. falcata have declined regionally, now persisting at a fraction of historical numbers (Nehlsen et al., 1991; Naiman et al., 2002; Gavin et al., 2018), perhaps further influencing present-day M. falcata populations.
Study goals
Given that M. falcata is a species of conservation concern in Oregon, if Coast Range populations in the state are indeed responding to this unique watershed context, it may be especially important to understand the specific factors influencing their distribution, persistence, abundance, and condition. Additionally, developing and making use of cost-effective approaches to understand habitat and environmental features that may limit mussel populations is critical in long-term conservation of these imperiled species. Therefore, in this project, we used publicly available and previously collected multi-year stream survey datasets (Constable & Suring, 2022) to conduct a mixed method analysis of regional ecological characteristics of M. falcata populations in small coastal drainages in the Coast Range ecoregion of Oregon. Our goals were to understand:
-
(1)
What is the current distribution and occupancy of M. falcata in Oregon’s small coastal drainages?
Catalogued historic and recent, and generally opportunistic, mussel observations throughout the coastal region of Oregon (Xerces Society and CTUIR, 2021) suggest a wide distribution pattern of occurrence across the region, and we expect that mussel occurrence data collected through a randomized, multi-year stream survey effort in the region (Constable & Suring, 2022) will mirror this wide distribution pattern.
-
(2)
Which reach-scale habitat variables best predict mussel occupancy across the four strata of the Coast Range Ecoregion?
We expect that among the habitat variables measured for each coastal stream reach in this study, variables influencing mussel occupancy will include those associated with low stream velocity, such as areas with lower gradient and sand/silt substrates (Howard & Cuffey, 2006; Stone et al., 2004; Hegeman et al., 2014).
-
(3)
Is there a relationship between host fish abundance and mussel presence at sample locations?
In freshwater mussel studies more broadly, the expected positive association between host species and freshwater mussels has rarely been observed in field experiments (in many cases the relationship is likely confounded by environmental variables or host attributes), though high levels of correlation have been observed in controlled settings (Haag & Stoecktel, 2015; Inoue et al., 2017). The reproductive success of freshwater mussels reliant on host attraction strategies such as broadcast spawning or use of conglutinates (a M. falcata trait) is thought to be influenced to a greater degree by host species abundance (Haag, 2012). As such, we expect to see a positive relationship between counts of Coho Salmon [Oncorhynchus kisutch (Walbaum, 1792)], a native salmonid distributed across Coast Range streams and a potential host fish (Karna & Millemann, 1978; Stone et al., 2004), and M. falcata presence.
-
(4)
In addition to distribution and occupancy, does mussel condition vary across the sampling range?
We expect mussel condition to vary among sites due to an assortment of factors contributing to mussel condition that also varies across Coast Range watersheds, such as food/nutrient availability, environmental stressors, disease, and legacy impacts. However, because this analysis was not able to account for these factors, we make no specific predictions about patterns of condition.
Methods
Study area and geography
Our study area was Oregon’s Coast Range Ecoregion, commonly referred to as the Coast Range. The Coast Range is a unique biogeographic and climatic area encompassed by 26 distinct coastal drainages south of the Columbia River estuary to Cape Blanco (Fig. 1). Since freshwater mussel larval transport across open ocean with subsequent freshwater habitat colonization has not been documented, freshwater mussel populations within each of these distinct coastal drainages is considered functionally isolated (Seposki & Rex, 1974; King et al., 1999). Each drainage is within one of the four salmonid “biogeographic strata”, which comprise the Oregon Coast Coho evolutionarily significant unit (Weitkamp et al., 1995). These strata include the North Coast (Nehalem, Nestucca, Necanicum, Tillamook area watersheds), Mid Coast (Siletz, Yaquina, Alsea, Siuslaw Rivers), Umpqua (the Lower, North, and South Umpqua River and the Smith River), and Mid-South Coast (Floras, Sixes, Coos, and Coquille Rivers). See Figure S1 (Supplementary Material—SM, Figure S1) for full list of rivers surveyed within each stratum. The Coast Range ecoregion does not include portions of the Umpqua River above the confluence with Elk Creek, including the North and South Umpqua Rivers, and also does not include the portion of the Siuslaw River above Siuslaw Falls. By further differentiating the Coast Range, the four strata provide a useful framework for evaluating regional variation in mussel observations and habitat characteristics.
Survey data collection
The Oregon Department of Fish and Wildlife Aquatic Inventories Project (ODFW AQI) annually collects data through a multi-year survey effort in 1st through 3rd order streams across Western Oregon, including Coast Range streams, as part of monitoring efforts supporting the Oregon Plan for Salmon and Watersheds (EO 99-01, 1999; Constable & Suring, 2022). During surveys, snorkelers document the presence of juvenile salmonids, the presence and relative abundance of freshwater mussels, and a suite of habitat variables. As part of the AQI sampling design, sampling locations were randomly selected with spatial balance throughout the study area using a Generalized Random Tessellation Stratification (GRTS) survey design (Stevens & Olsen, 2004). Relative numbers of surveys within each region are presented in Fig. 3A. Selected stream segments (1 km in length) were sampled using a rotating panel design, dividing locations equally between four survey rotations (single visit, annual, 3 year, or 9 year; Stevens, 2002). Between 2010 and 2020 (the time period examined in this analysis), an average of 130 locations (1,426 surveys overall and 680 unique stream reaches) were surveyed by ODFW staff per year during base flow periods (mid-July to early-October, depending on rainfall) within the Coast Range. Throughout this time period, sites were visited 2.1 times on average (minimum = 1, maximum = 11).
A Survey location pins divided into larger regional categories: North Coast, Mid Coast, Umpqua, and Mid-South Coast. B Frequency of sites with detections/observations summarized by watershed and displayed as a choropleth map divided and classified using natural breaks (Jenks & Caspall, 1971). C Proportions of detections from the total sites summarized within each watershed and classified as a function of standard deviation
During surveys, the presence and abundance of live mussels were recorded at sites. Although live mussels were not identified by species, empty mussel shells were collected at ODFW survey locations from 2010 to 2020 and identified by mussel experts within the agency. Shells from all three extant taxonomic freshwater mussel groups in Oregon were identified but all shells collected in the Coast Range (n = 129) were identified as M. falcata (Shelly Miller and Al Smith, ODFW, personal communication). Based on this expert ID coupled with verified observations of mussel beds present in the Coast Range Ecoregion (at 75 of 84 sites), as well as expert knowledge of other mussel species’ distributions in coastal Oregon (Xerces Society and CTUIR, 2021), it is safe to assume that all freshwater mussel observations included in this analysis are M. falcata. For the purposes of this analysis, mussel data was categorized in terms of presence/absence during each survey effort.
Habitat data were collected concurrently with snorkel surveys according to protocols designed by Moore et al. (1997) at a subset of survey locations (n = 566; 81%). Through the combination of snorkel and habitat surveys, data on in-stream habitat condition (i.e., substrate type, water temperature at each visit, percent pool habitat, course woody debris quantity), juvenile salmonid abundance, mussel presence and relative abundance, and geomorphic characteristics were recorded (Table 1).
Mussel condition sampling
Between 2017 and 2018, individual biological samples of M. falcata were collected as part of an assessment of bivalve pesticide contaminants (Scully-Engelmeyer et al., 2021). These samples originated from seven Coast Range watersheds (eight total collection sites). The numbers of collection sites and samples were limited to reduce potential impacts to sensitive populations, and sites were purposely chosen for their location within smaller drainages and their accessibility. To evaluate condition at each collection site, fifteen individuals were collected by hand, wading or during snorkel dives. All samples were held in ambient water collected on site and transported in a cooler with wet ice to the Applied Coastal Ecology (ACE) Laboratory at Portland State University (Portland, OR) or the Hatfield Marine Science Center (Newport, OR) for processing. Individual mussels were weighed, shucked, drained, and final shell and tissue wet weights and shell lengths were recorded (Crosby & Gale, 1990).
Statistical analyses
Survey data
The ODFW AQI dataset, including M. falcata presence, abundance, and covariate data, was analyzed using an occupancy modeling approach, which estimates true occupancy (i.e., accounting for imperfect encounter rate), and enables exploration of the relationship between presence/absence of mussels and reach-scale habitat variables. Prior to estimating true occupancy, we analyzed proportional observation frequency at each site (n = 680) to understand relative distribution (naïve occupancy) of M. falcata among and within the Coast Range. Using ArcMap 10.7.1, we calculated the number of sites per catchment with detections (frequency) across the sampling period (11 years). We then mapped the naïve occupancy within each watershed (% of sites with at least one mussel observation) in relation to standard deviation to examine the relative spatial distribution of the species within watersheds.
Of the 680 total survey locations, 566 had complete habitat data accompanying mussel occurrence data, and reach level habitat profiles were developed at the 566 sites by averaging repeated measurements over multiple visits. Means and ranges of continuous variables and proportions of categorical variables across sites are summarized in Table 2.
To estimate true occupancy and analyze habitat covariates, we applied a static occupancy model. Occupancy modeling was limited to the 211 sites for which habitat data were available and which were visited more than once during the survey period. Due to the long lived and sessile nature of M. falcata mussel beds, with individual maximum mussel life expectancy exceeding 100 years (Haag, 2012), the eleven-year sampling period was considered closed to changes in mussel occupancy (closed period). We therefore modeled detection (P) as constant based on repeated annual visits. We modeled occupancy (ψ) probabilities using habitat variables (in-stream and adjacent geomorphic; Table 1) that were averaged across site visits to account for differences in surveyor estimations and uneven habitat data collection frequencies across sites. Watershed size was also included as a site covariate to explore population isolation, where smaller watersheds were considered more isolated. Prior to modeling, habitat variables were compared via correlation matrices, and one of each pair of highly correlated variables were excluded (Pearson’s correlation coefficient > 0.40). Final covariates were scaled.
We estimated reach level detection (P) and occupancy (ψ) probabilities using R-Studio (version 1.2.5033; unmarked, AICmodavg, and MuMIn packages). Since all covariates could be influential in mussel occupancy, we developed an “all subsets” candidate model set based from a global model and compared summed model weights to determine relative covariate importance (Arnold, 2010). We compared candidate models ranked based on Akaike Information Criterion (AIC), and the best models were selected for averaging based on AIC weights within 0.1 of the highest ranking model (Burnham & Anderson, 2002; MacKenzie et al., 2017). “Single season” global occupancy model goodness of fit and overdispersion parameter (c-hat) was simulated using 1000 bootstrapped samples (MacKenzie & Baily, 2004).
In order to assess whether regional habitat differences explain mussel presence, the highest ranking covariates identified via model averaging of AIC weights were then compared among the four regions of the study area using non-parametric analysis of variance (Kruskal–Wallis). Additionally, when significant variance was found among regions, we compared the covariates between regions using pairwise Wilcoxon tests, correcting for multiple testing using Bonferroni adjustment.
Fish counts and mussel presence or absence was recorded at every survey location, and as a result, all 680 sites were used in the development of the binomial logistic regression model. Since more sites were available for analysis, we approached our exploration of the host fish/mussel relationship with a separate analysis. Relationships between presence/absence of mussels and counts of salmon species (O. kisutch) observed in snorkel surveys were investigated using binomial logistic regression analysis. Fish counts at sites were averaged across sampling events.
Mussel condition analysis
For the condition analysis, basic physiological health among organisms was summarized by calculating a live mussel body condition index (BCI) metric based on measurements of collected mussels (full organism weight, shell length) (Nobles & Zhang, 2015). Standardized methods for freshwater mussel BCI have been suggested (Crosby & Gale, 1990) using dry tissue weight and shell volume for metric calculation. The allometric mussel data available for this analysis did not include those measurements, as the organisms were collected and sacrificed using another sample processing method, making the collection of those measurements impractical.
BCI was compared between sampling locations using Kruskal–Wallis non-parametric analysis of variance to determine if measurable differences in health were detectable among sampled populations. Site differences in condition were compared to the sample mean using multiple pairwise Wilcoxon tests, corrected for multiple testing using the Bonferroni adjustment, and visualized with boxplots. Organism allometry, the scaled relationship between variation in organism morphology and organism size (Gayon, 2000), can be a useful metric in measuring how organisms function in environments (feeding/growth rates, water filtration, etc.) (Kreeger, 2011). Bivalve molluscs are known to have highly correlated relationships between shell length and tissue weight, and documenting these relationships in sacrificed organisms is helpful for future non-lethal biomass sampling. We performed least squares regression to explore the allometric length–weight relationship between shell length (mm) and wet tissue weight (g).
Results
Mussel observation frequency and distribution
Of the 680 sites sampled within the Coast Range during the 2010–2020 sampling period, mussels were observed at least once at 84 of the sites, with shells collected at 75 (all identified as M. falcata), for a naïve occupancy proportion of 12.4%. Spatial frequency of mussel observations varied across the study area, with the highest frequencies seen in the Coquille watershed, and lowest frequencies observed on the North Coast (Fig. 3B). When standardized by watershed size (converted to observation frequency within each watershed) and compared with the coastwide observation frequency, southern coast watersheds displayed the highest deviations above average, but smaller catchments such as Floras Creek and Tahkenitch Lake were significantly elevated compared with larger watersheds (Fig. 3C).
Occupancy and habitat analysis
Prior to modeling, percent pools, VWI and bedrock variables were removed based on multicollinearity with other covariates (gradient, sand and organic matter, and bedrock respectively). The null model determined the probability of detection (P) = 0.44 (95% CI 0.38–0.54), which was included as constant in all subsequent occupancy models. Models incorporating the covariates gradient, percent secondary channel, and boulders, in ψ estimates frequently rated high, had the highest cumulative AIC weights based on all combinations of models (n = 128 candidate models) (Table 3), and were the three variables in the top model. The top model (p (.) ψ (gradient + secondary channel area + boulders)) suggested negative relationships between predicted occupancy and channel gradient (− 2.52, CI − 3.52 to − 1.53) and percent secondary channel area (− 0.62, − 1.26 to − 0.03), and positive relationship between occupancy and boulder counts (1.01, CI 0.47–1.55). Goodness of fit simulation estimated a P value of 0.414 and c-hat of 1 from 1000 bootstrapped samples, suggesting no indication of oversimplification based on MacKenzie and Bailey Goodness-of-Fit for single season occupancy models (Supplementary Materials, Figure S2).
Gradient, secondary channel area, and boulders, the highest ranking habitat covariates explaining mussel presence, were compared across the four regions to explore whether regional variation in habitat characteristics could help explain spatial variability observed in mussel naïve occupancy (see Fig. 4B, C). Kruskal–Wallis analysis of variance indicated at least one significant difference between regions for each habitat covariate (Fig. 5), so multiple pairwise Wilcoxon tests were performed between regional groups (corrected for multiple testing). Wilcoxon tests indicated that gradient at North Coast survey locations was significantly higher than the other regions (Fig. 5A). Pairwise tests between regions of percentage of secondary channel indicated that North Coast sites had significantly more secondary channel area than sites in other regions, and Mid Coast sites had signifiantly more than survey locations in the Umpqua watershed (Fig. 5B). Pairwise analysis of boulder counts between regions found that Mid-South Coast sites had significantly higher counts than Mid Coast sites (Fig. 5C).
Average gradient as a function of the sampled reach (1 km) was the strongest covariate in predicted mussel occupancy (A), followed by percentage of secondary channel area (B) and count of boulders (C). The solid line represents changes in predicted mussel occupancy based on the covariate, grey area represents confidence intervals
Regional comparison of the highest ranking covariates in predicting mussel presence, A average gradient, B percent secondary channel area, and C count of boulders. Boxes indicate interquartile range, with the central line indicating sample median. Lines represnent the sample ranges without outliers, which are shown as dots. Kruskal–Wallis tests suggest significant differences between regional groups for each variable. Pairwise wilcoxan tests with bonferonni adjustment for multiple testing offer insight into regional comparisons. Significant relationships are displayed using the following symbology ****P val ≤ 0.0001, ***P val ≤ 0.001, **P val ≤ 0.01
Host fish analysis
O. kisutch species counts predicted mussel observations (binomial logistic regression; Fig. 6) significantly better than the null model (chi squared = 19.15, with 1 degree of freedom; P < 0.0001), exhibiting a positive relationship with mussel observation probability (Fig. 6). The concordance index was 0.641, indicating a predictive capacity exceeding random (Table 4).
Mussel Condition Index Comparison
Fifteen mussels were collected at seven sites, and ten mussels were collected at one site (collection was modified due to low abundance at that site; SZ) across seven watersheds within three sub-regions of the Coast Range. Body condition indices (BCI) were significantly different among sites (Kruskal–Wallis, chi-squared = 44.482, df = 7, P value < 0.001), with Siletz and Big Elk (Yaquina) sites significantly lower than the mean, and Fall Creek (Alsea), Smith, and Weatherly (Umpqua) sites significantly higher (Fig. 7). BCI variables (shell length and full organism wet weight) displayed a strong positive relationship, and the largest/heaviest mussels were found at sites with the smallest upstream catchments (Fig. 8). Allometric length–weight measurements were fit using least squares regression and log–log transformation, resulting in a significant regression equation (F (4,144) = 1,113 DF, P val < 0.001), with an R2 of 0.97. Predicted log mussel weight (g) is equal to − 9.24 + 2.99 (log shell length), where length is measured in mm.
Discussion
Although research focused on western North American species of freshwater mussels is generally limited, this study provides a model for how information on mussel presence collected as part of a multi-year survey effort focused on salmonids and coastal stream habitat, can contribute important insights into mussel detection, occupancy, and habitat associations at an ecoregional scale. Advantages of this approach include accomplishing multi-species monitoring objectives in a cost-effective manner, all of which is available through a publicly accessible data set. Other benefits of this approach include:
-
the randomization of site selection, which results in both presence and absence data for M. falcata, as well as an unbiased approach to collecting habitat data;
-
the use of an efficient method for documenting mussel beds (mussel counts appended to ongoing monitoring efforts), which can be difficult to survey for across the large network of Coast Range streams;
-
the ability to assess detection probability based on multiple site visits and the use of novel surveyors each year;
-
the selection of a survey distance (1 km) of sufficient length to reduce the chance that any shift in bottom substrate, and thus mussel beds, outside of the surveyed area would result in violation of the closed period assumption.
As such, this study provides important insight relevant to an in-progress effort to develop a western freshwater mussel visual survey protocol (BLM, 2022). Additionally, this study, which used a mixed-methods analysis to examine distribution, detection, occupancy, habitat associations, host fish associations, and condition of Coast Range populations of M. falcata, has revealed important information about the species across Oregon coastal watersheds and regions.
Current distribution and occupancy of M. falcata in Coast Range Watersheds
Overall, naïve mussel occupancy across sites was low (12.4% of sites), and modeling indicated observed occupancy underrepresented predicted occupancy (ψ = 0.24, CI 0.19–0.31) due to detection probability of around 45% over the eleven year assumed closed period (P = 0.447, CI 0.38–0.52). The low detection probability observed here is not surprising, given the elusive nature of freshwater mussels as burrowing benthic organisms. Other research applying occupancy/detection methods to 15 freshwater mussel species during a single season closed period estimated a similar average detection probability (P = 0.42, CI 0.37–0.47) (Pandolfo et al., 2016). This similarity in detection probably suggests that our application of modeled mussel occupancy over a multiple year closed period is a practical means to account for imperfect detection of long-lived freshwater mussels using traditional stream survey methodologies. As described above, detection probability examined in this randomized multi-year approach is further strengthened as the locations are surveyed by multiple surveyors over the repeated visits, unlike many directed mussel surveys that seek to optimize mussel detection, and are of sufficient length (1 km) to capture possible mussel bed-shifting in dynamic stream habitat.
M. falcata population decline has been observed throughout parts of its native range (Blevins et al., 2017), but limited historical baseline information presents challenges in understanding the true extent of this decline. Due to their long life spans and slow growth rates, isolated populations may be slow to adapt to changes in the environment, accruing extinction debt that may not be perceptible at shorter timeframes (Newton et al., 2008). Additionally, climate change is projected to alter flow regimes and increase instream temperatures, which may further disrupt extant populations via direct and indirect impacts to mussels and host fish species (Terui et al., 2014; Blevins, 2018). Relatively low predicted occupancy was observed at high order stream survey locations (ψ = 0.24, CI 0.19–0.31) using a randomized study design, providing a new baseline for M. falcata populations in Oregon’s coastal watersheds that could serve as a comparison to interpret future occupancy monitoring information.
Reach-scale habitat covariates
Research in the western US has identified habitat factors and environmental variables that influence age structure and distribution, which provides critical first steps in assessing intrinsic habitat potential of streams and rivers within subregions (Anderson, 2002; Brim-Box et al., 2003; Howard & Cuffey, 2003, 2006b; Stone et al., 2004; Davis et al., 2013). Considering the variability in watershed types throughout this species’ range, identifying region-specific functional habitat variables and the relationships between mussels and host fish inform important elements of the regional landscape ecology of mussel species (Newton et al., 2008). Developing cost-effective approaches to understand habitat and environmental features that may limit mussel populations is critical in long-term conservation of these imperiled species.
Stream gradient (averaged over 1 km survey length), percent secondary channel area, and presence of boulders were strong predictors of mussel occupancy in Oregon coastal headwater streams, which aligns with previous research in Washington state indicating the importance of areas of lower shear stress (preference towards boulder-dominated substrate) in mussel habitat requirements (Stone et al., 2004). Boulder-stabilized substrate has also been linked to juvenile micro-habitat preferences in closely related Margaritifera margaritifera (Linnaeus, 1758) (Hastie et al., 2000). Percent secondary channel, which is a reflection of wetted surface area outside of the main channel, showed a negative association with mussel occupancy in this study. This relationship is not an indication of whether mussels occupy secondary channel in these areas, or which portion of the streams mussels are found, but rather that sites with greater amounts of secondary channel areas are less likely to support mussels, as indicated in the regression plot (Fig. 4B).
Pool formation and sediment storage in watersheds has been shown to decrease in volume and prevalence with greater channel gradient, which is driven by tendency for debris flow scour in these systems (Buffington et al., 2002). Therefore, the volume and frequency of pools in higher gradient segments is largely associated with increased stream complexity and the presence and size of large wood debris (LWD), which trap and store sediment (Beechie & Sibley, 1997; Buffington et al., 2002; Rosenfeld & Huato, 2003). This dynamic mechanism of channel morphology in coastal watersheds relies on upstream sources of large wood, and is influenced by riparian area complexity (Collins et al., 2012). Further in-depth investigation into connections between mussels and underlying processes such as this, which generate and maintain habitat features utilized by M. falcata, is critical for future conservation of these species, especially in isolated catchments where productive downstream migration may be limited by watershed size. In this study of 1st–3rd order streams, occupancy did not appear to be limited by relative watershed isolation (as represented by watershed size in covariate analysis), suggesting other factors (such as identified habitat variables) are stronger predictors of mussel occupancy in this case.
Regional differences within the Coast Range
North Coast watersheds exhibited low occupancy in randomly surveyed 1–3rd order streams, both in counts of sites with detections and as deviations from coast-wide average observation proportions. To investigate whether habitat characteristics may be contributing to regional differences in mussel observations, we compared the highest ranking reach-level habitat variables across the sampling regions. Headwater stream segments surveyed in North Coast watersheds have significantly higher average gradient compared to other regions surveyed in this analysis. Difference in flow and shear stress are known to influence benthic habitat stability and thought to be linked with mussel mortality and/or downstream transport of mussels (Strayer, 1999; Niraula et al., 2016), which may explain why noticeably fewer mussels were observed in steeper North Coast headwater survey locations compared to other areas. Sites surveyed in this analysis suggest some regional differences in the amount of secondary channel area in Coast Range headwater streams, with North Coast sites containing significantly more than other regions, and Mid Coast sites containing more than Umpqua to the south (Fig. 5B). This regional pattern is similar to that we observed for average gradient; suggesting secondary channels may in this case be an expression of the dynamic nature of high gradient streams. Mussel aggregations respond to habitat needs and hydrological variables across micro and meso, scales within watersheds (Newton et al., 2008), but regional associations provide useful information for species conservation, especially in light of connectivity constraints among populations. Lack of mussel observations in North Coast headwater streams does not indicate that mussels are not present in the region, but that they may be confined to lower portions of some watersheds.
Though this study identified habitat characteristics associated with mussel presence in 1st–3rd order (headwater) streams and found regional differences in habitat availability, it is unclear how regional habitat differences affect mussel populations lower in watersheds, where channel morphology and gradient may provide more consistent habitat over time compared with dynamic headwaters. Habitat characteristics and substrate suitability are important considerations in understanding patch dynamics of freshwater mussel populations, but the complex (and lengthy) life history of M. falcata requires consideration of additional controlling factors in their persistence such as host fish and population condition (Strayer et al., 2004).
Host fish abundance and mussel presence
O. kisutch abundance exhibited a strong positive association with mussel observations based on binomial logistic regression. The timing and type of juvenile salmon present during the summer months raises an important point regarding co-occurrence with mussel populations during periods of glochidial release into the water column. Timing of M. falcata conglutinates can be variable, but have been detected in water samples between late March and June in Oregon (Karna & Millemann, 1978; O'Brien et al. 2013; Allard et al., 2017). Allard et al. (2017) attributed the timing of glochidial release to seasonal changes in daily water temperature fluctuation, which may be asynchronous with host species co-occurrence. Freshwater pearl mussels (M. margaritifera) possess subpopulation-level adaptations to different host species based on coinciding historical presence and conditions (Salonen et al., 2017). M. falcata may exhibit similar subpopulation adaptations, but regional relationships of M. falcata and host-species adaptations have not been investigated. Considering the richness and diversity of life histories and species of potential salmonid hosts throughout the Pacific Northwest, significant data gaps remain in current understanding about M. falcata host species relationships and potential subpopulation adaptations.
The strong positive relationship we observed between O. kisutch abundance and M. falcata presence suggest habitat preference similarities between the species during the sampling season. Though LWD volume was not a high-ranking predictor of mussel occupancy in our occupancy analysis, debris jams and LWD presence have been identified as critical pool forming habitat features in coastal headwater streams, supporting higher densities of O. kisutch during the summer (Roni & Quinn, 2011). These patterns of pool occupancy during the summer may, in part, explain the relative counts and associations we observed in our co-occurrence analysis. Gradient, an indicator of pool presence known to affect presence of O. kisutch in Oregon Coast Range streams (Hicks & Hall, 2003), was also the strongest predictor of mussel occupancy; further suggesting shared habitat preferences/limitations between mussels and host species in steep and highly dynamic watersheds like those found in parts of the Oregon Coast Range.
Mussel condition and allometric comparisons
Understanding M. falcata presence/absence across the Coast Range is useful in determining distribution of extant populations, but does not include an indication of overall health or condition of those populations. Our condition analyses indicate that population fitness differs across coastal watersheds/scales, with some populations exhibiting significantly higher or lower BCI when compared to the sample mean (Fig. 7). Given the relatively small sample size across a large geographic area we did not analyze, and therefore, cannot make draw associations about regional influence on mussel condition, though we did observe the most condition variability in our northern sites. Upstream watershed size, indicated by varying dot size in the comparison of shell length and organism weight (Fig. 8), indicates the largest and heaviest mussels collected in this study originated from locations with smaller upstream catchment areas. As anticipated, shell length was strongly correlated with body weight, consistent with previous findings about freshwater Unionida allometry (Atkinson et al., 2020), though we explored relationships using wet instead of dry mass. Documentation of length-mass relationships provides useful information that could inform any future biomass assessment of M. falcata, as a non-lethal means to measure function and contribution of populations to ecosystems (Atkinson et al., 2020).
Study limitations/research directions
In general, freshwater mussel populations inhabiting coastal drainages are thought to be functionally isolated from each other and from other larger watershed networks (Sepkoski & Rex, 1974; King et al., 1999; Karlsson et al., 2014; Archambault et al., 2018). Several theories propose movement pathways of freshwater mussels between unconnected drainages in the eastern US, varying from aerial bird transport to initial colonization being reliant on geomorphic stream capture processes (Ortmann, 1913; Sepkoski & Rex, 1974). Initial colonization of coastal drainages likely took place thousands of years ago, facilitated by altered entrapment and river connectivity between basins. There is some evidence that a subset of salmonid life histories involve movement between catchments before ocean migration, which is a potential route of dispersion for mussels during their parasitic stage, but there are no data to verify if mussels are able to move between drainages this way (Strayer, 1987). Understanding the current status of isolated populations of freshwater mussels is particularly important as remnant populations may contain unique genetic diversity (Mock et al., 2013, 2010; Wacker et al., 2019; Österling et al., 2020; Walton et al., 2020).
Mussel observations during AQI surveys were collected as incidental data to fish counts, which allowed for this preliminary analysis of distribution and occupancy of mussels in 1st–3rd order streams throughout coastal watersheds. Changes in mussel occupancy over time and assessment of colonization and extinction rates, which could vary according to land use/land management practices or other habitat variables expected to change over time (e.g., temperature, disturbance events), could not be investigated with these data, but are important variables worth investigating in future M. falcata monitoring in coastal watersheds. Asymmetrical dispersal patterns are of particular interest in small coastal watersheds with dynamic sediment movement regimes, as downstream migration of mussels over time may deplete reproductive subpopulations of mussels in headwaters, which can be important for metapopulation dynamics (Terui et al., 2014). Mussel occupancy was held at constant throughout the survey period to explore survey detection probabilities, but this may underappreciate mussel “migration” in morphologically dynamic headwaters.
This study identified a subset of coastal watersheds in which mussels were not observed in 1st–3rd order streams during the eleven year AQI survey period (SM, Figure S1). Environmental DNA (eDNA) monitoring technology has evolved as an effective monitoring tool to assess presence/absence of aquatic species in watersheds, and recent applications incorporate freshwater mussels assays (Rodgers et al., 2020; Preece et al., 2021). Identified watersheds should be prioritized for future monitoring to determine the status of mussel presence and/or extirpation in coastal watersheds to guide future efforts in population dynamics and extinction debt research in functionally isolated populations.
Data availability
Upon publication, data will be made publicly available on pdx.scholar.
Code availability
Not Applicable.
References
Arnold, T. W. 2010. Uninformative parameters and model selection using Akaike's Information Criterion. The Journal of Wildlife Management, 74(6): 1175–1178.
Allard, D. J., T. A. Whitesel, S. C. Lohr & M. L. Koski, 2017. Western Pearlshell mussel life history in Merrill creek, Oregon: reproductive timing, growth, and movement. Northwest Science Northwest Scientific Association 91: 1–14.
Anderson, L. P., 2002. Population genetics and conservation of the freshwater mussel Margaritifera falcata from the northwestern United States. 47.
Archambault, J. M., W. G. Cope & T. J. Kwak, 2018. Chasing a changing climate: reproductive and dispersal traits predict how sessile species respond to global warming. Diversity and Distributions 24: 880–891.
Atkinson, C. L., T. B. Parr, B. C. van Ee, D. D. Knapp, M. Winebarger, K. J. Madoni & W. R. Haag, 2020. Length-mass equations for freshwater unionid mussel assemblages: implications for estimating ecosystem function. Freshwater Science the University of Chicago Press 39: 377–390.
Beechie, T. J. & T. H. Sibley, 1997. Relationships between channel characteristics, woody debris, and fish habitat in Northwestern Washington streams. Transactions of the American Fisheries Society Taylor & Francis 126: 217–229.
Blevins, E., 2018. Opportunities for Freshwater Mussel Conservation in the Pacific Northwest and Intermountain West in a Changing Climate. Xerces Society for Invertebrate Conservation 49.
Blevins, E., S. Jepsen, J. B. Box, D. Nez, J. Howard, A. Maine & C. O’Brien, 2017. Extinction risk of western north American freshwater mussels: Anodonta nuttalliana, the Anodonta Oregonensis/Kennerlyi Clade, Gonidea Angulata, and Margaritifera Falcata. Freshwater Mollusk Biology and Conservation Freshwater Mollusk Conservation Society 20: 71–88.
Brim-Box, J., D. Wolf, J. Howard, C. O’Brian, D. Nez, & D. Close, 2003. Distribution and Status of Feshwater Mussels in the Umatilla River System. Bonneville Power Administration 2002–2003 Annual Report, Project No. 200203700, http://docs.streamnetlibrary.org/BPA_Fish_and_Wildlife/00011402-1.pdf.
Buffington, J. M., T. E. Lisle, R. D. Woodsmith & S. Hilton, 2002. Controls on the size and occurrence of pools in coarse-grained forest rivers. River Research and Applications 18: 507–531.
Bureau of Land Management (BLM), 2022. Draft visual survey protocol framework for Western North American freshwater mussels, Version 1.2.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, Springer, New York.
Collins, B. D., D. R. Montgomery, K. L. Fetherston & T. B. Abbe, 2012. The floodplain large-wood cycle hypothesis: a mechanism for the physical and biotic structuring of temperate forested alluvial valleys in the North Pacific coastal ecoregion. Geomorphology 139–140: 460–470.
Constable, R. J. Jr., & E. Suring, 2022. Juvenile salmonid monitoring in Coastal Oregon and Lower Columbia streams, 2021. Monitoring Program Report Number OPSW-ODFW-2022-1, Oregon Department of Fish and Wildlife, Salem, Oregon.
Crosby, M. P. & L. D. Gale, 1990. A review and evaluation of bivalve condition index methodologies with a suggested standard method. Journal of Shellfish Research 9: 233–237.
Davis, E. A., A. T. David, K. M. Norgaard, T. H. Parker, K. McKay, C. Tennant, T. Soto, K. Rowe & R. Reed, 2013. Distribution and abundance of freshwater mussels in the mid Klamath Subbasin, California. Northwest Science Northwest Scientific Association 87: 189–206.
EO 99-01, 1999. Governor’s executive order: E 99-01. Salem, Oregon. https://digital.osl.state.or.us/islandora/object/osl:62141 (accessed 5–15–22). 17 p.
Gavin, D., J. Kusler & B. Finney, 2018. Millennial-scale decline in coho salmon abundance since the middle Holocene in a coastal Oregon watershed, USA. Quaternary Research 89: 1–14.
Gayon, J., 2000. History of the concept of allometry. American Zoologist 40: 748–758.
Haag, W. R., 2012. North American Freshwater Mussels: Natural History, Ecology, and Conservation, Cambridge University Press:
Haag, W. R. & J. A. Stoeckel, 2015. The role of host abundance in regulating populations of freshwater mussels with parasitic larvae. Oecologia 178(4): 1159–1168.
Hastie, L. C., P. J. Boon & M. R. Young, 2000. Physical microhabitat requirements of freshwater pearl mussels, Margaritifera margaritifera (L.). Hydrobiologia 429: 59–71.
Hegeman, E. E., S. W. Miller, K. E. Mock & V. Trenkel, 2014. Modeling freshwater mussel distribution in relation to biotic and abiotic habitat variables at multiple spatial scales. Canadian Journal of Fisheries & Aquatic Sciences Canadian Science Publishing 71: 1483–1497.
Hicks, B. J. & J. D. Hall, 2003. Rock type and channel gradient structure salmonid populations in the Oregon Coast Range. Transactions of the American Fisheries Society 132(3): 468–482.
Howard, J. K., & Cuffey, K. M. 2003. Freshwater mussels in a California North Coast Range river: occurrence, distribution, and controls. Journal of the North American Benthological Society, 22(1): 63–77.
Howard, J. K. & K. M. Cuffey, 2006. Factors controlling the age structure of Margaritifera falcata in 2 northern California streams. Journal of the North American Benthological Society the University of Chicago Press 25: 677–690.
Inoue, K., K. Stoeckl & J. Geist, 2017. Joint species models reveal the effects of environment on community assemblage of freshwater mussels and fishes in European rivers. Diversity and Distributions 23(3): 284–296.
IUCN Red List of Threatened Species, 2021.IUCN Red List of Threatened Species. https://www.iucnredlist.org/en.
Jenks, G. F., & Caspall, F. C. 1971. Error on choroplethic maps: definition, measurement, reduction. Annals of the Association of American Geographers, 61(2): 217–244.
Karlsson, S., B. M. Larsen & K. Hindar, 2014. Host-dependent genetic variation in freshwater pearl mussel (Margaritifera margaritifera L.). Hydrobiologia 735: 179–190.
Karna, D. W. & R. E. Millemann, 1978. Glochidiosis of salmonid fishes. III. Comparative susceptibility to natural infection with Margaritifera margaritifera (L.) (Pelecypoda: Margaritanidae) and associated histopathology. The Journal of Parasitology [The American Society of Parasitologists, Allen Press] 64: 528–537.
King, T. L., M. S. Eackles, B. Gjetvaj & W. R. Hoeh, 1999. Intraspecific phylogeography of Lasmigona subviridis (Bivalvia: Unionidae): conservation implications of range discontinuity. Molecular Ecology 8: S65–S78.
Kreeger, D. A., 2011. Physiological processing of suspended matter by freshwater mussels in rivers of eastern Oregon. A final report for the Freshwater Mussel Research and Restoration Project. Prepared for: Confederated Tribes of the Umatilla Indian Reservation and U.S. Department of Energy, Bonneville Power Administration.
Lydeard, C., R. H. Cowie, W. F. Ponder, A. E. Bogan, P. Bouchet, S. A. Clark, K. S. Cummings, T. J. Frest, O. Gargominy, D. G. Herbert, R. Hershler, K. E. Perez, B. Roth, M. Seddon, E. E. Strong & F. G. Thompson, 2004. The global decline of nonmarine mollusks. BioScience American Institute of Biological Sciences 54: 321–330.
MacKenzie, D. I. & L. L. Bailey, 2004. Assessing the fit of site-occupancy models. Journal of Agricultural, Biological, and Environmental Statistics 9: 300–318.
MacKenzie, D. I., J. D. Nichols, J. A. Royle, K. H. Pollock, L. Bailey, & J. E. Hines, 2017. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence. Elsevier.
May, C. L. & R. E. Gresswell, 2003. Processes and rates of sediment and wood accumulation in headwater streams of the Oregon Coast Range, USA. Earth Surface Processes and Landforms 28: 409–424.
Mock, K. E., J. C. B. Box, J. P. Chong, J. K. Howard, D. A. Nez, D. Wolf & R. S. Gardner, 2010. Genetic structuring in the freshwater mussel Anodonta corresponds with major hydrologic basins in the western United States. Molecular Ecology 19: 569–591.
Mock, K. E., J. C. B. Box, J. P. Chong, J. Furnish & J. K. Howard, 2013. Comparison of population genetic patterns in two widespread freshwater mussels with contrasting life histories in western North America. Molecular Ecology 22: 6060–6073.
Moore, K.M.S, K.K. Jones, & J.M. Dambacher. 1997. Methods for Stream Habitat Surveys. Oregon Department of Fish & Wildlife Information Report 97–4. Portland, OR 40p.
Naiman, R. J., R. E. Bilby, D. E. Schindler & J. M. Helfield, 2002. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 5: 399–417.
Nehlsen, W., J. E. Williams & J. A. Lichatowich, 1991. Pacific salmon at the crossroads: stocks at risk from California, Oregon, Idaho, and Washington. Fisheries 16: 4–21.
Newton, T. J., D. A. Woolnough & D. L. Strayer, 2008. Using landscape ecology to understand and manage freshwater mussel populations. Journal of the North American Benthological Society the University of Chicago Press 27: 424–439.
Niraula, B. B., J. M. Hyde, J. M. Miller & P. M. Stewart, 2016. Differential sediment stability for two federally threatened and one common species of freshwater mussels in Southeastern Coastal Plain Streams, USA. Journal of Freshwater Ecology. https://doi.org/10.1080/02705060.2016.1248501.
O’Brien, C., D. Nez, D. Wolf & J. B. Box, 2013. Reproductive biology of Anodonta californiensis, Gonidea angulata, and Margaritifera falcata (Bivalvia: Unionoida) in the Middle Fork John Day River, Oregon. Northwest Science 87(1): 59–72.
Omernik, J. M. 1987. Ecoregions of the conterminous United States. Annals of the Association of American geographers, 77(1): 118–125.
Ortmann, A. E., 1913. The Alleghenian divide, and its influence upon the freshwater fauna. Proceedings of the American Philosophical Society American Philosophical Society 52: 287–390.
Österling, M., M. Lopes-Lima, E. Froufe, A. H. Hadzihalilovic & B. Arvidsson, 2020. The genetic diversity and differentiation of mussels with complex life cycles and relations to host fish migratory traits and densities. Scientific Reports Nature Publishing Group 10: 17435.
Pandolfo, T. J., T. J. Kwak, W. G. Cope, R. J. Heise, R. B. Nichols & K. Pacifici, 2016. Species traits and catchment-scale habitat factors influence the occurrence of freshwater mussel populations and assemblages. Freshwater Biology 61: 1671–1684.
Preece, E. P., M. Bryan, S. M. Mapes, C. Wademan & R. Dorazio, 2021. Monitoring for freshwater mussel presence in rivers using environmental DNA. Environmental DNA 3(3): 591–604.
Rodgers, T. W., J. C. Dysthe, C. Tait, T. W. Franklin, M. K. Schwartz & K. E. Mock, 2020. Detection of 4 imperiled western North American freshwater mussel species from environmental DNA with multiplex qPCR assays. Freshwater Science the University of Chicago Press 39: 762–772.
Roni, P. & T. P. Quinn, 2011. Density and size of juvenile salmonids in response to placement of large woody debris in western Oregon and Washington streams. Canadian Journal of Fisheries and Aquatic Sciences. https://doi.org/10.1139/f00-246.
Rosenfeld, J. S. & L. Huato, 2003. Relationship between large woody debris characteristics and pool formation in small coastal British Columbia streams. North American Journal of Fisheries Management Taylor & Francis 23: 928–938.
Salonen, J. K., P.-L. Luhta, E. Moilanen, P. Oulasvirta, J. Turunen & J. Taskinen, 2017. Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) differ in their suitability as hosts for the endangered freshwater pearl mussel (Margaritifera margaritifera) in northern Fennoscandian rivers. Freshwater Biology 62: 1346–1358.
Scully-Engelmeyer, K., E. F. Granek, M. Nielsen-Pincus, A. Lanier, S. S. Rumrill, P. Moran, E. Nilsen, M. L. Hladik & L. Pillsbury, 2021. Exploring biophysical linkages between coastal forestry management practices and aquatic bivalve contaminant exposure. Toxics Multidisciplinary Digital Publishing Institute 9: 46.
Sepkoski, J. J. & M. A. Rex, 1974. Distribution of freshwater mussels: coastal rivers as biogeographic islands. Systematic Zoology 23: 165–188.
Stevens, D. L., 2002. Sampling Design and Statistical Analysis Methods for the Integrated Biological and Physical Monitoring of Oregon Streams, Oregon Department of Fish and Wildlife, Portland, OR:
Stevens, D. L. & A. R. Olsen, 2004. Spatially balanced sampling of natural resources. Journal of the American Statistical Association 99: 262–278.
Stone, J., S. Barndt & M. Gangloff, 2004. Spatial distribution and habitat use of the western Pearlshell mussel (Margaritifera falcata) in a Western Washington Stream. Journal of Freshwater Ecology Taylor & Francis 19: 341–352.
Strayer, D., 1987. Ecology and zoogeography of the freshwater Mollusks of the Hudson river basin. Malacological Review 20: 1–68.
Strayer, D. L., 1999. Use of flow refuges by Unionid mussels in rivers. Journal of the North American Benthological Society the University of Chicago Press 18: 468–476.
Strayer, D. L., J. A. Downing, W. R. Haag, T. L. King, J. B. Layzer, T. J. Newton & J. S. Nichols, 2004. Changing perspectives on pearly mussels, North America’s most Imperiled animals. BioScience 54: 429–439.
Terui, A., Y. Miyazaki, A. Yoshioka, K. Kaifu, S. S. Matsuzaki & I. Washitani, 2014. Asymmetric dispersal structures a riverine metapopulation of the freshwater pearl mussel Margaritifera laevis. Ecology and Evolution 4: 3004–3014.
Wacker, S., B. M. Larsen, S. Karlsson & K. Hindar, 2019. Host specificity drives genetic structure in a freshwater mussel. Scientific Reports Nature Publishing Group 9: 10409.
Walton, J., K. Mock, S. Brownlee, J. Mageroy, G. Wilson, & I. Walker, 2020. Genetic variation at the species and population levels in the Rocky Mountain ridged mussel (Gonidea angulata) – Supplementary Material. Browse all Datasets. https://digitalcommons.usu.edu/all_datasets/124.
Weitkamp, L. A., T. C. Wainwright, G. J. Bryant, G. B. Milner, D. J. Teel, R. G. Kope, & R. S. Waples, 1995. Status review of coho salmon from Washington, Oregon, and California. NOAA Technical Memorandum NMFS-NWFSC-24. Northwest Fisheries Science Center, Seattle, WA
Wimberly, M. C. & J. L. Ohmann, 2004. A multi-scale assessment of human and environmental constraints on forest land cover change on the Oregon (USA) coast range. Landscape Ecology 19: 631–646.
Xerces Society, & Confederated Tribes of the Umatilla Indian Reservation (CTUIR), 2021. Western Freshwater Mussel Database | Xerces Society. https://www.xerces.org/endangered-species/freshwater-mussels/database.
Nobles, T., & Zhang, Y. 2015. Survival, growth and condition of freshwater mussels: Effects of municipal wastewater effluent. PloS one, 10(6): e0128488.
Acknowledgements
We thank the Aquatic Inventories Project team with the Oregon Department of Fish and Wildlife for their countless survey hours and Matt Strickland for his assistance with habitat data. We thank Oregon Sea Grant for their financial support of this research.
Funding
Financial support was received from Oregon Sea Grant [Grant # NB325E-B].
Author information
Authors and Affiliations
Contributions
Conceptualization: KS-E, EB, EG; Methodology: KS-E, EB, EG, RC; Formal analysis and investigation: KS-E; Writing—original draft preparation: KS-E; Writing—review and editing: EB, EG, RC; Funding acquisition: EG.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. The funders had no role in the design of the study, collection, analyses, or interpretation of data, the writing of the manuscript, or in the decision to publish the results.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scully-Engelmeyer, K., Blevins, E., Granek, E.F. et al. Freshwater mussel populations in Pacific Coast Watersheds (Oregon, USA): occurrence, condition, habitat, and fish species overlap. Hydrobiologia 850, 821–839 (2023). https://doi.org/10.1007/s10750-022-05127-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05127-w