Abstract
For evaluating hydraulic stress reduction strategies of caddisfly larvae, our study has three goals. First, creating a database on Reynolds numbers (Re) and drag coefficients valid for Limnephilidae larvae with cylindrical mineral cases. Second, evaluating the effects of submerged weight and biometry in cases with comparable length/width ratios. And third, collecting field data in an alpine environment for gaining insights into the hydraulic niches occupied by thirteen Drusinae species. Biometric data were subsequently combined with published Reynolds numbers and mean flow velocity data measured immediately upstream of Limnephilidae larvae at the moment of dislodgement. This provides drag coefficients for the range of Reynolds numbers obtained in the field. Data reveal that heavy cases strongly benefit from compensating drag by submerged weight, thereby enabling species to utilize high velocity spots, an important benefit for filtering species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Each roughness element at the bottom of a stream modifies its surrounding hydraulic field and is, in turn, subject to hydraulic stress exerted by the flow (Dingman, 1984). This applies to both sediment particles and macrozoobenthic organisms, such as lotic insect larvae. In the latter, hydraulic stress is mostly compensated by active efforts of the larvae to cling to the substrate and to submerged body weight, which can be largely increased by shells and cases constructed by the larvae. In the present study, we focus on the ecological implications of caddisfly cases as means of compensating hydraulic stress. Such an approach involves experiments and field measurements based on reliable descriptors of case shape and performance, some simple ones such as geometry, weight, volume, and length–width ratios, and some complex ones, such as drag and lift coefficients. Such an extensive database is the prerequisite to address the structural diversity observed in Trichoptera.
In fact, there is an amazing variety in caddisfly cases (Wiggins, 1996; Wallace et al., 2003; Holzenthal et al., 2007; Rinne & Wiberg-Larsen, 2017; Morse et al., 2019): cross-sections range from triangular (e.g., Anabolia brevipennis (Curtis, 1834)) over quadrangular (e.g., Brachycentrus montanus Klapálek, 1892) to circular (e.g., Beraea pullata (Curtis, 1834)). There are also turtle- and purse-shaped cases (e.g., Agapetus fuscipes Curtis, 1834; Hydroptila occulta Eaton, 1873), and construction materials cover a wide spectrum from mineral particles of various grain sizes over mollusc and testacean shells to woody debris, algal filaments, and plant and freshwater sponge fragments.
In lotic habitats, a tubular case constructed of mineral particles is the most abundant type (Waringer & Graf, 2011). Even so, tubular cases are very different in length, width, weight, curvature, and roughness. Generally they are always tapered, because after larval molts the larger instars add new case sections at the anterior end. This particular feature adds a certain degree of streamlining to the overall tubular case design. Moreover, as the legs of the larvae which hold on to the substrate extend from the anterior case opening, the longitudinal axis of the case aligns parallel with the flow (Waringer et al., 2021).
From a fluid dynamical perspective, such cases may be abstracted as near-wall, submerged, cylinder-like bodies exposed to a fluid flow where the maximum (anterior) case diameter defines how far the larva extends into the log-law layer in which turbulent fluctuations become important (Hoerner, 1965; Kuhlmann, 2014; Waringer et al., 2020). For the biota in question, the hydraulic stress on a body exposed to a flow is a force per area of the body which results from the fluid dynamical pressure (normal stress) and from viscous effects (normal and tangential [shear] stresses) (Scott, 2005). Unfortunately, basic biometric and hydraulic data sets necessary for estimating hydraulic stress parameters applicable to field conditions are lacking. The aim of the present study is, therefore, (1) to offer information on drag coefficients valid for the typical range of Reynolds numbers encountered by lotic caddisfly larvae, (2) to provide data on the type and amount of hydraulic stress for both viscous and pressure drag dominance, and (3) to detect and evaluate strategies of hydraulic stress reduction, based on case biometry and on series of in situ velocity measurements in the immediate, harsh, high-alpine, hydraulic environment of Drusinae larvae.
Materials and methods
Biometric parameters
A database on biometric parameters (case length, case width, anterior case surface) is necessary for the computation of hydraulic parameters such as Reynolds number and drag which describe case performance in the flow; weight and volume data are necessary for obtaining density of larvae and cases. In total, there are five larval instars in Limnephilidae, with mineral, cylindrical cases becoming longer and wider with each subsequent larval molt; for the biometrical database, we took larvae sampled in their alpine habitats and preserved in 70% Ethanol. Fresh weight of 72 final instar larvae in their cases belonging to thirteen Drusinae species were measured on a laboratory balance (to the nearest mg). For obtaining volume data of larvae plus cases (to the nearest 0.01 ml) a burette filled with water was used. For each individual specimen we removed excess conservative fluid with filter paper and took the difference between volume readings without and with larvae. Aspect ratios Γ were calculated by dividing mean case length L by anterior case width 2R. Length and width data (to the nearest 0.01 mm) were measured under a dissecting microscope using an ocular micrometer. Calculations of the projected frontal surface areas were based on maximum (anterior) case diameters. For submerged weight reduced by buoyancy, the volume measurements of larvae plus cases were multiplied by the density of water at 10 °C and subsequently subtracted from fresh weight. The corresponding biometric data for the five larval instars of Allogamus auricollis (Pictet 1834) and fifth instars of Potamophylax cingulatus Stephens 1837 as well as velocity data acting exactly at the moment of dislodgement (drift entry) were taken from Waringer (1989, 1993) and supplemented with additional unpublished measurements. The biometric database is included in Tables 1 and 2.
Hydraulic stress parameters
In streams and rivers, the unidirectional mean flow is driven by gravity and depends on the slope of the river bed. Lotic organisms on the stream bed are, therefore, subject to forces acting on the surface of their body (Morisawa, 1968; Vogel, 1981; Gordon et al., 1992). In the first step, we wanted to explore the character of those forces around Drusinae larvae in their cases by computing the relative importance of viscous and pressure forces expressed by the Reynolds number of the body.
where U (m s−1) is the mean flow velocity to which the larva is exposed, L is a characteristic length of the larva (e.g., case length), and ν is the kinematic viscosity which is temperature dependent (lab: ν15°C = 1.15 × 10−6 m2 s−1; field: ν10°C = 1.30 × 10−6 m2 s−1). At very low Reynolds numbers, viscous forces (skin friction) on the body dominate. With increasing Re, pressure forces (form drag) become the dominant component of total drag.
In the second step, the magnitude of the hydraulic forces acting on the larvae was investigated. The force component parallel (streamwise) to the direction of the (mean) flow is called the drag, whereas the cross-stream component is the lift (Kuhlmann, 2014). Both are usually expressed by the non-dimensional drag and lift coefficients CD and CL, which result when the drag and lift are scaled by the frontal stagnation pressure times a characteristic area A, which depends on body shape. For thick, blunt bodies, such as spheres, cylinders, and cars, the area projected in streamwise direction is typically selected, ‘that is, the area one sees when looking toward the body from upstream’ (White, 2011), which also applies for caddis larvae with cylindrical cases. For an approximation of the projected area, we take A = πR2, where R is the maximum radius of the case. For a blunt body, the total drag force FD is usually expressed in terms of the stagnation point pressure times the cross-sectional area A which leads to
where the drag coefficient CD is introduced. ρ is the density of the water which is temperature dependent (lab: ρ15°C = 999.13 kg m−3; field: ρ10°C = 999.70 kg m−3). For moderately large Reynolds numbers (> 100) CD is of the order of 1 with \(A\rho {U}^{2}/2\) providing the order of magnitude of the drag force. The exact drag coefficient CD depends on the shape of the body and the Reynolds number Re or, equivalently, on U (for constant L and ν). Typically, the drag coefficient is determined for a homogeneous flow (moving airfoil in quiescent air). But if the oncoming flow is turbulent the drag coefficient will change.
In the third step, we explored the stabilizing forces of a larva in its case, based on its submerged weight. For a body resting on the stream bed, the total drag force (2) is counteracted by a static friction force FF. Based on the submerged weight of the larvae in their cases this force can be estimated by
where V is the volume (larva + case; m3), g is the acceleration due to gravity (9.81 m s−2), ρL is the density of the larva including the case (kg m−3), ρ is the density of water, and f is an empirical friction factor which is f = 0.69 for mineral Trichoptera cases on mineral substrates (Waringer, 1989). In flattened body shapes, hydrodynamic lift may also be substantial. However, in benthic animals that are less or not at all dorsoventrally flattened, e.g., Plecoptera or Trichoptera larvae, the combined pressure and viscous skin friction drag by far outweigh the lift force, with the latter actually close to zero (Weissenberger et al., 1991).
As outlined above, the drag coefficient CD, necessary for calculating drag, will change if the oncoming flow is turbulent, which is frequently the case in the natural habitats of Drusinae larvae. In the fourth step, therefore, we had to provide information on CD over the range of Reynolds numbers typical for lotic caddisfly habitats. For this, we used the particular situation in which FD = FF. This is the dislodgement condition at the moment of drift entry
It occurs at a particular velocity U* or at a particular Reynolds number \({Re}^{*}\). Solving for the drag coefficient we obtain as follows:
or with U* = ν Re*/L,
For a cylinder-like body, we can assume V = cAL with constant c (c = 0.39) as shape parameter. The constant accounts for the fact that V is based on the anterior (maximum) case diameter which overestimates V for conical cases. Because we consider only the same universal shape, we can drop the dependence of CD on c and obtain as follows:
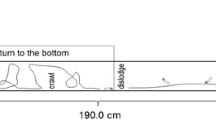
In Eq. (7), the coefficient K is approximately constant over all taxa and instars, based on the fact that density variations were negligible in Drusinae larvae and cases. Therefore, the relation C*D ~ K (L3/Re*2) captures the essential dependence of the drag coefficient at the moment of dislodgement on the size of the larva, parameterized by its length L. For this experimental determination of CD*, dead lotic Trichoptera larvae (preserved in 70% Ethanol) were used, because the dislodgement conditions expressed by Eq. (4) are only valid without active efforts of the larvae to cling to the substrate. We exposed the larvae in their cases to increasing flow velocities, using a shallow laboratory flume (described in detail by Waringer 1989a; water depth = 5–7 mm) and measured the dislodgement velocity U* at which they drifted from their initial position. Larvae were exactly aligned parallel to the incoming flow, heads upstream. Velocity at the moment of dislodgement was in the range of 0.03–0.75 m s−1 and measured immediately in front of the larvae.
In the step five of our analysis we deduced the expected drag as a function of the size of larvae and cases using a generalization of Eq. (7), where we need to know the mean velocity entering Re*. For this, Le Roux (2004) provided an integrated equation valid for the transitional flow regime between hydrodynamically smooth (= viscous sublayer thicker than roughness elements) and rough conditions (= viscous sublayer thinner than roughness elements):
with Re = \(\frac{\rho k({{gSy)}^{*}}^{0.5}}{ \mu }\), where S is the slope (dimensionless), y is the water depth (m), µ is the dynamic viscosity (N s m−2), and k is the substrate roughness (m).
Field measurements
Flow velocity data were collected in the field from 7 June to 25 July 2019, from 15 to 23 May 2021, and from 10 to 15 July 2021. The study sites were situated in high-gradient, low-order, unpolluted, shallow, and summer-cold mountain brooks 50 to 100 cm wide, with adjoining catchments consisting mostly of alpine grassland and, less frequently, mixed forest at some stations below the treeline. A synopsis of the sites is provided in Supplementary Material 1. In total, 101 data sets were taken for fifth instar larvae. The flow to which larvae are exposed in their natural setting is highly fluctuating (usually turbulent; Waringer et al., 2020) and even the mean velocity profiles (as functions of the distance from the ground) may vary. Furthermore, these flow details are very difficult to measure under field conditions. We were able, however, to measure the velocity ahead of the larvae by a tripod-mounted Schiltknecht MiniWater 20 Micro-velocimeter probe to the nearest 0.01 m s−1 (time resolution = 1 measurement per s). This probe has a circular cross section of 10 mm. The tripod enabled setting the measuring head, touching the bottom, directly at front center of a larva, at water depths ranging from 10 to 30 mm, thereby yielding a good approximation to the velocity field vertically averaged over the height of the larva. The mean velocity U is obtained as the arithmetic mean of the recorded fluctuating signal for each data set. Information on the number of single-velocity measurements per data set is included in Table 3.
Results
Biometry
The biometric database includes information for 16 taxa of Limnephilidae larvae (subfamilies Drusinae and Limnephilinae–Stenophylacini), most in the last (fifth) instar, but also for instars 1–4 in Allogamus auricollis (Pictet 1834). For the close species pair Drusus rhaeticus (Schmid 1955) and Drusus flavipennis (Pictet 1834), data were pooled. Based on weight and overall size of fully grown larvae, the largest taxon included was Potamophylax cingulatus Stephens 1837, whereas D. rhaeticus/flavipennis was the smallest. Taxa-specific data on case biometry necessary for the calculations of hydraulic parameters, such as static friction and Reynolds numbers corresponding to the drift entry velocity U*, are summarized in Tables 1 and 2.
Drag coefficient
Drag coefficients CD* are calculated using Eq. (7) and are based on dislodging flow velocities ranging from 0.030 m s−1 in first instar A. auricollis larvae to 0.209 m s−1 in fifth instars when the longitudinal body axes were exactly aligned with flow, heads directed upstream. Due to their high submerged fresh weight of up to 255 mg, which is six times higher than in A. auricollis (fifth instars), drift entry of final instar larvae of P. cingulatus was measured at drift entry flow velocities (U*) of 0.71 m s−1 (Tables 1 and 2). Reynolds numbers at the moment of dislodgement (Re*) were below 100 in first instar larvae of A. auricollis, between 100 and 1000 in instars 2 and 3, and between 1400 and 3100 in instars 4 and 5; for final instar larvae of P. cingulatus, Re* was one order of magnitude higher than in A. auricollis (Re* = 13,364; Fig. 1, Table 1). This reflects the rapidly increasing static friction and lower anterior surface (A) increase of larvae during ontogenesis. Fresh weight and, therefore, static friction were low in first to fourth instars (0.89–30.36 mg and 1.48–36.55 × 10–6 N, respectively), but strongly increased in fifth instar larvae of A. auricollis and P. cingulatus (181.00–781.44 mg and 279.22–1722.01 × 10–6 N, respectively), thereby increasing U* and Re* accordingly.
Projected frontal case area A (log mm2; circles), static friction (log × 10–6 N: triangles), and drag coefficient CD* (Eq. (7) with c = 0.39; squares) as a function of the Reynolds number Re* at the moment of dislodgement for first to fifth instar larvae of Allogamus auricollis (Pictet 1834) and fifth instar larvae of Potamophylax cingulatus (Stephens 1837). Solid regression line for data points of CD* according to equation: CD* = 193 * (Re*)−.0.75 (P < 0.000; r2 = 0.92). Data are based on unpublished and experimental flume data (Waringer 1989, 1993). Diamonds: data of numerical OpenFoam simulations conducted with surface models of D. alpinus Meyer-Dür, 1875, D. bosnicus Klapálek 1899, D. discolor (Rambur 1842), D. monticola McLachlan, 1876, and D. nebulicola (McLachlan 1867) by A. Vieira and H.C. Kuhlmann.
The anterior projected area A increased from 0.77 to 17.36 mm2 in first to fifth instar larvae of A. auricollis and was as high as 31.46 mm2 in final instars of P. cingulatus, reflecting the enlargement of the cases at a ratio of 1.3 to 1.9 in length between two successive molts. As surface increases with the power of 2 of the length, but volume with the power of 3, static friction due to submerged weight increased more rapidly than A in Fig. 1.
Influence of case size on drag
In order to accommodate a given body volume, case sizes vary during ontogeny and between species (e.g., Table 1). Despite a high range of fresh weights (0.89–781.44 mg; Table 2), variation in aspect ratios (Γ; case length divided by anterior case width) was negligible, ranging from 3.24 to 4.67 (Table 2). To explore the interplay between hydraulic stress and submerged weight as well as maximum case height, we computed static friction FF using Eq. (3) and total drag FD using Eq. (2) for two water depths (0.1 and 0.01 m), two slope situations (0.004 and 0.016), and for case diameters in the range of 0.2 to 7 mm, using biometric data included in Tables 1 and 2. The drag force, shown in Fig. 2, is calculated using the fit for the drag coefficient. All evaluations are based on velocities valid for anterior case center (R) using Eq. (8) valid for the transitional flow regime between hydrodynamically smooth and rough conditions. In the species set investigated in the present study (Tables 1, 2), maximum anterior case diameters were always 2R ≥ 0.95 mm. Therefore, the drag gradients for case diameters 2R = 0.2–0.8 mm included in Fig. 2 are intended just to illustrate drag for putative small larvae.
Log-linear relationship between maximum anterior diameter (mm) of cylindrical cases composed of mineral particles, static friction force FF (gray line), and total drag force FD(lines in color) for four chosen situations: slope = 0.016 (‘slope high’) and 0.004 (‘slope low’); and mean water depth = 0.1 m (‘deep’) and 0.01 m (‘shallow’). Graphs are based on Eq. (8) and the following parameters: aspect ratios of cases = 3.5; water temperature = 10 °C; and mean density of larvae and cases = 1251.43 ± 47.09 kg m−3. Cases aligned with flow, larval heads directed upstream
Generally, the drag force for all case diameters is higher when the slope is high. The drag increases asymptotically with increasing case diameter (Fig. 2). This is due to the fact that small larvae benefit from their small anterior case diameters which experience less drag due to their smaller projected anterior surface A and low spot velocities near to the sediment surface. In the region below the interception from the gray static friction line and the total drag force curves, dislodgement of the larvae is not possible because total drag is lower than stabilizing static friction. This situation applies to hypothetical larvae with a case diameter of 2R = 0.2–0.4 mm in situation ‘shallow, slope low,’ and to case diameters 2R ~ 6 mm in all situations except ‘deep, slope high.’ In the regions above the interception from the friction line and the total drag curves, there is a need of active stabilizing efforts by the larvae in order to remain stationary.
Drag experienced by Drusinae larvae in the field
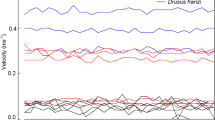
The 101 data sets taken at the locations of 13 Drusinae species at high-alpine sampling locations in Austria, Italy and Switzerland were used for exploring actual drag encountered by cased caddis larvae in the field. During all measurements, larvae were always aligned with flow, heads directed upstream. With respect to flow velocity, significant (P = 0.000; Kruskal–Wallis-ANOVA) differences were observed between feeding types as defined by Pauls et al. (2008) and Vitecek et al. (2015): in shredders which feed on roots, water mosses, riparian vegetation, and coarse particulate organic matter, mean velocities (± 95% CL) were as low as 0.09 ± 0.01 m s−1. In grazing species using epilithic biofilms as primary food source, we observed intermediate mean velocities of 0.19 ± 0.02 m s−1; highest mean velocities were recorded in carnivorous filter feeders (0.33 ± 0.03 m s−1) (Fig. 3). Whereas some time-resolved data series included several zero readings at low-stress larval locations, flow velocities in certain species were exceedingly high: in Drusus katagelastos up to 0.93 m s−1 and in D. nebulicola up to 1.38 m s−1. Corresponding Reynolds numbers ranged from 0 to 11,146 (Table 3). For shredders, mean Re (± 95% CL) was 756 ± 87, for grazers 1632 ± 136, and for filter feeders 3125 ± 162.
Calculated mean static friction using Eq. (3) and biometric information provided by Table 2 for the Drusinae species set studied in the field ranged from 25.25 × 10–6 N in the Drusus flavipennis/rhaetica (= Metanoea) species pair to 255.26 × 10–6 N in D. chrysotus (Table 3), indicative for the heavy cases and large larvae of the latter species. Mean drag exerted on the larvae was as low as 3.34 × 10–6 N in D. flavipennis/rhaetica, but up to 236.98 × 10–6 N in D. katagelastos; finally, maximum drag ranged from 27.98 × 10–6 N in D. flavipennis/rhaetica to 832.58 × 10–6 N in D. katagelastos (Table 3).
When grouped into feeding guilds established for Drusinae (Pauls et al., 2008; Vitecek et al., 2015), we noticed significant (P < 0.05) differences in mean and maximum drag forces, whereas differences in static friction were not significant (P > 0.05; Kruskal–Wallis-ANOVA). Static friction and mean drag increased from shredders over grazers to filtering collectors (Fig. 4). Interestingly, mean drag was smaller than static friction in shredders, indicating that Drusus alpinus and D. franzi frequented hydraulic spots where their submerged weight prevented drift entry and dislodgement. In grazers and filtering collectors, larvae have to cling actively to the substrate in order to stay stationary. Maximum drag in filtering collectors was as high as 674.17 × 10–6 N; in shredders, observed maximum drag was higher than in grazers (371.80 versus 217.52 × 10–6 N) (Fig. 4).
Flow velocities (m s−1) measured at front center of larvae of 13 Drusinae species in the field. The intervals between single measurements were 1 s, and the number of single-velocity measurements for each of the 101 data series is indicated in Table 3. White squares = means, black rectangles = 25/75% quartiles, error bars = range without outliers, circles = outliers
Discussion
In their seminal paper on hydraulic stream ecology, Statzner et al. (1988) highlighted their observation that body shapes of many lotic zoobenthic organisms are not well adapted to minimize the hydrodynamic forces on their body. This also fully applies to the lotic caddisfly larvae of the present study: for example, in Drusus chrysotus, a deep concavity on the head capsule is present, and the pronota of Drusinae are frequently fitted with dorsal humps and ridges, thereby increasing the frontal area directed toward flow (Waringer & Graf, 2011). Despite those morphological drawbacks, the same species are abundant in stream sections where flow velocity and hydraulic stress are high simply because of nutritional and/or respirational needs (Waringer et al., 2021). In the present study we address this ecological trade-off by providing data on the hydraulic forces exerted on lotic caddisfly larvae and by highlighting avoidance strategies against hydraulic stress.
Biometry
For the range of lotic Limnephilidae larvae measured (Table 2), the mean density for larvae plus cases, based on fresh weight, was found to be 1251.43 ± 47.09 kg m−3 which is significantly higher than the density of water at 10 °C (= 999.70 kg m−3), a water temperature frequently recorded in the field at the sampling sites of the species set. However, this stabilizing effect of higher density cannot be fully utilized by lotic caddisflies, because the proportion of submerged weight to fresh weight (mean ± 95% CL) was only (21.02 ± 2.30) %, illustrating the fact that submerged cased caddis larvae loose up to four-fifths of their fresh weight due to buoyancy which greatly reduces static friction, the most important stabilizing force in streams. Projected frontal surface areas (A) ranged from 0.77 ± 0.05 in first instars of Allogamus auricollis to 31.46 ± 4.01 mm2 in fifth instar larvae of Potamophylax cingulatus, almost 41 times as large as in the former taxon. As drag and lift are scaled by the frontal stagnation pressure times frontal surface area (A), this biometric parameter also strongly influences hydraulic stress experienced by the larvae: small cases generally experience less drag due to their smaller projected anterior surfaces.
Drag coefficient
Knowledge of the range and magnitude of the drag coefficient CD is a prerequisite for computing hydraulic forces acting on lotic caddisfly larvae. By taking case length as length parameter L in the equation of the organismic Reynolds number (Eq. 1), our laboratory flume experiments show that CD = > > 1 for Re = 84 (Fig. 1), indicating the dominance of viscous (skin friction) drag. Experimental data for passive drift entry of first to fifth instar larvae of A. auricollis at Re* = 84 to 3057 resulted in CD* values decreasing from 4.3 to 0.73; in fifth instars of P. cingulatus at Re* = 13,364, CD* was as low as 0.22. Our numerical simulation data (unpublished) on fifth instar larvae of the 5 Drusinae species included in Fig. 1 (Re = 1902–3741, CD = 1.29–0.84) were close to the established relationship of CD*(Re*). It must be kept in mind that Eq. (7) was derived for the condition of dislodgement only. It does not provide an expression for the drag as a function of the Reynolds number CD(Re). Only if the functional dependence CD(Re) for all larvae investigated would be the same, the data for the drag coefficient at the moment of dislodgement C*D would sample the function CD(Re) at the sampling points Re*. However, as the numerical simulations performed independently fit the regression line from the dislodgement experiments quite well, the assumption C*D(Re*) = CD(Re) can be made. This means that CD values for all species investigated are the same at a given Re, based on the uniformity of their mineral, conical cases, and the same aspect ratios. Therefore, the regression equation included in the legend of Fig. 1 can be taken to obtain CD values for hydraulic parameter computations based on our data obtained in the field.
Of course, CD and CD* always consist of a pressure and viscous drag component which could not be separated in the laboratory flume experiments. However, data generated by numerical simulations revealed that in Drusus alpinus Meyer-Dür, 1875 at Re = 3680 pressure drag contributed 43% and viscous drag 57% to total drag.
By taking a close look at the drag coefficients over the Re* range of 80 to 14,000 (Fig. 1), CD* strongly increases at Reynolds numbers smaller than 200, because viscous effects become more and more important. At Re 1000–2000, the CD* gradient becomes weaker. When looking at individual data points (black squares), a plateau effect becomes visible at Re 1000–2000, with a subsequent stronger decrease at Re > 2000 (Fig. 1). This pattern matches the CD(Re) gradient of a sphere in a flow field, where a plateau (or even minimum) of ~ 0.4 was also observed at intermediate Re. At low Re; however, CD is much higher for the rougher and longer larvae than for spheres: at an increase of the aspect ratio Γ from 1 (sphere) to 4 (larvae), skin friction drag is also four times as high in the cylindrical cases than in spheres.
Influence of case size on drag
In addition to the relationship CD (Re) discussed above, our data summarized in Fig. 2 illustrate the effect of flow velocity on cases with the same length-to-width ratio. Generally, a smaller larva with a smaller anterior case diameter experiences less drag due to its smaller projected anterior surface A and because it is closer to the bottom where friction and the no-slip condition produces strongly declining spot velocities at the level of the case center (Fig. 2). As volume increases to the power of 3 of the length, static friction due to submerged weight strongly increases with the size of the larva and becomes dominant over total drag; this is shown in Fig. 2 where curve sections to the right of intersections between drag and static friction lines define situations where dislodgement is not possible. To the left of intersections, however, there is a need of active stabilizing efforts by the larvae in order to remain stationary. The data suggest that in intermediate-sized larvae the investment of muscle energy in their legs to remain stationary is highest and such size classes are more prone to dislodgement than small and very large larvae. This is well illustrated by P. cingulatus (fifth instars) where the volume gain (volume of larvae + cases divided by projected anterior area) is up to 16.77 mm3 mm−2 when compared with first instar larvae of A. auricollis (0.87 mm3 mm−2). Bulky cases benefit from compensating a large amount of drag: in P. cingulatus, a flow velocity of 0.71 ± 0.06 m s−1 is needed for drift entry of dead larvae in their cases aligned with flow (Waringer, 1993). Another strategy to remain stationary in the flow was observed in Brachycentrus spp.: in this caddisfly genus, larvae attach their cases to the substrate using silk to release their legs for filtering and other activities (Gallepp, 1977).
Drag experienced by Drusinae larvae in the field
For our field measurements in the high-gradient, turbulent channels well above the treeline inhabited by the Drusinae larvae studied in detail, it is important to keep in mind that our considerations did not take into account the shape of the mean velocity profile of the turbulent flow. The velocity profile will enter the drag force and thus the drag coefficient such that CD will no longer be unique. Furthermore, we did not account for any variation of the larva shape but assumed that all larvae had the same aspect ratios and are characterized by a common constant c. Finally, the mean velocity at the height of the larvae has only been approximated by our field measurements and are strictly valid only for 5-mm wall distance. Our data revealed that Drusinae species were able to deal with quite high flow velocities: D. nebulicola was able to withstand flow velocities of up to 1.38 m s−1. As all sampling locations were shallow, our flow velocity measurements are valid for the hydraulic situation at the larval positions. In D. nebulicola, the anterior case diameter is only 1.0–2.5 mm (Bohle, 1987); the latter, combined with a very high active effort of the larva in clinging to the substrate, resulted in withstanding a maximum drag of 672.14 × 10–6 N, equal to 25 times its static friction, illustrating the capacity of filter feeders to expose themselves to high flow velocities. The opposite trend was observed within the hydraulic environment of D. flavipennis/rhaeticus where the lowest flow velocity range (0–0.11 m s−1) and the lowest mean Reynolds number (Re = 138) were observed. At such Re values, the species pair is situated in the descending left limb of the CD graph. The highest submerged weight of 37.71 mg of the Drusinae species set was measured in D. chrysotus; although in this species the active effort of the larvae to cling to the substrate was one of the lowest of the species studied (only 2.4 times of submerged weight; Table 3), their high static friction and, hence, Re of up to 5707 shifted CD values well into the right descending limb of Fig. 1.
At the level of feeding guilds, our new data are in line with the findings of Waringer et al. (2021). We noticed increasing trends in static friction and mean drag from shredders over grazers to filtering collectors (Fig. 4). Generally, mean drag was smaller than static friction in shredders, indicating their preferences for hydrodynamic low-stress patches within their predominantly low-stress (hypo-)krenal habitats where the accumulations of detrital particles, roots and riparian vegetation are highest. Grazers prefer hydrodynamic intermediate stress patches where the autotrophic biofilms and epilithic algae growing on mineral substrates are not yet eroded by flow velocities high enough to set sediments in motion. In line with the latter, maximum drag was lowest in this feeding guild (Fig. 4). Finally, species of the filtering clade frequent high stress patches because high flow velocity is required to operate their filtering apparatus efficiently, resulting in the highest observed maximum drag values in this group. Interestingly, in the filtering Drusinae clade, Zittra et al. (2022) detected modifications of the internal head capsule morphology such as an increasing simplification of the tentorium. In this regard, it can be assumed that the modified head capsules of the filtering carnivore Drusinae, e.g., dimples separated by fortified ridges, offer greater mechanical stability due to their structured surface as compared to the simply rounded head capsules of the other Drusinae, rendering the dorsal branch of the tentorium superfluous. This interpretation of the observed structures on the head capsule are doubtlessly capable of withstanding greater hydraulic forces, identifying the latter as important drivers for the evolution of morphological structures.
Conclusion
Our study revealed that high-alpine caddisfly larvae cover a wide spectrum of hydraulic microhabitats in their steep and hard substrate channels. For the chosen set of thirteen species of Drusus and Ecclisopteryx species, our field measurements revealed Reynolds numbers ranging from 0 to 11,146, corresponding to instantaneous flow velocities at the larval locations from 0 to 1.38 m s−1. Drag coefficients calculated from experimental data in a laboratory flume (Waringer, 1989) yielded precise information on the drag acting on the larvae in the field. When grouping the chosen species set in functional feeding guilds, shredders frequented hydraulic spots where their submerged weight alone prevented drift entry and dislodgement, without the need for investing muscle energy in order to stay stationary. In grazers and filtering collectors, larvae have to actively cling to the substrate to prevent dislodgement. Grazer clade species expose themselves to only very low Reynolds numbers (e.g., Drusus flavipennis/rhaeticus). They achieve stress reduction by their small cases which experience less drag due to their smaller projected anterior surfaces and because close to the bottom, friction and the no-slip condition produce strongly declining spot velocities at the level of the case center. Another grazer strategy is taken by voluminous larvae constructing heavy, bulky cases such as D. monticola and Ecclisopteryx madida; they benefit from compensating a large amount of drag by submerged weight, thereby minimizing energy investments in active flow compensation. Filter feeders need to expose themselves to high hydraulic stress in order to efficiently operate their filtering apparatus.
Data availability
The data sets generated during the current study are available from the corresponding author on reasonable request.
References
Bohle, H. W., 1987. Drift-fangende Köcherfliegen-Larven unter den Drusinae (Trichoptera: Limnephilidae). Entomologica Generalis 12: 119–132.
Dingman, S. L., 1984. Fluvial Hydrology. Freeman and Company, New York:
Gordon, N. D., T. A. McMahon & B. L. Finlayson, 1992. Stream Hydrology, Wiley, Chichester:
Gallepp, G. W., 1977. Response of caddisfly larvae (Brachycentrus spp.) to temperature, food availability and current velocity. The American Midland Naturalist 98: 59–84.
Hoerner, S. F., 1965. Fluid-Dynamic Drag: Practical Information on Aerodynamic Drag and Hydrodynamic Resistance, Hoerner Fluid Dynamics, Bakersfield:
Holzenthal, R. W., R. J. Blahnik, A. L. Prather & K. M. Kjer, 2007. Order Trichoptera Kirby, 1813 (Insecta), Caddisflies. Zootaxa 1668: 639–698.
Kuhlmann, H., 2014. Strömungsmechanik. Eine kompakte Einführung für Physiker und Ingenieure. 2,. Aktualisierte Pearson Studium. Pearson Education, Deutschland:
Le Roux, J. P., 2004. An integrated law of the wall for hydrodynamically transitional flow over plane beds. Sedimentary Geology 163: 311–321.
Morisawa, M. E., 1968. Streams, Their Dynamics and Morphology, McGraw-Hill, New York:
Morse, J. C., P. B. Frandsen, W. Graf & J. A. Thomas, 2019. Diversity and ecosystem services of Trichoptera. Insects. https://doi.org/10.3390/insects10050125.
Pauls, S. U., W. Graf, P. Haase, H. T. Lumbsch & J. Waringer, 2008. Grazers, shredders and filtering carnivores—the evolution of feeding ecology in Drusinae (Trichoptera: Limnephilidae. Insights from a molecular phylogeny. Molecular Phylogenetics and Evolution 46: 776–791.
Rinne, A. & P. Wiberg-Larsen, 2017. Trichoptera Larvae of Finland, Trificon Publishers, Tampere:
Scott, J. 2005. Golf ball dimples & drag. http://www.aerospaceweb.org/question/aerodynamics/q0215.shtml. Accessed 24 February 2022.
Statzner, B., J. A. Gore & V. H. Resh, 1988. Hydraulic stream ecology: observed patterns and potential applications. Journal of the North American Benthological Society 7: 307–360.
Vitecek, S., W. Graf, A. Previšić, M. Kučinić, J. Oláh, M. Bálint, L. Keresztes, S. U. Pauls & J. Waringer, 2015. A hairy case: the evolution of filtering carnivorous Drusinae (Limnephilidae, Trichoptera). Molecular Phylogenetics and Evolution 93: 249–260.
Vogel, S., 1981. Life in Moving Fluids, Willard Grant Press, Boston:
Wallace, I. D., B. Wallace & G. N. Philipson, 2003. A Key to the Case-bearing Caddis Larvae of Britain and Ireland: Freshwater Biological Association Scientific Publication 61, Freshwater Biological Association, Ambleside:
Waringer, J., 1989. Resistance of a cased caddis larva to accidental entry into the drift: the contribution of active and passive elements. Freshwater Biology 21: 411–420.
Waringer, J., 1993. The drag coefficient of cased caddis larvae from running waters: experimental determination and ecological applications. Freshwater Biology 29: 419–427.
Waringer, J. & W. Graf, 2011. Atlas of Central European Trichoptera Larvae, Erik Mauch Verlag, Dinkelscherben:
Waringer, J., S. Vitecek, J. Martini, C. Zittra, S. Handschuh, A. Vieira & H. Kuhlmann, 2020. Hydraulic stress parameters of a cased caddis larva (Drusus biguttatus) using spatio-temporally filtered velocity measurements. Hydrobiologia 847: 3437–3451.
Waringer, J., S. Vitecek, J. Martini, C. Zittra, S. Handschuh, A. Vieira & H. C. Kuhlmann, 2021. Hydraulic niche utilization by larvae of the three Drusinae clades (Insecta: Trichoptera). Biologia 76: 1465–1473.
Weissenberger, J., H.-C. Spatz, A. Emanns & J. Schwoerbel, 1991. Measurement of lift and drag forces in the mN range experienced by benthic arthropods at flow velocities below 1.2 m s-1. Freshwater Biology 25: 21–31.
White, F. M., 2011. Fluid Mechanics, 7th ed. McGraw-Hill, New York:
Wiggins, G. B., 1996. Larvae of the North American Caddisfly Genera (Trichoptera), 2nd ed. University of Toronto Press, Toronto:
Zittra, C., S. Vitecek, T. Schwaha, S. Handschuh, J. Martini, A. Vieira, H. C. Kuhlmann & J. Waringer, 2022. Comparing head muscles among Drusinae clades (Insecta: Trichoptera) reveals high congruence despite strong contrasts in head shape. Scientific Reports. https://doi.org/10.1038/s41598-022-04790-2.
Acknowledgements
We would like to thank Nikola Falk and Emil Birnstiel for helping with the field measurements–Emil was crucial for the sites in Switzerland. This paper is part of the project “Intricate bodies in the boundary layer” (Project Number P31258-B29, PIs: J. Waringer, H. C. Kuhlmann) funded by the Austrian Science Fund (FWF).
Funding
Open access funding provided by Austrian Science Fund (FWF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The authors followed all accepted principles of ethical and professional conduct that are relevant to the content of this article.
Additional information
Handling editor: Sally A. Entrekin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waringer, J., Vitecek, S., Martini, J. et al. Case design and flow resistance in high-alpine caddisfly larvae (Insecta, Trichoptera). Hydrobiologia 849, 4259–4271 (2022). https://doi.org/10.1007/s10750-022-04981-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04981-y