Abstract
Detritus is an important energy source of stream food webs. Being a mix of allochthonous and autochthonous sources, it is often unknown, which components contribute to the growth of stream organisms. This study focussed on the comparison of two different detritus types (riparian detritus and stream detritus) with respect to food quality and effects on growth as a fitness parameter of juvenile freshwater pearl mussels (FPM). We performed feeding experiments with juvenile FPM under laboratory conditions using the two detritus types from four different natural sources each. Food quality was determined by analysing the fatty acid composition. Stream detritus (conditioned to stream environment including autochthonous microbes) resulted in significantly higher growth rates of juvenile FPM than predominately terrestrial-based riparian detritus indicating higher food quality. Significantly positive correlations were found between mussel growth and different groups of polyunsaturated fatty acids (PUFA). This suggests that especially trace substances such as long-chained n-3 PUFAs and a high ratio of n-3 to n-6 PUFAs enhance the food quality of stream detritus for juvenile FPM. These results highlight the importance of instream conditioning of detritus for the food mix in headwater streams and the importance of PUFAs for the development of juvenile FPM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How land and water interact has been one of the prime questions in freshwater biology, both for purely ecological reasons, but also for many regulatory questions within the water framework directive and the protection of freshwater streams harbouring numerous endangered species. In the context of land water interaction, the analysis of stream detritus as food source has become one of the prime questions in freshwater ecology. Detritus mainly directly originating from land or wetland ecosystems (allochthonous material) has been shown in several studies to be an important energy source for stream organisms in aquatic food webs (Fisher & Likens, 1973; Reid et al., 2008). Being a mix of both of allochthonous and autochthonous sources, it is often unknown, which components of detritus contribute to the growth of stream organisms. In recent years, studies indicated the importance of within stream (autochthonous) material for freshwater food webs as it provides macroinvertebrates with trace substances of high food quality (Guo et al., 2016a). Consequently, how the origin of detritus determines its food quality for stream organisms remains a question of exceptional importance.
The freshwater pearl mussel [FPM, Margaritifera margaritifera (Linnaeus, 1758)] is a species which is believed to strongly rely on terrestrial-based detritus especially during the juvenile phase (Hruška, 1995, 1999). Freshwater mussels in general are considered one of the species groups most affected by biodiversity loss (Auerswald et al., 2019) and therefore one of the most endangered freshwater species groups worldwide (Strayer et al., 2004; Lopes-Lima et al., 2017). The FPM in particular is listed as critically endangered (Moorkens, 2011) and is one of the most endangered freshwater mussels in Europe. Therefore, a wide range of protective measures has been implemented over the last 30 years including several captive breeding programmes throughout Europe (McIvor & Aldridge, 2008; Schmidt & Vandré, 2010; Thomas et al., 2010; Gum et al., 2011; Thielen, 2011; Scheder et al., 2014).
In addition to conservation measures, several studies have been carried out analysing the risk factors that are currently leading to the continuous decline in FPM populations (Geist & Auerswald, 2007; Österling et al., 2010; Denic & Geist, 2015; Baldan et al., 2021; Hoess & Geist, 2021). Whilst most studies focus on the required sediment properties and water quality, only a few studies have analysed the nutritional requirements of FPM.
Former studies documented the importance of detritus as a main food component for FPM (Hruška, 1995, 1999). In our study we distinguished between two types of detritus: riparian detritus and stream detritus. Riparian detritus is basically detritus from wet patches closely located (only a few meters away) to a stream. During rain events or with the subsurface flow, this type of detritus is washed into streams. Stream detritus, on the other hand, describes detritus that is found directly within the stream. It is usually found in flow-calmed areas, e.g. behind large boulders or tree roots where fine suspended particles can sediment and accumulate. Obviously, riparian detritus entering the stream will contribute to the stream detritus and therefore will be part of the available food resources for FPM. A stable isotope analysis conducted by Brauns et al. (2021) showed that terrestrial particulate organic matter as well as benthic organic matter contributed substantially to the diet of FPM supporting this assumption. The partial dependency on allochthonous organic matter in the headwater streams in which FPM occur would fit to the River Continuum concept (Vannote et al., 1980). Nevertheless, recent studies have shown that invertebrate grazers (e.g. Ecdyonurus sp.) and even shredders such as Gammarus sp. retain mainly but not exclusively algal carbon, most likely by feeding on the biofilm on leaf litter (Kühmayer et al., 2020). These microorganisms that are associated with biofilms (including bacteria, fungi, algae) play an important role in controlling food quality for invertebrate consumers and are leading to a “trophic upgrading” of benthic organic material by synthesising essential components such as polyunsaturated fatty acids (PUFA; Anderson et al., 2017; Kühmayer et al., 2020).
In most current captive breeding programmes, in which juvenile FPM are fed for a certain time period, detritus is provided as food (Gum et al., 2011), and the most commonly used food source is riparian detritus as it has been described and suggested by Hruška (1995, 1999, 2001). To our knowledge the difference between stream detritus and riparian detritus concerning its food quality for aquatic invertebrates and specifically for juvenile FPM has not been analysed in detail [but see Lange & Selheim (2011) for seston]. Given that autochthonous organic material from within the stream is also incorporated it can be hypothesised that stream detritus is a more suitable food for juvenile FPM than the pure riparian detritus. Stream detritus includes decomposed organic material from riparian vegetation but also from biofilms, macrophytes and algae. In particular, algae such as diatoms could play an important role because of their potentially high content of PUFA and other trace substances such as vitamins and sterols (Gatenby et al., 1997; Nichols & Garling, 2000). Long-chain polyunsaturated fatty acids (LC PUFA) such as eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) are particularly associated with improving growth rates of freshwater invertebrates (Guo et al., 2016a, b) including juvenile freshwater mussels (Wacker et al., 2002). These LC PUFAs are known to increase the fluidity of cell membranes (adaptation to low temperatures) and have anti-inflammatory and pro-resolving effects (Brett & Müller‐Navarra, 1997; von Elert & Fink, 2018; Steinberg, 2022).
Eybe et al. (2013) and Hyvärinen et al. (2020) showed that algae play an important role as food component for juvenile FPM (contradicting Hruška 1995, 1999) and commercial algae such as Shellfish Diet 1800® and Nanno 3600® (Reed Mariculture Inc., Campbell, California, USA) are widely used as food additives in breeding programmes. Eybe et al. (2013) showed that juvenile FPM grow best and have good survival rates when fed a mixture of riparian detritus and the mentioned commercial algae. They assumed that the detritus acts both as a food source for juvenile FPM and as a source of biologically active compounds that keeps particularly harmful ions such as ammonium and nitrite at low levels.
We analysed the growth rate of juvenile FPM as a function of food quality because current data supports the hypothesis that high growth rates during early development results in a higher survival probability of juvenile FPM individuals, at least during the first winter. During the first growing phase mussels which reaches a shell length of at least 1 mm have a higher survival probability for the first winter (Buddensiek, 1995) and consequently the growth rate is directly related to the survival probability of this species. Especially during this first growth period of the juvenile FPM, should neither trace substances nor the amount of food limit the growth of these young individuals.
Therefore, we combined the analysis of the food quality of eight different detritus samples from riparian and stream detritus sources with an analysis of food quantity of juvenile FPM. We hypothesised that (a) stream detritus (including autochthonous material), is a more suitable food source for juvenile FPM than a predominantly terrestrial-based riparian detritus and (b) that stream detritus is of higher food quality leading to higher growth rates of the young mussels. We further hypothesised that increased growth rates of juvenile FPM are associated with a higher content of polyunsaturated fatty acids in the respective food mixture, determining the food quality. Finally, we analysed the relationship between growth rates of juvenile FPM and increasing concentrations of food, to investigate food quantity effects.
Methods
Source of juvenile mussels and mussel growth rates
Following the method described by Gum et al. (2011) mussels were collected from infested brown trout (Salmo trutta Linnaeus, 1758) in the freshwater pearl mussel rearing station (Vogtland, Germany) in May–June (2017 and 2019). The fish, which were reared in a local hatchery, had been infested with glochidia from free-living gravid female mussels in July–August the year before the respective experiments and were kept in fishponds. Experiment 1 was performed between August and October in year 2017 and experiment 2 between June and September in year 2019.
The cultivation of the collected FPM was carried out according to the method developed by Hruška (1995, 1999). Juvenile mussels were kept for the first 1–2 month in small plastic boxes (500 ml) in the rearing station at 15°C. They were fed with a mixture of water from a local creek, riparian detritus from a natural riparian detritus source closely located to a river and an addition of Nanno 3600® (Nannochloropsis sp.). The riparian detritus was sieved to a fraction smaller 75 µm. The ratio between water, riparian detritus and Nanno 3600® was 9 l:1 l:0.2 ml. The food mixture was renewed twice a week and dead mussels were removed. After this first rearing time the mussels were transferred to the laboratory, where the experiments for this study were conducted.
Mussels were always kept in groups of 25 individuals (experiment 1) resp. 30 individuals (experiment 2) in one plastic beaker (total volume of 100 ml) representing one replicate of a respective treatment. The plastic beakers have proven to be suitable for the cultivation of juvenile FPM in previous experiments. The mussels were photographed at the start and the end of the experiments to determine their length with an accuracy of 10 µm using the image analysis software ImageJ (https://imagej.nih.gov/ij/) and to calculate the mean growth rates for mussels of each beaker. To eliminate outliers, the upper and lower 10% percentiles of the measured mussel lengths were removed from each beaker and mean growth rates (µm day−1) were calculated for each replicate of a treatment.
Sampling of detritus sources
Riparian detritus and stream detritus samples used for the experiment were collected from headwater mountain streams [referred here to S1, S2, S3, S4 (stream)] or their riparian zone [R1, R2, R3, R4 (riparian)] located in the Vogtland region (Saxony, Germany). Samples from S2, R2 and R3 were taken from the same stream system, as well as samples from S1 and R1. The samples S3, S4 and R4 were taken from three different stream systems. The land use in the whole catchment was dominated by coniferous forestry (around 50–60%), arable land use (around 20–30%) and grassland use (around 10–20%).
Riparian detritus was collected from wet patches closely located to headwater streams by carefully scooping the detritus with a ladle as described by Hruška (1999, 2001) and M. Lange (pers. comm. 2017). The vegetation at these patches was dominated by Carex sp., Poa sp., Salix sp. and Alnus glutinosa (L.) Gaertn. At R3 and R4 also some coniferous trees of the genus Picea sp. were present and at R1 and R2 also the neophyte Impatiens glandulifera Royle occurred. The sources R1 and R4 came closest to Hruška’s (1995, 1999) description, but still deviated from an ideal riparian detritus source for which we would expected to obtain the highest growth rates of juvenile FPM. The riparian detritus was sieved to the fraction < 500 µm (to eliminate macrozoobenthos and roots) and stored under low light conditions, 15°C and constant aeration until further processing.
Stream detritus was collected directly from the upper surfaces of the riverbeds. Due to sedimentation, it is concentrated at flow-calmed areas, e.g. behind larger root systems of alder trees reaching into the riverbed. The stream detritus (upper layer of around 1 cm thickness) was collected using a self-build suction device, that consists of a canister with two hoses connected. One hose connects the canister to a double-lift pump to produce a negative pressure inside the canister. The opening of the other hose was used to carefully suck in the stream detritus from the riverbed into the canister. The collected stream detritus was sieved to the fraction < 500 µm and stored under low light conditions, 15°C and constant aeration until further processing.
Study design
Detritus type-dependent growth (1st experiment)
Eight different samples of detritus (four stream detritus samples and four riparian detritus samples with five replicates each) were used to test for differences in growth and survival rates of juvenile FPM feeding on different natural food resources. Detritus samples were taken as described before every 2 weeks and transported into the laboratory. At the second and fourth sampling, additional samples were taken from each detritus sample and frozen at − 20°C for later analysis of the fatty acid composition.
In the lab the detritus samples were sieved to a particle size smaller 200 µm and diluted with stream water (from a Vogtland stream successfully used for breeding mussels) which was sieved to a particle size smaller 70 µm before to generate a 1 mm thick detritus layer after sedimentation at the bottom of a small plastic beaker filled with 100 ml food mixture (mix of stream water with the respective detritus). The dilution ratio to produce the 1 mm thick detritus layer was determined by creating separate dilution series and measuring the thickness of the sedimented detritus layers for each detritus sample every 2 weeks after taking new samples from the field.
30 mussels each were kept in the plastic beakers stored in the dark in a climate cabinet (Binder KB 53, Binder GmbH, Tuttlingen, Germay) at a constant temperature of 15°C. The experiment was started 81 days after the mussels had detached from the host fish and lasted for 77 days (August–October). The overall mean size of the mussels at the beginning of the experiment was 897 ± 60 µm. Twice a week, when the food mixture was changed, mussels were visually inspected with a binocular microscope.
Effects of the fatty acid content of food mixtures on growth (1st experiment)
The fatty acid composition was determined for samples of the eight different detritus types of the detritus type-dependent growth experiments. During this experiment samples from two sampling dates (August and September) were frozen at − 20°C in order to examine them later for their fatty acid composition. After defrosting the samples were sieved to the same particle size as the food mixture in the beaker (< 200 µm). A subsample was taken to analyse the dry mass and particulate organic carbon (POC) concentration of the samples using a carbon analyser (C844, LECO, St. Joseph, USA). The rest of the sample was freeze-dried using a Lyophilizer (Delta 1–24 LSC, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany).
Fatty acid analysis
Quantitative analysis of fatty acids of freeze-dried detritus with particle size smaller 200 µm was performed according to von Elert (2020) and Werner et al. (2021). In general, 50–100 mg of detritus were extracted with 5 ml of a (2:1, v:v) mixture of dichloromethane/methanol in the presence of 2 µg or 4 µg heptadecanoic acid methyl ester (C17:0 ME) in isohexane as internal standard. The extract was evaporated under a stream of nitrogen at 40°C, and the residue was then transesterified with 5 ml of 3 N methanolic HCl at 70°C for 20 min to obtain fatty acid methyl esters (FAMEs). The 3 N methanolic HCl was prepared by cooling 200 ml of methanol to 0°C using an ice bath and subsequent careful addition of 48 ml acetylchloride under gentle stirring. After transesterification, FAMEs were extracted twice by adding approximately 2 ml of isohexane twice to the samples, vortexing, and then collecting the hexane layer after each extraction step. Both extracts were pooled, evaporated to dryness under a stream of nitrogen at 40°C and redissolved in 100 μl of isohexane. An aliquot of 1 μl was injected splitlessly into a 6890-N GC System (Agilent Technologies, Waldbronn, Germany) equipped with a DB-225 capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific). Instrument settings were as follows: injector and FID temperatures 220°C; initial oven temperature 60°C for 1 min, followed by a 120°C/min temperature ramp to 180°C, then a ramp of 50°C/min to 200°C followed by 10.5 min at 200°C, followed by a ramp of 120°C/min to 220°C. Helium with a flow rate of 1.5 ml/min was used as the carrier gas. FAMEs were identified by comparing retention times with those of the reference compounds and then quantified using the internal standard and previously established calibration functions for each individual FAME (von Elert, 2002). Each detritus type was analysed in triplicate. Relative proportions of individual fatty acids or fatty acid groups were calculated by normalising the mass concentrations of an individual fatty acids or groups to the total fatty acid amount. Similarly, the ratio of n-3 PUFAs to n-6 PUFAs was calculated.
The measured fatty acid concentrations (FAs per 100 mg dry mass) were afterwards adjusted by the used dilution factors when creating the food mixture and multiplied with the dry mass of the sample (in the fraction smaller 200 µm) to calculate the total amount of fatty acids in the beaker the mussels were in (Eq. 1).
Food quantity-dependent growth and survival (2nd experiment)
For analysing the food quantity-dependent growth and survival rates the young mussels were divided into six test groups with 2–4 replicates each (each replicate containing 25 individuals) that differed in food quantity and the particle size spectrum (Table 1). All food mixtures (mix of stream water and stream detritus) were prepared with stream detritus from S2 (same detritus source as in the 1st experiment, which resulted in the highest growth rates of juvenile FPM), which was collected from the field every 2 weeks. The different particle sizes of the food mixture were produced by sieving the food mixture with sieves of respective mesh widths (30 µm and 200 µm) directly before feeding the mussels. 25 mussels each were kept in small plastic beakers (with a total volume of 100 ml food mixture) and stored in the dark in a climate cabinet (Binder KB 53, Binder GmbH, Tuttlingen, Germay) at a constant temperature of 15°C. The overall mean size of the mussels at the beginning of the experiment was 687 ± 19 µm. Every week, the mussels were visually inspected with a binocular microscope. The experiment lasted for 88 days (between June–August).
In order to prepare the different food mixtures, the POC concentration of the stored stream detritus was measured weekly using a carbon analyser (C844, LECO, St. Joseph, USA). Afterwards, the stream detritus was diluted with stream water of the same stream (stream water filtered to a size fraction of < 30 µm) to prepare the three different POC concentrations (low: ~ 30 mg l−1, medium: ~ 120 mg l−1 and high: ~ 360 mg l−1). Every 3 weeks the POC concentration of the final food mixtures was also analysed with a carbon analyser (C844, LECO, St. Joseph, USA).
Data analysis
Differences between growth rates of mussels depending on the sample site and the two detritus types (riparian and stream detritus) within the detritus type-dependent growth experiment (1st experiment) were analysed by performing a two-way ANOVA type II.
In order to identify fatty acid groups which correlate with the mussel growth rates, two principal component analyses were performed (for the fatty acid concentrations and the relative proportions of fatty acids) using the R-package vegan (Oksanen et al., 2020). The relation between growth rates of the mussels and the different fatty acid groups or the relative proportion of the fatty acid groups were then analysed using linear regressions.
Differences in fatty acid composition depending on the different detritus type and sampling sites were analysed performing an adonis permutation test (1000 permutations) and a redundancy analysis using the R-package vegan (Oksanen et al., 2020). To analyse differences regarding the concentrations and relative proportions of fatty acid groups between the two different detritus types, the relative effect size (Cohen’s d) was calculated with values larger 0.8 indicating a strong effect (Cohen, 1988). To test for significant differences between stream detritus and riparian detritus with respect to the ratio of n-3 to n-6 PUFAs, a Welch two sample test was performed. The effect of the three different POC concentrations groups (low, medium and high) and the two different particle fractions (< 30 µm and < 200 µm) on the survival rates of the juvenile FPM were analysed using a two-way ANOVA type II.
In order to account for a possible saturation of juvenile FPM growth rates with increasing POC concentration and to estimate a maximum possible growth rate, we fitted a Holling type II function to the data set of food quantity-dependent growth and survival (2nd experiment).
All statistical analyses were performed using R (R Core Team, 2020), with the significance level set to P < 0.05.
Results
Detritus type-dependent growth (1st experiment)
To investigate the growth of juvenile FPM dependent on the type of detritus, mussels were fed with detritus from four different stream detritus sources and four different riparian detritus sources over a period of 3 months, with five replicates each.
A two-way ANOVA type II revealed significant effects of the detritus sample sites (F(5,32) = 18.8, P < 0.001) and the detritus type (riparian or stream detritus; F(1,32) = 85.0, P < 0.001) on the mussel growth rates.
Mussels feeding on stream detritus showed significantly higher growth rates than mussels feeding on riparian detritus (Fig. 1). The mean growth rates calculated for the whole experiment duration of 3 months differed between the treatments ranging from 3.6 ± 0.4 µm day−1 (mean ± standard error, n = 5) if feeding on stream detritus from S2 to 0.4 ± 0.3 µm day−1 (mean ± standard error, n = 5) if feeding on riparian detritus from R1 (Fig. 1). Growth rates of mussels feeding on riparian detritus varied between 1.9 ± 0.4 µm day−1 and 0.4 ± 0.3 µm day−1 (mean ± standard error, n = 5) whilst growth rates of mussels feeding on stream detritus showed a higher mean variation ranging between 3.6 ± 0.4 µm day−1 and 1.3 ± 0.6 µm day−1 (mean ± standard error, n = 5). The final mean length of mussels fed with stream detritus at the end of the experiment was 1097 ± 77 µm and of mussels fed with riparian detritus 968 ± 47 µm.
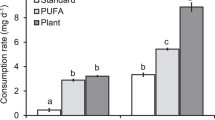
a Growth rates (mean ± standard deviation, n = 5) of juvenile freshwater pearl mussel (FPM) fed with 8 different natural food resources (four stream detritus S1–S4, four riparian detritus R1–R4). b Growth rates of juvenile freshwater pearl mussels (FPM) fed with stream detritus and riparian detritus (Box-Whisker plots, median, quartiles, 10th and 90th percentiles; ***P value < 0.001, two-way ANOVA type II, n = 20) (1st experiment)
The overall survival rate over the 3-month experiment was 98% and did not differ between the different treatments. The lowest observed survival rate was 97.3% and the highest was 100%.
Effects of the fatty acid content of food mixtures on growth (1st experiment)
In order to analyse the food quality of the eight different detritus sources from the detritus type-dependent growth analysis, the fatty acid content of these samples was measured at two time points within this experiment. Fatty acid groups (concentrations and relative proportions) indicating a positive or negative correlation with mussel growth within a principal component analysis (see Fig. 6 in the Online Appendix) were then analysed for correlation using linear regression analysis.
We found significantly positive linear correlations between the growth rates of juvenile FPM fed with different natural food sources (see Fig. 1) and the concentration of different groups of fatty acids in these food mixtures (Fig. 2). Growth rates increased significantly with the total amount of polyunsaturated fatty acids (PUFA) in the 100 ml food mixture. According to this linear regression the mean growth rates of juvenile FPM increased by 0.44 µm day−1 if the amount of available PUFAs was increased by 1 µg 100 ml−1 in the food mixture. Within the group of total PUFAs, positive linear regressions were found for the concentrations of gamma-linolenic acid (GLA, 18:3n-6) and of n-3 PUFAs. Amongst the group of n-3 PUFAs, positive linear regressions were found for the concentrations of alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3) and the stearidonic acid (SDA, 18:4n-3). Beside these PUFA groups, a significant positive linear correlation was found only for the saturated fatty acid palmitic acid (C16:0).
Concerning the relative proportion of the fatty acid groups (normalised to the total amount of fatty acids) significantly positive linear correlations were detected for the total amounts of PUFAs, of n-3 PUFAs and of the two n-3 PUFAs ALA and EPA (Fig. 3). For all four groups the effect was similar and indicated that the mean growth rate of the juvenile FPM increased by 0.26–0.33 µm day−1 if the proportion of the respective fatty acid group increased by 1%. The only negative correlation concerned the relative content of the monounsaturated fatty acid oleic fatty acid (C18:1n-9c).
A strongly significant positive correlation was also found between growth rates of the juvenile mussels and the ratio of n-3 to n-6 PUFAs in the food mixtures (Fig. 4). This indicates that juvenile FPM grew stronger if the proportion of n-3 PUFAs was higher in comparison to the proportion of n-6 PUFAs. Accordingly, the mean growth rate increased by 0.69 µm day−1 if the ratio increased by a value of 1.0.
a Growth rates of juvenile freshwater pearl mussels as a function of the ratio of n-3 to n-6 polyunsaturated fatty acids (PUFAs) in the food mixture (1st experiment). The line indicates a significant linear regression. b Ratio of n-3 to n-6 PUFAs in stream detritus and riparian detritus samples (**P value < 0.01, Welch two sample test, n = 8)
Differences in fatty acid composition depending on the different detritus types and sampling sites were analysed using an adonis permutation test. This revealed no statistically significant effects on the fatty acid concentrations but significant effects of sampling site (adonis: r2 = 0.56, P < 0.001) and detritus type (adonis: r2 = 0.21, P < 0.001) on the relative proportion of the fatty acids. A redundancy analysis showed that the sampling sites and the detritus type explain 43.7% of the variance of the fatty acid composition regarding the fatty acid concentrations and 77.3% of the variance of the fatty acid composition regarding the relative proportion of fatty acids. Therefore, most differences between stream detritus and riparian detritus samples became apparent when comparing the relative proportion of fatty acid groups (Table 2).
Stream detritus samples were characterised by a significantly higher ratio of n-3 to n-6 PUFAs (Fig. 4), a higher mean amount of the n-3 PUFA ALA in 100 ml food mixture and the total absence of the long-chain n-6 PUFAs dihomo-gamma-linolenic acid (DGLA, C20:3n-6), arachidonic acid (AA, C20:4n-6) and docosadienoic acid (C22:2n-6). Regarding the relative proportion of fatty acids, stream detritus was characterised by a higher relative content of the saturated fatty acids pentadecylic acid (C 15:0), palmitic acid (C16:0), stearic acid (C18:0), heneicosanoic acid (C21:0), the n-3 PUFA ALA and lower relative proportions of the monounsaturated fatty acids oleic acid (C18:1n-9 c) and hypogeic acid (C16:1n-9) (Table 2).
Food quantity-dependent growth and survival (2nd experiment)
In order to analyse the food quantity-dependent growth and survival of juvenile freshwater pearl mussels (FPM), mussels were fed with stream detritus from S2 which was diluted to three different POC concentrations (low, medium and high) and offered in two different particle size fractions (< 30 µm and < 200 µm) over a period of 88 days.
The growth rates of juvenile mussels increased with the amount of available potential food. This was observed for the concentration of POC in the particle fraction smaller 30 µm (Fig. 5) as well as for the dry mass in the same particle fraction and for the total amount of POC and dry mass in all available particle size fractions in the food mixture over a period of 88 days (data not shown).
Growth rates of juvenile freshwater pearl mussels (FPM) within a period of 88 days depending on the POC concentration (mean ± standard error) of the food resource (stream detritus from S2) in the size fraction < 30 µm. The dotted line represents the function (Holing-model type II) fitted to the data (2nd experiment)
Mussels from the treatments with a medium POC concentration and a particle fraction smaller 200 µm as well as all mussels from treatments with the high POC concentration reached a similar mean final length of 975 ± 13 µm at the end of the experiment. On the other hand, mussels from the treatments with a medium POC concentration and a particle fraction smaller 30 µm as well as all mussels from treatments with the lowest POC concentration reached only a mean final length of 887 ± 27 µm.
The dependence of growth rates on the POC concentration was not linear but followed a logarithmic curve (Fig. 5). By applying a Holing-model type II to the data the maximum possible growth rate was estimated. Therefore, the maximum growth rate which can be reached by increasing the amount of POC in the fraction smaller 30 µm would be around 3.7 µm day−1. Half of the maximum growth rate could be reached at a POC concentration of around 24 mg l−1 stream detritus from stream 2. The carbon content of the dry mass was very similar as it ranged from 6.4 to 7.0% across all two particle fractions.
A two-way ANOVA type II revealed a significant influence of the factor particle fraction on the survival rates of the juvenile mussels (F(1,8) = 8.59, P < 0.05). The POC concentration on the other hand had no significant effect on the survival rates. Mussels which were fed with stream detritus in a fraction smaller 30 µm showed the highest mean survival rate (99%, n = 6) within their 2nd–4th month (over 88 days) after excystment. The mean survival rate of mussels fed with stream detritus in a fraction smaller 200 µm was lower (96%, n = 8) but still high.
Discussion
Generally, the results of this study indicate that detritus originating from within the stream (detritus conditioned to stream environment and including autochthonous microbes) is of higher food quality than (predominantly allochthonous) riparian detritus. The amount of polyunsaturated fatty acids (PUFA), especially n-3 PUFAs, and the ratio of n-3 to n-6 PUFAs in the food mixture showed a positive relationship with the growth rates of juvenile FPM underlining the importance of trace substances, which determine food quality. This was also reflected in the differences in fatty acid composition between stream detritus and riparian detritus samples and probably explains the higher growth rates of juvenile FPM fed with stream detritus compared to mussels which were fed with riparian detritus. We assume that the higher food quality of stream detritus is a result of a “trophic upgrading” of the predominantly terrestrial-based organic material entering the streams (including riparian detritus) by autochthonous microbes (including algae, bacteria, fungi), which are rich in long-chained n-3 PUFAs (LC n-3 PUFAs) (Anderson et al., 2017).
Effects of detritus type on mussel growth
The results showed that mussels fed with stream detritus had higher growth rates than mussels fed with riparian detritus. This is consistent with our hypothesis that stream detritus is a more suitable food for juvenile FPM than riparian detritus but appears to contradict the assumption of Hruška (1995, 1999). He postulated that detritus derived from terrestrial plant material, as well as detritus produced by breaking off tiny hair roots of the rhizosphere of riparian vegetation protruding into the streambed, is the main food of FPM. Riparian detritus is therefore most commonly used to feed juvenile FPM in captive breeding programmes during the first phase of laboratory rearing (Gum et al., 2011). However, Hruška (1995) pointed out that the food quality of the riparian detritus depends strongly on the land use from which the detritus originated. It is therefore possible that the current land uses in the catchments of the streams from which detritus samples were taken differed to strongly from the ideal land use described by Hruška (flooded meadows with Alopecurus pratensis L. and Poa trivialis L. being the dominant vegetation) and therefore leads to the contradicting result that riparian detritus is a less suitable food source for juvenile FPM. This was probably the case even with the riparian detritus sources R1 and R4, which came closest to Hruška's description of ideal land use, but still differed from it and therefore showed contradicting results with the lowest observed growth rates of juvenile FPM feeding on these sources. Furthermore, these sources had also low concentrations of n-3 PUFAs. One explanation for this could be a rather low flow through of these sources and therefore a higher water temperature as it is assumed in previous studies that algae from temperate headwater streams probably contain more long-chained PUFAs than algae from warmer waters (Guo et al., 2016a, 2017).
That terrestrial organic material contributes significantly to the nutrition of FPM and that therefore intact riparian areas are of great importance for FPM has already been shown by Brauns et al. (2021). Once riparian detritus enters the stream, it is most likely processed further by macroinvertebrates and the microbial community and becomes mixed with other organic compounds from the stream, e.g. algae from biofilms or connected ponds. Colonisation of in-stream microbes (including bacteria, fungi, algae) may result in a “trophic upgrading” by synthesising essential components (Anderson et al., 2017). We were able to confirm that stream detritus is of higher food quality for juvenile FPM resulting in higher growth rates of the mussels. This was also reflected by the comparison of the fatty acid profiles of the two detritus types and could have important implications for other aquatic invertebrates living in similar ecological niches. Stream detritus had a much higher relative proportion of n-3 PUFAs and lacked long-chain n-6 PUFAs, resulting in a significantly higher ratio of n-3 to n-6 PUFAs, which is considered a good indicator for higher food quality (Galloway & Winder, 2015). Therefore, we consider the natural stream detritus to be a more suitable food source for juvenile FPM than pure riparian detritus and suggest to use the ratio of n-3 to n-6 PUFAs to determine the food quality of detritus sources.
This explicitly does not mean that we assume that riparian detritus and thus the renaturation of riparian areas are unimportant for FPM. It could still be an important carbon source and provides the FPM with other important trace substances that were not analysed in this study, e.g. minerals such as calcium, but which should also be investigated in future studies. However, since riparian detritus is also part of the stream detritus, we recommend to assess the possibility to use stream detritus for mussel cultivation in captive breeding programmes instead.
Effects of fatty acid composition on mussel growth
Fatty acids, especially PUFAs, have been shown to be of great importance in assessing food quality for freshwater invertebrates (Brett & Müller‐Navarra, 1997; Guo et al., 2016a). This is in concordance with our results which showed that especially the total amount and relative proportion of n-3 PUFAs as well as a high ratio of n-3 to n-6 PUFAs in the food mixture of juvenile FPM are indicating high food quality, as they lead to higher growth rates. The most abundant n-3 PUFA was alpha-linolenic acid (ALA, C18:3n-3), which also had a significantly higher concentration in stream detritus compared to riparian detritus. ALA is a precursor for long-chain essential fatty acids (LC EFAs) such as eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3; Steinberg, 2022) which are particularly associated with enhanced growth rates of freshwater invertebrates (Wacker & von Elert, 2008; Guo et al., 2016a, b). However, the ability to convert ALA into LC EFAs is rather low, which underlines the importance of the direct uptake of dietary LC EFAs (Guo et al., 2016a).
EPA was found in higher concentrations only in stream detritus samples from S2, which also resulted in the highest growth rates of juvenile FPM fed with it. DHA was not detected in any stream detritus or riparian detritus sample. All samples examined were deficient in LC EFAs and thus no sample was of optimal food quality.
Our results indicated a limitation in food quality due to a suboptimal amount of n-3 PUFAs but not due to a too low amount of n-6 PUFAs. This was reflected in the positive correlation of the ratio of n-3 PUFAs to n-6 PUFAs with growth rates. During the metabolic processing of n-6 PUFAs to more pro-inflammatory lipid mediators and n-3 PUFAs to less potent pro-inflammatory and more pro-resolving lipid mediators, both PUFA groups compete for the same class of enzyme (Steinberg, 2022). This probably explains why the ratio can be considered a good indicator of the food quality of juvenile FPM, with high levels of n-3 PUFAs increasing their growth rates.
During the rearing of juvenile FPM in captive breeding programmes, the food quality can be enriched by supplementing the food mixture with n-3 PUFA-rich algal species, which must fall in the size class of ingestible particles. One possible species is the microalgae Nannochloropsis sp., which is already used in several FPM breeding programmes as a food additive (Nanno 3600®) and is known for its high content of EPA. Natural sources for n-3 LC PUFAs with a respective high ratio of n-3 to n-6 PUFAs are especially diatoms, dinoflagellates and cryptophytes (Taipale et al., 2013; Galloway & Winder, 2015; Peltomaa et al., 2019). Green algae on the other hand are considered to be of intermediate and cyanobacteria of poor food quality for aquatic invertebrates in terms of their PUFA content (von Elert et al., 2003; Guo et al., 2017). In a laboratory experiment Kühmayer et al. (2020) demonstrated that aquatic invertebrates (Ecdyonurus sp. and Gammarus sp.) took up EPA directly from biofilm associated algae, which consisted of over 90% diatoms. This underlines the importance of autochthonous material determining the food quality for invertebrates in headwater streams. Therefore, we conclude that algae probably also play an important role in determining the food quality for juvenile FPM by being a source for LC n-3 PUFAs. This hypothesis is supported by the findings of Eybe et al. (2013) and a recent study analysing the diet of adult FPM and showing that diatoms are one of the most abundant algae groups found in the gut content of the adult mussels (Komulaynen, 2021). This contradicts the assumption of Hruška (1995, 1999) that algae do not play a major role in the nutrition of FPM. Due to seasonal fluctuations in algae composition, it can be assumed that also the fatty acid composition and therefore the food quality of detritus will differ over time. In our experiment we analysed samples from only two time points which showed low differences concerning the relative proportion of fatty acids and stronger differences with regard to the fatty acid concentration (see Fig. 6 in the Online Appendix). This potential fluctuation of food quality should therefore be part of further investigations.
Next to algae also aquatic ascomycetes are known to be rich in PUFAs and therefore could also be a potential PUFA source for juvenile FPM. However they seem to lack the capability to synthesise LC PUFAs (Funck et al., 2015).
Effects of food quantity on mussel growth
Beside the effects of food quality, we also observed effects of food quantity on mussel growth. A positive relationship between the growth rates of mussels and the POC concentration of the food mixture has already been described (Wacker & von Elert, 2008; Bracken et al., 2012). In contrast to these studies, our data indicated a saturation curve and not a linear positive relationship. Saturation was reached at a carbon concentration of 200–300 mg C l−1 in the particle fraction < 30 µm but it is likely that this range of carbon concentrations is specific for the used detritus samples.
In this experiment the lowest carbon concentration of 30 mg C l−1 seems to limit mussel growth. At this carbon concentration each of the 25 mussels in a beaker with a total volume of 100 ml food mixture would have had 17 µg C per day and mussel available. Therefore, each mussel could consume 119 µg C per week. During an additional experiment (in May 2020) in which we tried to measure the carbon consumption rate of juvenile mussels, a significant difference of 40 µg of carbon between the starting conditions and treatments after 1 week could be detected (see Fig. 7 in the Online Appendix). Nevertheless, it was not possible to detect a significant difference between treatments with and without juvenile FPM (after 1 week) even though the mussels grew well during this experiment (6.7 ± 2.4 µm day−1 in shell length, n = 23) not indicating starvation at any stage. Hence, it was not possible to measure the carbon consumption rates of juvenile FPM but we could conclude that it is smaller than these 40 µg C per week. Therefore, the offered food amount in the group of the lowest carbon concentration (30 mg C l−1, 1st experiment) should have been high enough to not limit mussel growth due to a to low POC concentration. In consequence, this means that the mussels were not limited by the available amount of POC in this experiment but by some trace substances within the food mixture (e.g. PUFAs) whose total amount in a beaker also increases if the total amount of food is increased.
Assuming that juvenile FPMs are able to use compensatory feeding or selective feeding to take up food particles of high quality, this could explain why high POC concentration levels lead to higher mussel growth rates even though the carbon consumption rate of juvenile FPMs is assumed to be very low. Earlier studies showed that at least some freshwater mussels are capable of selective feeding (Baker & Levinton, 2003; Beck & Neves, 2003; Brendelberger & Klauke, 2009; Bracken et al., 2012; Rosa et al., 2018). Whilst most studies like Baker & Levinton (2003) only revealed a particle size selectivity, the results by Fung & Ackerman (2020) also indicate a species specific feeding selectivity. Fung & Ackerman (2020) demonstrated that juvenile individuals of the unionid mussel Lampsilis siliquoidea (Barnes, 1823) specifically selected diatoms, which are considered to be of higher food quality (Taipale et al., 2013; Galloway & Winder, 2015; Guo et al., 2017) compared to chlorophytes.
We therefore conclude that the growth rates of juvenile FPM can increase with increasing total amount of trace substances to be selected by the mussels, but growth would also increase if the relative proportion of trace substances increases (i.e. if food quality increases) whilst the amount of carbon (food quantity) remains the same.
In the context of selective feeding, the size of ingestible particles is an important factor to be considered. According to Lavictoire et al. (2018) and Schartum et al. (2017) the lower size range of particles mussels can filter from the water is between 1.54 and 2.3 µm. The upper size range is most likely depending on the mouth width of the mussels. To our knowledge the only available information is an electron microscope image of the mouth region of a 1-month-old FPM (Lavictoire et al., 2018). According to this, the upper size range would be around 10–30 µm. This suggests that even small microalgae as Nannochloropsis sp. (2–4 µm in diameter) would fall into the size spectrum of ingestible food particles for juvenile FPM. However, it can be assumed that smaller particles can also be filtered out of the water and be ingested if they are associated with larger particles and the conglomerate does not exceed the upper size range of ingestible particles. But, particles larger 10–30 µm probably also play an important role when food particles of an ingestible size class are attached to these particles and can be grazed from them by the mussel using its foot during pedal feeding activity. Even though we found a significant effect of the particle fraction on the survival rates of the juvenile FPM, the effect was not very strong. In both cases, the mussels showed very high survival rates in all treatments (90–100% over 88 days), with a mean survival rate which was three percentage points higher in the treatments with a particle fraction < 30 µm.
Conclusion
In conclusion our study demonstrated that considerable differences exist between riparian and stream (predominantly allochthonous vs. conditioned to stream environment and including autochthonous microbes) detritus regarding its food quality for invertebrate stream organisms like juvenile FPM. Stream detritus was characterised by a significantly higher amount of n-3 PUFAs and a higher ratio of n-3 to n-6 PUFAs, leading to a higher food quality of stream detritus compared to riparian detritus. The most likely source for the n-3 PUFAs (especially for the LC n-3 PUFAs) are algae such as diatoms, dinoflagellates and cryptophytes (Taipale et al., 2013; Galloway & Winder, 2015; Peltomaa et al., 2019). Our results therefore underline the importance of autochthonous material even in headwater streams, which influences food quality for invertebrates such as juvenile FPM and is leading to a nutritional improvement of the allochthonous dominated organic material found in these habitats. In addition, the results provide new insights into the nutritional needs of juvenile FPM and can therefore contribute to the improvement of conservation measures such as captive breeding and the identification and restauration of suitable habitats for this highly endangered freshwater mussel.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Anderson, T. R., D. W. Pond & D. J. Mayor, 2017. The role of microbes in the nutrition of detritivorous invertebrates: a stoichiometric analysis. Frontiers in Microbiology 7: 2113. https://doi.org/10.3389/fmicb.2016.02113/full.
Auerswald, K., P. Moyle, S. P. Seibert & J. Geist, 2019. HESS Opinions: socio-economic and ecological trade-offs of flood management—benefits of a transdisciplinary approach. Hydrology and Earth System Sciences 23: 1035–1044.
Baker, S. M. & J. S. Levinton, 2003. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia 505: 97–105.
Baldan, D., J. Kiesel, C. Hauer, S. C. Jähnig & T. Hein, 2021. Increased sediment deposition triggered by climate change impacts freshwater pearl mussels habitats and metapopulations. Journal of Applied Ecology 58(9): 1933–1944.
Beck, K. & R. J. Neves, 2003. An evaluation of selective feeding by three age-groups of the rainbow mussel Villosa iris. North American Journal of Aquaculture 65: 203–209.
Bracken, M., B. Menge, M. Foley, C. Sorte, J. Lubchenco & D. Schiel, 2012. Mussel selectivity for high-quality food drives carbon inputs into open-coast intertidal ecosystems. Marine Ecology Progress Series 459: 53–62.
Brauns, M., T. Berendonk, S. Berg, F. Grunicke, D. Kneis, S. Krenek, T. Schiller, J. Schneider, A. Wagner & M. Weitere, 2021. Stable isotopes reveal the importance of terrestrially derived resources for the diet of the freshwater pearl mussel (Margaritifera margaritifera). Aquatic Conservation: Marine and Freshwater Ecosystems 31(9): 2496–2505. https://doi.org/10.1002/aqc.3619.
Brendelberger, H. & C. Klauke, 2009. Pedal feeding in freshwater unionid mussels: particle-size selectivity. SIL Proceedings 1922–2010(30): 1082–1084.
Brett, M. & D. Müller-Navarra, 1997. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biology 38: 483–499.
Buddensiek, V., 1995. The culture of juvenile freshwater pearl mussels Margaritifera margaritifera L. in cages: a contribution to conservation programmes and the knowledge of habitat requirements. Biological Conservation 74: 33–40.
Cohen, J., 1988. Statistical Power Analysis for the Behavioral Sciences. Routledge, London.
Denic, M. & J. Geist, 2015. Linking stream sediment deposition and aquatic habitat quality in pearl mussel streams: implications for conservation. River Research and Applications 31: 943–952.
Eybe, T., F. Thielen, T. Bohn & B. Sures, 2013. The first millimeter—rearing juvenile freshwater pearl mussels (Margaritifera margaritifera L.) in plastic boxes. Aquatic Conservation: Marine and Freshwater Ecosystems 23: 964–975.
Fisher, S. G. & G. E. Likens, 1973. Energy flow in bear brook, New Hampshire: an integrative approach to stream ecosystem metabolism. Ecological Monographs 43: 421–439.
Funck, A. J., A. Bec, F. Perrière, V. Felten & M. Danger, 2015. Aquatic hyphomycetes: a potential source of polyunsaturated fatty acids in detritus-based stream food webs. Fungal Ecology 13: 205–210.
Fung, V. & J. D. Ackerman, 2020. The effects of river algae and pore water flow on the feeding of juvenile mussels. Journal of Geophysical Research: Biogeosciences 125: e2019JG005302. https://doi.org/10.1029/2019JG005302.
Galloway, A. W. E. & M. Winder, 2015. Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS ONE 10: e0130053. https://doi.org/10.1371/journal.pone.0130053.
Gatenby, C. M., B. C. Parker & R. J. Neves, 1997. Growth and survival of juvenile rainbow mussels, Villosa iris (Lea, 1829) (Bivalvia: Unionidae), reared on algal diets and sediment. American Malacological Bulletin 14: 57–66.
Geist, J. & K. Auerswald, 2007. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshwater Biology 52: 2299–2316.
Gum, B., M. Lange & J. Geist, 2011. A critical reflection on the success of rearing and culturing juvenile freshwater mussels with a focus on the endangered freshwater pearl mussel (Margaritifera margaritifera L.). Aquatic Conservation: Marine and Freshwater Ecosystems 21: 743–751.
Guo, F., M. J. Kainz, F. Sheldon & S. E. Bunn, 2016a. The importance of high-quality algal food sources in stream food webs—current status and future perspectives. Freshwater Biology 61: 815–831.
Guo, F., M. J. Kainz, F. Sheldon & S. E. Bunn, 2016b. Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 181: 449–462.
Guo, F., S. E. Bunn, M. T. Brett & M. J. Kainz, 2017. Polyunsaturated fatty acids in stream food webs—high dissimilarity among producers and consumers. Freshwater Biology 62: 1325–1334.
Hoess, R. & J. Geist, 2021. Effect of fish pond drainage on turbidity, suspended solids, fine sediment deposition and nutrient concentration in receiving pearl mussel streams. Environmental Pollution 274: 116520.
Hruška, J., 1995. Problematik der Rettung ausgewählter oligotropher Gewässersysteme und deren natürlicher Lebensgemeinschaften in der Tschechischen Republik. Lindberger Hefte 5: 98–123.
Hruška, J., 1999. Nahrungsansprüche der Flussperlmuschel und deren halbnatürliche Aufzucht in der Tschechischen Republik. Heldia 4: 69–79.
Hruška, J., 2001. Experience of semi-natural breeding program of freshwater pearl mussel in the Czech Republic. Die Flussperlmuschel in Europa: Bestandssituation und Schutzmaßnahmen. Kongressband. WWA Hof, Albert-Ludwigs Universität, Freiburg 69–75.
Hyvärinen, H. S. H., M. M. R. Chowdhury & J. Taskinen, 2020. Pulsed flow-through cultivation of Margaritifera margaritifera: effects of water source and food quantity on the survival and growth of juveniles. Hydrobiologia. https://doi.org/10.1007/s10750-020-04225-x.
Komulaynen, S. F., 2021. Diet of European freshwater pearl mussels Margaritifera margaritifera (Mollusca: Bivalvia: Unionoida) in small river (Republic of Karelia, Russia). Proceedings of the Zoological Institute RAS 325: 502–515.
Kühmayer, T., F. Guo, N. Ebm, T. J. Battin, M. T. Brett, S. E. Bunn, B. Fry & M. J. Kainz, 2020. Preferential retention of algal carbon in benthic invertebrates: stable isotope and fatty acid evidence from an outdoor flume experiment. Freshwater Biology 65: 1200–1209.
Lange, M. & H. Selheim, 2011. Growing factors of juvenile freshwater pearl mussels and their characteristics in selected pearl mussel habitats in Saxony (Germany). Ferrantia 64: 30–37.
Lavictoire, L., A. D. Ramsey, E. A. Moorkens, G. Souch & M. C. Barnhart, 2018. Ontogeny of juvenile freshwater pearl mussels, Margaritifera margaritifera (Bivalvia: Margaritiferidae). PLoS ONE 13: e0193637. https://doi.org/10.1371/journal.pone.0193637.
Lopes-Lima, M., R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, K. Douda, E. Froufe, D. Georgiev, C. Gumpinger, A. Karatayev, Ü. Kebapçi, I. Killeen, J. Lajtner, B. M. Larsen, R. Lauceri, A. Legakis, S. Lois, S. Lundberg, E. Moorkens, G. Motte, K.-O. Nagel, P. Ondina, A. Outeiro, M. Paunovic, V. Prié, T. von Proschwitz, N. Riccardi, M. Rudzīte, M. Rudzītis, C. Scheder, M. Seddon, H. Şereflişan, V. Simić, S. Sokolova, K. Stoeckl, J. Taskinen, A. Teixeira, F. Thielen, T. Trichkova, S. Varandas, H. Vicentini, K. Zajac, T. Zajac & S. Zogaris, 2017. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews 92: 572–607.
McIvor A & Aldridge D, 2008. The cultivation of the freshwater pearl mussel, Margaritifera margaritifera. CCW Contract Science Report No. 849, Countryside Council for Wales/Environment Agency, Bangor.
Moorkens, E., 2011. Margaritifera margaritifera. The IUCN Red List of Threatened Species 2011: e.T12799A3382660. , https://www.iucnredlist.org/species/12799/3382660.
Nichols, S. J. & D. Garling, 2000. Food-web dynamics and trophic-level interactions in a multispecies community of freshwater unionids. Canadian Journal of Zoology 78: 871–882.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, & H. Wagner, 2020. vegan: Community ecology package. https://CRAN.R-project.org/package=vegan.
Österling, M. E., B. L. Arvidsson & L. A. Greenberg, 2010. Habitat degradation and the decline of the threatened mussel Margaritifera margaritifera: influence of turbidity and sedimentation on the mussel and its host. Journal of Applied Ecology 47: 759–768.
Peltomaa, E., H. Hällfors & S. J. Taipale, 2019. Comparison of diatoms and dinoflagellates from different habitats as sources of PUFAs. Marine Drugs 17: 233.
R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Reid, D. J., G. P. Quinn, P. S. Lake & P. Reich, 2008. Terrestrial detritus supports the food webs in lowland intermittent streams of south-eastern Australia: a stable isotope study. Freshwater Biology 53: 2036–2050.
Rosa, M., J. E. Ward & S. E. Shumway, 2018. Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. Journal of Shellfish Research 37: 727–746.
Schartum, E., S. Mortensen, K. Pittman & P. J. Jakobsen, 2017. From pedal to filter feeding: ctenidial organogenesis and implications for feeding in the postlarval freshwater pearl mussel Margaritifera margaritifera (Linnaeus, 1758). Journal of Molluscan Studies 83: 36–42.
Scheder, C., B. Lerchegger, M. Jung, D. Csar & C. Gumpinger, 2014. Practical experience in the rearing of freshwater pearl mussels (Margaritifera margaritifera): advantages of a work-saving infection approach, survival, and growth of early life stages. Hydrobiologia 735: 203–212.
Schmidt, C. & R. Vandré, 2010. Ten years of experience in the rearing of young freshwater pearl mussels (Margaritifera margaritifera). Aquatic Conservation: Marine and Freshwater Ecosystems 20: 735–747.
Steinberg, C. E. W., 2022. Essential fatty acids—‘fueling versus controlling’ Aquatic animal nutrition. Springer, Cham: 673–721. https://doi.org/10.1007/978-3-030-87227-4_27.
Strayer, D. L., J. A. Downing, W. R. Haag, T. L. King, J. B. Layzer, T. J. Newton & S. J. Nichols, 2004. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 54: 429.
Taipale, S., U. Strandberg, E. Peltomaa, A. Galloway, A. Ojala & M. Brett, 2013. Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquatic Microbial Ecology 71: 165–178.
Thielen, F., 2011. Rearing of unionoid mussels (with special emphasis on the freshwater pearl mussel Margaritifera margaritifera). Ferrantia 64, Musée national d’histoire naturelle, Luxembourg: 66.
Thomas, G., J. Taylor & C. Garcia de Leaniz, 2010. Captive breeding of the endangered freshwater pearl mussel Margaritifera margaritifera. Endangered Species Research 12: 1–9.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
von Elert, E., 2002. Determination of limiting polyunsaturated fatty acids in Daphnia galeata using a new method to enrich food algae with single fatty acids. Limnology and Oceanography 47: 1764–1773.
von Elert, E., 2020. Polyunsaturated fatty acids in decomposing leaf litter. In Bärlocher, F., M. O. Gessner & M. A. S. Graça (eds), Methods to Study Litter Decomposition: A Practical Guide. Springer, Cham: 147–155. https://doi.org/10.1007/978-3-030-30515-4_17.
von Elert, E. & P. Fink, 2018. Global warming: testing for direct and indirect effects of temperature at the interface of primary producers and herbivores is required. Frontiers in Ecology and Evolution 6: 87.
von Elert, E., D. Martin-Creuzburg & J. R. Le Coz, 2003. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proceedings of the Royal Society of London Series b: Biological Sciences 270: 1209–1214.
Wacker, A. & E. von Elert, 2008. Body size and food thresholds for zero growth in Dreissena polymorpha: a mechanism underlying intraspecific competition. Freshwater Biology 53: 2356–2363.
Wacker, A., P. Becher & E. von Elert, 2002. Food quality effects of unsaturated fatty acids on larvae of the zebra mussel Dreissena polymorpha. Limnology and Oceanography 47: 1242–1248.
Werner, C., K. A. Otte & E. von Elert, 2021. Phenotypic convergence in a natural Daphnia population acclimated to low temperature. Ecology and Evolution 11: 15312–15324.
Acknowledgements
We thank Antje Kuhr, Claudia Volke, Thomas Schiller and Dr. Jana Schneider for their help in performing the lab experiments and with the fieldwork and Thomas von Einem for performing the fatty acid analyses. Furthermore, we would like to thank Dr. Thomas Petzoldt for his statistical support and Thomas Thoß and Wolfgang Schmettlach for their work in the vogtlandic FPM breeding station. The study was embedded in the joint project ArKoNaVera and funded by a grant from the Federal Ministry of Education and Research, grant no. 01LC1313B and 01LC1313A. Mussels used in this study originated from a captive-breeding program and were used with the permission of the authority for nature conservation of the federal state of Saxony.
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding was provided by Bundesministerium für Bildung und Forschung (Grant nos. 01LC1313B and 01LC1313A).
Author information
Authors and Affiliations
Contributions
FG: Methodology, Investigation, Software, Formal analysis, Writing—Original Draft, Writing—Review & Editing. AW: Conceptualisation, Methodology, Formal analysis, Supervision, Writing—Review & Editing. EvE: Methodology, Investigation, Formal analysis, Supervision, Writing—Review & Editing. MW: Conceptualisation, Funding acquisition, Writing—Review & Editing. TB: Conceptualisation, Funding acquisition. Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10750_2022_5120_MOESM1_ESM.eps
Supplementary file2 Fig. 6 Principal component analysis of fatty acid data as concentrations (top) and relative proportions (bottom), samples are grouped into riparian (blue) and stream (red) detritus samples. The samples are coded with sampling site and month of sampling. (EPS 700 kb)
10750_2022_5120_MOESM2_ESM.eps
Supplementary file3 Fig. 7 Carbon consumption of juvenile freshwater pearl mussels (FPM). Depicted is the total amount of POC (mg) in the assay volume of 4 mL at the start and after 7 days in the control (without FPM) and in the feeding trial (with FPM). Box-Whisker plots, median, quartiles, 10th and 90th percentiles, n = 23 for each group, Box-Whisker plots sharing the same letter do not differ significantly (Post hoc test after ANOVA with x10-transformed data). (EPS 48 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grunicke, F., Wagner, A., von Elert, E. et al. Riparian detritus vs. stream detritus: food quality determines fitness of juveniles of the highly endangered freshwater pearl mussels (Margaritifera margaritifera). Hydrobiologia 850, 729–746 (2023). https://doi.org/10.1007/s10750-022-05120-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05120-3