Abstract
Cyanobacteria periodically dominate phytoplankton composition in lakes, and produce a wide array of toxic secondary metabolites. Blooms of cyanobacteria often coincide with infections of zooplankton by microparasites (such as Metschnikowia bicuspidata, a parasitic yeast of Daphnia), and prior research has shown that cyanobacteria-based diets could mitigate fungal infections of the host. Here, we tested whether cyanotoxins could exert detrimental effects against free-living parasite stages: we inoculated two genotypes of the host Daphnia galeata × longispina with fungal spores, which were previously exposed to cyanobacterial extracts or to a placebo solution. Additionally, to test for interactive effects of cyanotoxins through environmental exposure and host consumption, Daphnia from each treatment were fed using either green algae or the same cyanobacterium. Exposing spores to cyanobacterial extracts did not reduce their infectivity; instead, parasite infectivity was increased, but only on one host genotype. The effect of host diet on parasite growth was also host-genotype dependent, with only one Daphnia genotype showing impaired spore production under a toxic diet. Our results suggest that dissolved cyanobacterial compounds released during blooms may not exert any detrimental effect on fungal spore banks, but likely influence transmission of the parasite when incorporated as part of the host’s diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are one of the most ancient organisms on Earth (Garcia-Pichel, 1998; Sánchez‐Baracaldo & Cardona, 2020). With an evolutionary origin dating back to at least 2000 Ma, their photosynthetic and nitrogen-fixing abilities have contributed to greatly affecting the Earth’s atmospheric composition and carbon cycle at a global scale (Knoll, 2008). Acting as main primary producers throughout most of the Proterozoic, cyanobacteria have since remained important—and sometimes dominant—components of phytoplankton communities within both marine and freshwater ecosystems (Sergeev et al., 2002). The seasonal ability of cyanobacteria to proliferate and dominate phytoplankton species assemblages during short periods of time is commonly referred to as ‘blooms’, which have been recorded increasingly throughout the twentieth century (Hallegraeff, 1993; Gobler, 2020) and elevated to a greater level of concern throughout the past three decades (Van Dolah et al., 2001; Moore et al., 2008; Grattan et al., 2016; Sukenik & Kaplan, 2021). In part thanks to their ancient evolutionary history, cyanobacteria exhibit a number of competitive traits, allowing them to outcompete other phytoplankton (such as green algae) when conditions are favourable. This includes their buoyancy regulation—allowing them to occupy superficial niches in the water column, thus optimizing light harvesting while shading competitors (Carey et al., 2012)—and faster growth under conditions of elevated temperatures, high concentrations of inorganic carbon (CO2) or replete nutrient availability (Mantzouki et al., 2016; Ji et al., 2017). Incidentally, such environmental conditions have been associated with contemporary ecological shifts of anthropogenic origin: human activity is causing harmful blooms to become more frequent, due to a combination of greenhouse gas effects and the eutrophication of aquatic ecosystems (Mantzouki et al., 2016), although increasing evidence show that cyanobacteria can also thrive in relatively low-nutrients environments (Reinl et al., 2021).
Besides the inherent phenology and population dynamics of cyanobacteria, their interactions with primary consumers (i.e. zooplankton) are also expected to change in a warming, increasingly eutrophic world (Benndorf & Henning, 1989; Ger et al., 2014). Cyanobacteria act as a relatively poor food source for common zooplankton taxa, such as copepods (DeMott & Moxter, 1991) or various species of cladocerans (Hanazato & Yasuno, 1987; Lundstedt & Brett, 1991; Smith & Gilbert, 1995). This is commonly imputed to low nutritional value, due to a lack of poly-unsaturated fatty acids (PUFAs) and/or sterols (DeMott & Müller-Navarra 1997; Ravet et al., 2003), in addition to their toxicity (DeMott et al., 1991). A major concern regarding the increasing occurrence of harmful algal blooms, cyanobacteria are renowned for their exceptional bioactivity and toxin production (Namikoshi & Rinehart, 1996; Huang & Zimba, 2019). Over time, cyanobacteria have evolved a variety of secondary metabolites and bioactive compounds (Welker & Von Döhren, 2006; Agha & Quesada, 2014), some of which can cause adverse effects to other organisms (including humans) and have thus been referred to as cyanotoxins (Carmichael, 1992; Chorus, 2012). These are proposed to have evolved as biochemical defences against invertebrate grazers (Ghadouani et al., 2004; Rohrlack et al., 2004; Czarnecki et al., 2006), following an evolutionary pathway akin to that of terrestrial plants (Kirk & Gilbert, 1992). Indeed, it was shown that cyanobacterial dominance can lead to seasonal decreases in zooplankton biomass (Hegg et al., 2022), while long-term exposure to high concentrations of cyanotoxins may promote the selection of more resistant species or genotypes (Wojtal-Frankiewicz et al., 2013).
In addition to their anti-grazing properties, a wider allelopathic activity was also documented in several compounds produced by cyanobacteria, reportedly efficient against algae and other bacteria (Berry et al., 2008). Associating such properties with the ancient evolutionary origin of cyanobacteria (Knoll, 2008), Sánchez et al. (2019) hypothesized that cyanotoxins could have evolved under the selective pressure of more ancestral antagonists, predating the apparition of their zooplanktonic (e.g. metazoan) grazers. Seeing as some cyanobacteria are infected by highly specialized parasites, such as fungi of the order Chytridiomycota (Canter, 1950) and viruses (i.e. cyanophages; Suttle, 2000), it is not surprising that a number of cyanobacterial secondary metabolites also display strong antifungal and antiviral properties (Volk & Furkert, 2006; Shishido et al., 2015; Marrez & Sultan, 2016). While the maintenance of cyanotoxins and other bioactive compounds may be driven by the selective pressure of such parasites (Rohrlack et al., 2013), several other hypotheses have been put forward regarding the primary biological functions of these metabolites, including: nutrient (Fe) scavenging (Utkilen & Gjølme, 1995), protection against oxidative stress (Zilliges et al., 2011), allelopathy (Schagerl et al., 2002; Sukenik et al., 2002; Leão et al., 2010) and quorum sensing (Kehr et al., 2006; Schatz et al., 2007). Given a somewhat lax binding specificity and their ability to act as inhibitors for a wide spectrum of enzymes (Teta et al., 2015), cyanotoxins could have later gained an adaptive value when competitors (prokaryotes, algae) and grazers (metazoan) emerged, thus promoting their conservation and diversification in some extant taxa.
Among the many grazers involved in the consumption of cyanobacteria in freshwater ecosystems, cladocerans of the genus Daphnia have been suggested as a potential source of control for their proliferation in eutrophic ponds and lakes (Chislock et al., 2013; Urrutia-Cordero et al., 2016). As nonselective filter feeders (DeMott, 1990), Daphnia are likely to feed on a mixed diet seston, whose composition is expected to be dominated by cyanobacterial cells during blooms (Ferrão-Filho et al., 2000). Of particular interest, in this regard, is the temporal co-occurrence of cyanobacterial blooms, in temperate lakes of the Northern hemisphere, with seasonal epidemics of the yeast Metschnikowia bicuspidata (Metschnikoff) Kamienski, a generalist parasite of Daphnia causing typical outbreaks in the late summer to early autumn (Duffy et al., 2009; Hall et al., 2011; Wolinska et al., 2011). Thus far, information on how cyanobacteria-based diets could modulate fungal infection in Daphnia have provided fairly consistent observations, often resulting in impaired parasite transmission (Penczykowski et al., 2014; Sánchez et al., 2019; Manzi et al., 2020). However, it is not always clear whether such observations stem from cyanobacteria’s poor nutritional value – leading to lowered transmission rate and/or parasite growth within the host – or rather involve effects on host immunity and possibly direct antagonistic effects on the fungus.
Considering the effects of cyanobacterial diets reported thus far in the Daphnia-Metschnikowia system, the following prospective mechanisms may be considered: (i) cyanobacteria reduce the success of infection through indirect consequences of their low nutritional value on host growth (i.e. reduced body size and filtering rate), leading to decreased rates of exposure as well as reduced parasite growth in successful infections; (ii) when incorporated as part of the host’s diet, cyanobacteria could affect functions of host immunity (similar to how toxic stress induced by blooms of marine dinoflagellates was shown to increase haemocyte activity in oysters; Hégaret & Wikfors, 2005); or (iii) by way of their suspected antifungal properties, secondary metabolites of cyanobacteria might directly interfere with ingested spores, either in the gut lumen or the haemolymph, thus acting as ‘medicines’ against the parasite. If the latter is true, antagonistic processes which usually occur at the within-host level may also be replicated outside of the host’s internal environment. To our knowledge, this prediction was not previously tested in experimental infections of Daphnia. Yet, a number of environmental contaminants (e.g. heavy metals) and persistent organic pollutants have been shown to influence the longevity and infectivity of environmental stages of parasites, particularly in aquatic ecosystems (reviewed in Pietrock & Marcogliese, 2003; Morley et al., 2006). Moreover, other external factors were proven to influence the infectivity and environmental survival of Metschnikowia, independently of later encounters with the host, such as solar radiation (Overholt et al., 2012) or extreme temperatures (Shocket et al., 2019; Duffy & Hunsberger, 2019).
To explore the possibility of direct antagonistic interactions between cyanobacteria and a fungal parasite of zooplankton, we inoculated two genotypes of the common lake hybrid Daphnia galeata × longispina with spore suspensions of the parasitic yeast Metschnikowia bicuspidata. Prior to their inoculation, fungal spores were either pre-exposed to (i) cyanobacterial extracts or (ii) a placebo solution consisting of cyanobacterial culture medium; exposing spores to extracts allowed us to test whether secondary metabolites produced by cyanobacteria could affect fungal spores outside of the host’s internal environment. In addition, to disentangle such effects from the limited nutritional value of cyanobacteria, Daphnia from each treatment were later maintained under two separate diets, one of which consisted of a high-quality, algal-based diet and the other of a mixture of a green alga and the same toxic cyanobacterium. We measured three variables influencing the overall transmission success of the parasite. Based on the putative antifungal properties of the cyanobacterium, we hypothesized that (i) prior exposure of the parasite to cyanobacterial toxins will impair the success of later infections and (ii) combining prior exposure to toxins with a toxic host diet will further reduce infection success.
Methods
Study system
Two genotypes of Daphnia galeata × longispina hybrids (AMME_12 and AMME_51) were selected from a wider collection of clones isolated from Lake Ammersee, Germany; the same two host genotypes were previously used in a life-table experiment involving mixed cyanobacterial diets, which revealed distinct clonal responses to such treatments (Manzi et al., 2020). Within the D. longispina species complex, hybrid genotypes commonly occur, and sometimes even dominate community composition in permanent lakes (Keller et al., 2008). Hybrids may also occupy intermediate habitats beyond the ecological niche of their respective progenitor species (Griebel et al., 2015; Ma et al, 2018). Daphnia were maintained in synthetic SSS culture medium (Saebelfeld et al., 2017) at 19°C, under a 12:12 light–dark photoperiod and fed three times per week with 1 mg C/l of green algae Scenedesmus obliquus (Turpin) Kützing.
The yeast Metschnikowia bicuspidata (hereafter referred to as Metschnikowia) is a generalist parasite infecting several Daphnia species (Ebert, 2005; Dallas et al., 2016). Infections of Daphnia hosts by Metschnikowia are common in nature. Epidemics typically start in late summer/early autumn (Wolinska et al., 2011) and the parasite can reach high prevalence (i.e. up to 60%) in lake Daphnia populations (Cáceres et al., 2006). Infection takes place upon ingestion of spores by hosts when filter feeding. Mature, needle-shaped spores pierce the gut wall before reaching the haemolymph (Metschnikoff, 1884). Infection symptoms become clearly visible after 9 to 10 days; at this point, the parasite’s final developmental stage (elongated asci) can be seen throughout the entire body cavity (Stewart Merrill & Cáceres, 2018). As an obligate killer, damage to the cuticle or decomposition of the host’s corpse is necessary for the parasite to be released into the environment; infective stages can then encounter new hosts, or build up as spore banks in the sediment. During this ‘environmental’ phase of the parasite’s life-cycle, spores could potentially be exposed to dissolved cyanobacterial toxins. A single M. bicuspidata strain was used (METS_AMME_2008), isolated from the same lake as the host. This strain was later propagated on lab-reared Daphnia magna Straus, 1820 (genotype E17:07) for long-term maintenance (Hesse et al., 2012). Due to its low host specificity, the parasite can be raised on D. magna—a larger host species, providing high spore outputs—and later used to infect other Daphnia species (Hesse et al., 2012; Manzi et al., 2020).
Two phytoplankton species were used as different food sources for the host: the unicellular green alga Scenedesmus obliquus (long-standing laboratory culture, used as standard food for Daphnia) and the coccoid cyanobacterium Microcystis aeruginosa (Kützing) Kützing (strain MaGr01, isolated from Greifensee, Switzerland; Tellenbach et al., 2016), one of the most common bloom-forming taxa in freshwater lakes (Reynolds & Walsby, 1975). Laboratory cultures of MaGr01 lost their colonial morphology, thus displaying an optimal shape and size range for Daphnia ingestion. This strain was confirmed to produce microcystin, with a reported concentration of 696 fg per cell (Tellenbach et al., 2016): a family of heptapeptides, microcystins are the main group of cyanobacterial toxins associated with the toxicity to vertebrates and invertebrates (Carmichael, 1992). Scenedesmus cultures were maintained in modified Z-medium (Zehnder & Gorham, 1960), under constant light exposure at 19°C. Microcystis were maintained as long-standing batch cultures in Z8 medium (Kótai, 1972), exposed to constant light at 16°C. Prior to the experiment, a subset of Microcystis cultures were brought up to the same conditions as Scenedesmus (19°C) to accelerate growth and equate the rearing temperature of Daphnia.
Experimental setup
Prior to the start of the experiment, the two D. galeata × longispina genotypes were maintained for two generations under standard conditions (19°C, 12:12 light–dark photoperiod, fed daily with 1 mg C/l of S. obliquus). 19°C is the standard rearing temperature of stock cultures in the laboratory and matches the typical August/September epilimnion temperature in Ammersee, when infection by Metschnikowia is usually first observed (Wolinska, personal observation). Individual Daphnia from two parthenogenetic lines (AMME_12/AMME_51) were used in a full factorial design, including two food sources of varying quality (Scenedesmus/Microcystis), and two infection treatments (‘METS’: spores exposed to a placebo solution/ ‘METS + Extract’: spores exposed to Microcystis extracts). Either 20 replicates (AMME_12) or 16 replicates (AMME_51) were set up for each combination of food and infection treatments, accounting for a total of 144 experimental units. All 144 jars containing individual Daphnia were inoculated with the parasite.
Experimental Daphnia were born within a 48-h time span, after which mothers were removed from the common jars (day 1). On day 4, spore suspensions of the fungal parasite Metschnikowia were prepared; to determine spore concentrations, the suspension obtained from crushed infected D. magna was homogenized and loaded twice (2 × 10 µl) on an Improved Neubauer counting chamber. Total spore yield was estimated from the number of mature spores counted in four squares of 1 × 10−4 ml capacity, across two independent loads. Two Eppendorf tubes were prepared (each containing 500 000 spores in 1 ml medium), which were either completed with a Microcystis extract (1 ml) or with a placebo solution (Z8 medium, 1 ml). Microcystis aeruginosa (strain MaGr01) were taken from an exponentially growing stock culture (OD at 750 nm = 0.181; approx. 7,500,000 cells/ml), which was then diluted (using Z8 medium) to reach an optical density of 0.09 (approx. 3 750 000 cells/ml) in 5 ml. This whole volume was briefly submerged into a solution of liquid nitrogen, to induce cell lysis (Zheng et al., 2011). The solution was then incubated at 4°C for a period of 48 h, to maximize extraction. Following this incubation period, the extracted cell content was left to thaw at room temperature for two hours and later filtrated using a GF/F glass fiber filter, to exclude any cell debris. From the resulting filtrate, 1.0 ml was added to the spore suspension used in the ‘METS + Extract’ treatment, while the ‘METS’ treatment received 1.0 ml of sterile Z8 medium (placebo solution).
Based on the level of microcystin production for strain MaGr01 assessed by Tellenbach et al. (2016) and the average absorbance / biomass ratio of Microcystis aeruginosa (Kaebernick et al., 2000), we aimed for an incubation concentration approximating 1300 µg/l of microcystin. Assuming a 50% to 80% extraction success granted by the liquid nitrogen method (Zheng et al., 2011), however, we estimated a final incubation concentration in the range of 650–1040 µg/l in the ‘METS + Extract’ treatment (for detailed calculations, see Supplementary Material). Such a concentration exceeds usual levels of microcystin observed during blooms in open water (≤ 240 µg/L, Francy et al., 2015), though higher concentrations are occasionally reported upon termination of large blooms in enclosed sections of freshwater bodies (≤ 1800 µg/L, Jones & Orr, 1994). The two spore solutions were kept at 4°C for 24 h, allowing for infective stages of the parasite to be directly exposed to cyanobacterial cell content, prior to their encounter with the host. On day 5, all experimental Daphnia were transferred to individual jars containing 5 ml of fresh culture medium. Experimental jars were then inoculated with a dose of 1000 spores/ml (i.e. 5000 spores per individual Daphnia), from either of these two suspensions. As the present protocol only allowed for a single inoculation event, this concentration was chosen to roughly equate the amount of spores previously introduced in Manzi et al., 2020 (i.e. 1250 spores/ml split across two separate events of parasite exposure). Daphnia were exposed to spore-inoculated medium for a total of two days: no food was provided on the day of inoculation, as low food supplies were shown to promote spore uptake (Hall et al., 2007); on the second exposure day, all experimental units were fed with 0.5 mg C/l of Scenedesmus.
Throughout the first six days of the experiment, all Daphnia were fed daily with 0.5 mg C/l of S. obliquus (with the exception of inoculation day, see above). On day 7, Daphnia were transferred to 15 ml of fresh medium and split into their respective diets (Scenedesmus or Microcystis). At this point in time (i.e. 48 h after inoculation), the parasite should have been able to reach and settle into the haemolymph (Stewart Merrill & Cáceres, 2018), and the further ingestion of infective stages would not be possible, due to the change in medium. In the Microcystis treatment, a food mixture was used in which Microcystis contributed 75% of the total amount of carbon, with Scenedesmus making up the remaining 25% (similarly as in Manzi et al., 2020). The correlation between optical density and carbon content for each phytoplankton taxon was established and used to prepare food suspensions accordingly. According to Ferrão-Filho et al. (2000), mixed diets are a suitable approach to estimate the toxicity of cyanobacteria, since Daphnia are likely to feed on seston of mixed origin, even in lakes temporally dominated by cyanobacteria.
Daphnia were transferred to fresh medium every 4 days. Neonates were counted and removed daily. Starting from day 12 (i.e. 7 days after parasite inoculation), all animals that died were fixed in 3.7% formaldehyde, to score them later for the presence of parasite spores. The earliest record of infection symptom in this system is day 8 post-inoculation (Manzi et al., 2020). As no further deaths were observed following 16 days after parasite inoculation, the experiment was terminated on day 21; all surviving individuals were then fixed for later inspection.
Recorded parameters
Parasite infectivity (calculated as the proportion of successfully infected Daphnia) was assessed by checking fixed animals for the presence of infection symptoms. The presence of mature spores was confirmed in suspensions obtained from crushed individuals (Nikon SMZ25 stereomicroscope, 200 × magnification). Parasite reproduction (the total number of spores accumulated upon host death, calculated individually per infected host) was estimated from a suspension of crushed infected Daphnia using a counting chamber (see Experimental setup). Age at death was recorded for each individual Daphnia that died starting from day 6 (one day after the introduction of the parasite); animals that were fixed in formaldehyde on the last experimental day were considered to have died at the age of 21 days (no natural death occurred on that day).
Data analysis
Data were analyzed using R version 4.1.0 (R Core Team, 2021). Graphical outputs were produced using the ‘ggplot2’ (Wickham, 2016) and ‘Hmisc’ (Harrell & Harrell, 2019) packages. Analysis of variance (F-test or χ2 test) was performed with the ‘car’ package (Fox et al., 2012) using type III sums-of-squares for ‘parasite infectivity’ and ‘parasite growth’, and type II for ‘host lifespan’ (as no significant interaction was detected for this variable). Model selection was then performed by a stepwise regression approach based on Akaike’s Information Criterion (AIC).
Parasite infectivity was analyzed by performing a binomial logistic regression (0 = not infected, 1 = infected) with Exposure, Diet and Clone as explanatory variables. Only those individuals, which survived until at least day 8 post-inoculation (i.e. earliest observation of fungal asci in this experiment and one previous study, Manzi et al., 2020) were considered for infectivity, as reliable detection of infection symptoms was not possible prior to that day. To control for the potential influence of host lifespan on the cumulative number of spores produced by the parasite, parasite growth was estimated as the ratio of total spore yield over the number of days survived post-inoculation. Parasite growth and host lifespan post-inoculation were analyzed using generalized linear models with Exposure, Diet and Clone as explanatory variables. Normal distribution and homoscedasticity of the residuals were verified by visual inspection of quantile–quantile plots and residuals against fitted values, respectively. Only animals which became successfully infected by the parasite were included in these analyses.
Results
Parasite infectivity
Out of 144 Daphnia exposed to Metschnikowia spores, 63 individuals died before day 8 post-inoculation (categorized as early death). As infection could only be confirmed starting from day 8 post-inoculation, these individuals were not included in the determination of parasite infectivity. Two individuals were lost due to handling error, prior to their inspection under the microscope. Among the 79 remaining individuals, 53 were confirmed infected (67%) and 26 remained uninfected.
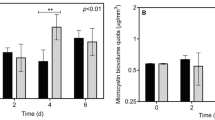
Spores which were pre-exposed to cyanobacterial toxins (‘METS + Extract’) had higher infectivity on clone AMME_51, as compared with the placebo treatment (‘METS’). However, no such effect was found on AMME_12 (significant Exposure × Clone interaction, Table 1). Specifically, pre-exposed spores led to 100% infection on clone AMME_51, while non-exposed spores infected less than 60% of this genotype (Fig. 1a). Otherwise, there was a tendency towards higher proportion of infected hosts under a Microcystis diet (Table 1, Fig. 1a).
Graphical representation of a parasite infectivity, computed as the proportion of infected hosts among those which survived long enough to allow for completion of the parasite’s life-cycle, b parasite growth, computed as the ratio of total spore yield upon host death over the number of days survived after inoculation, and c host lifespan post-inoculation, computed as the number of days survived by the host, following introduction of the parasite on day 5. Two genotypes of Daphnia galeata × longispina hybrids (AMME_12; AMME_51) were inoculated with 1000 spores/ml of the parasitic yeast Metschnikowia bicuspidata. These spore solutions were previously exposed to an extract of Microcystis aeruginosa (‘METS + Extract’), or to a placebo solution (‘METS’). Following completion of the inoculation process, individual Daphnia were then fed either with a standard diet, consisting of the green alga Scenedesmus obliquus or with a mixed diet, where the cyanobacterium Microcystis aeruginosa contributed 75% of the total carbon content. Error bars depict the standard error of the mean
Parasite growth
Pre-exposure of Metschnikowia to cyanobacterial toxins did not influence the parasite’s ability to produce spores (there were neither a main effect nor any significant interaction involving this factor, Table 1). Parasite growth was reduced by about two-fold when AMME_12 were fed with Microcystis, as compared with the Scenedesmus diet (Fig. 1b). Within clone AMME_51, however, parasite reproduction was comparable under both diets (significant Diet × Clone interaction, Table 1). In addition, the parasite’s ability to produce spores under a Microcystis diet was significantly higher on clone AMME_51 than AMME_12 (Tukey’s HSD test, P = 0.032).
Host lifespan post-inoculation
The maximum lifespan recorded among successfully infected individuals was 15 days post-inoculation. Pre-exposure of the parasite did not influence the lifespan of infected hosts (Table 1). However, those maintained on a Microcystis diet survived on average 2 days longer, compared to the standard diet (Fig. 1c).
Discussion
Given the complex evolutionary history and putative antifungal properties of cyanobacteria, we expected isolated cyanotoxins to exert direct antagonistic effects against a fungal parasite of Daphnia, possibly lowering its infection success upon later encounter with the host. While our experiment was not the first attempt to incorporate extracted cyanobacterial toxins into infection assays using the Daphnia-Metschnikowia system (Penczykowski et al., 2014; Sánchez et al., 2019), our protocol differed from previous studies by one key aspect. Specifically, the inclusion of cyanobacterial toxins was performed in such a way that would guarantee prolonged exposure of the parasite (i.e. 24 h), while limiting potential adverse effects on the host. By contrast, previous studies incorporated cyanobacterial compounds into the host’s diet, thus exposing both antagonists at the same time. This specificity allowed us to test for possible negative effects of secondary metabolites against infective stages of a fungal parasite, independently of their consumption by infected Daphnia.
Effects of parasite pre-exposure
Contrary to our expectations, pre-exposure to cyanobacterial toxins did not impair the parasite’s ability to infect in any treatment. Instead, we report a significant increase in infectivity for one of the tested Daphnia genotypes (AMME_51). While Daphnia from the ‘METS + Extract’ treatment were technically exposed to cyanobacterial toxins through the parasite inoculate (i.e. estimated concentration of microcystin in the experimental vial ranging from 0.00259 to 0.00418 µg/ml, see Supplementary Material), such a concentration remains largely inferior to the 48-h LC50 described for Daphnia hyalina Leidig, 1860 (11.6 µg/ml; DeMott et al. 1991); thus, the amount of cyanobacterial toxins introduced as part of the inoculate is unlikely to have caused direct detrimental effects on Daphnia upon exposure. However, we cannot rule out that some toxic compounds extracted from Microcystis could have adhered to the surface of fungal spores. Upon passing through the gut epithelium, lingering cyanotoxins may have thwarted the immune response of the host (i.e. mobilization of the haemocytes; Metschnikoff, 1884), thus directly acting against defence mechanisms of the host, rather than ‘enhancing’ the infectious potential of the parasite’s propagules. Although previous studies did not report such an increase in host susceptibility, the apparent lack of detrimental effect on the parasite remains consistent with the results of Penczykowski et al. (2014): the authors artificially coated green algal cells with cyanobacterial extracts, showing no reduction in either the transmission rate or spore yield of Metschnikowia.
Effects of the Microcystis-based diet
Based on previous results using this system, we expected a two- to three-fold reduction in parasite reproduction, when the host is maintained under a sustained Microcystis diet (Manzi et al., 2020). Surprisingly, this effect only applied to infections of the Daphnia genotype AMME_12. In genotype AMME_51, the Microcystis diet allowed the parasite to produce spores at a rate comparable to that of the high-quality diet. These types of host-genotype-by-environment (GH × E) interactions affecting parasitic infections are commonly reported in the Daphnia system, meaning that the outcome of infection can depend on environmental conditions, in a genotype-specific way (Wolinska & King, 2009). For instance, it was found that the infectivity and virulence of the bacterium Pasteuria ramosa Metschnikoff, 1888 would vary interactively between clonal identity of the host and either nutrition (Mitchell & Read, 2005; Little et al., 2007) or temperature (Mitchell et al., 2005; Little et al., 2007). Previously, we observed that the reduction in parasite reproduction induced by a Microcystis diet applied to both Daphnia genotypes, but was stronger on AMME_12 than AMME_51 (Manzi et al. 2020). While the disparity was more pronounced here, this reflects a similar interaction and thus remains consistent with our previous findings.
Additionally, we observed that a Microcystis-based diet allowed infected hosts to survive slightly longer than the Scenedesmus treatment (by about two days). A similar increase in the longevity of infected hosts was previously observed on a Microcystis-based diet, although this was found exclusively at 23°C (Manzi et al., 2020). Paradoxically, what could be interpreted as a protective effect for the host might in fact increase the parasite’s transmission success: in spite of a less-nutritious diet, the parasite can benefit from an extended infection time, allowing it to accumulate a higher spore yield upon host death; a paradox that has long been described as the virulence-transmission trade-off (Anderson & May, 1982; Acevedo et al., 2019). Because Microcystis strongly reduced the rate of spore production in AMME_12 hosts, the small increase in host longevity granted by this diet was not sufficient to outcompete spore outputs in the Scenedesmus treatment (Figure S1). By contrast, the mitigated effects of a toxic diet on AMME_51 allowed the parasite to perform equally under both levels of food quality (Figure S2).
Microcystis has been reported to induce feeding inhibition in cladocerans (even when integrated as low as 5% of a mixed diet), which may be imputed in part to behavioural avoidance of toxic cells (Ferrão-Filho et al., 2000) or diet-induced reduction of the host’s body size, leading to lower filtering rates (Penczykowski et al., 2014). In the latter study, a drastic reduction in the infection rate of Metschnikowia was attributed to both behavioural and size-related effects. In our experimental assay, we found an opposite trend, with systematically higher proportion of infected hosts under a Microcystis diet, although this tendency was not statistically significant (P = 0.08). However, this was expected, due to a different layout in our experimental design: because the Microcystis diet was only introduced after parasite exposure, any potential effect of food quality on parasite encounter per se can be ruled out. Thus, within the context of our study, the Microcystis diet could only have influenced within-host processes involved in the parasite’s infectivity, such as its ability to overcome the host’s immune response. Our results thereby suggest that maintaining the host on a toxic diet throughout the progression of the parasite’s development cycle does not impair the parasite’s infectivity. Instead, the greatest consequence of a poor diet under natural conditions should consist of limited exposure to the parasite, due to behavioural and/or physiological decreases in feeding rates, constraining the overall success of infection (Ferrão-Filho et al., 2000; Penczykowski et al., 2014).
Environmental relevance
When cyanobacteria are incorporated as part of Daphnia’s diet, endotoxins are delivered upon digestion of bacterial cells, during their passage through the gut lumen (Carmichael, 1992). Considering that Microcystis compounds are capable of binding to (thus inhibiting) digestive proteases within the gut lumen of Daphnia (Agrawal et al., 2005; von Elert et al., 2012), in the context of parasitic infections, the digested cell content of cyanobacteria may also get in contact with ingested spores. Therefore, cyanobacteria are likely to interact with spores of horizontally transmitted parasites, when both are consumed by the host and concurrently passing through the host’s gut.
Throughout the course of harmful algal blooms, cell lysis of dominant cyanobacteria results in the release of large amounts of (otherwise intracellular) bioactive compounds in the water column (Jones & Orr, 1994; Ha et al., 2009). The levels of cyanotoxins released during blooms have been remarked to approximate half the LC50 for Daphnia (DeMott et al., 1991; Sánchez et al., 2019), with concentrations of microcystins reportedly ranging from < 0.10 to 240 µg/l in freshwater lakes and recreational sites (Francy et al., 2015). Upon termination of a large bloom within enclosed water bodies, particularly high concentrations of microcystins (1300–1800 µg/l) have been noted to persist for up to nine days, prior to a rapid phase of degradation (Jones & Orr, 1994). Thus, such concentrations of dissolved compounds may be high enough for cyanobacteria to exert adverse effects on cladoceran hosts, independently of their consumption as live cells (Ibelings et al., 2005). As far as cyanobacterial toxins directly interfering with free-floating spores or spore banks in the sediment, however, the likelihood of such event is more difficult to infer. On the one hand, some waterborne parasites (e.g. fish helminths) are able to bioconcentrate chemicals and pollutants at a much higher rate than free-living species, even when these are present at very low concentrations in the environment (Sures, 2003; Nachev & Sures, 2016). However, it can be argued that cyanobacteria and parasite spore banks normally occupy different layers in the stratified water column. While cyanobacteria’s high buoyancy allows them to maintain close proximity to the surface, spores of the fungal parasite Metschnikowia are commonly recruited from the sediment, as infected hosts tend to sink to the bottom upon their death (Cáceres et al., 2006; Duffy & Hunsberger, 2019). Thus, by filter-feeding onto higher parts of the water column, cladocerans may sequestrate large amounts of dissolved cyanobacterial toxins and prevent those from reaching the sediment.
While our results did not provide support for our starting hypothesis (i.e. that the putative antifungal effects of Microcystis aeruginosa may not be limited to ‘medicinal effects’ mediated by diet but could instead directly interfere with the parasite, prior to their ingestion by Daphnia hosts), the specificity of our experimental design allowed us to raise the question of the parasite’s vulnerability to an environmental stress of its own. Considering that outbreaks of this parasite typically occur for the span of a few months (i.e. from late summer to early winter) in temperate lakes, infective propagules contributing to the next seasonal epidemic will remain for considerable amounts of time buried in the sediment (Decaestecker et al., 2004). In temporary ponds susceptible to draining, spore banks of the parasite can even survive extended periods of complete host absence, such as during the dry seasons (Ebert, 2005). This effectively means that most of the parasite’s lifespan (i.e. from release into the environment, to completion of a new reproductive cycle) is effectively spent outside of the host’s internal environment, where infective propagules of parasites may be prone to a number of environmental disturbances (Pietrock & Marcogliese, 2003; Sures et al, 2017). Specifically, resting stages of Metschnikowia were previously shown to degrade or decrease in infectivity, following their exposure to solar UV (Overholt et al., 2012), high temperatures (Shocket et al., 2019) or near-freezing conditions (Duffy & Hunsberger, 2019). Such studies demonstrate that the transmission potential of this parasite can indeed be compromised by external environmental factors acting prior to—and independently of—its encounter with the host. Our results suggest, however, that exposure to dissolved cyanobacterial toxins during blooms does not constitute one such mechanism. While cyanobacterial dominance may not directly interfere with the infectivity of fungal spore banks, other parasites of cladocerans belonging to distant taxa, such as the endospore-forming bacterium Pasteuria ramosa (Ebert et al., 1996) or the viral agent for White Fat Cell Disease (Toenshoff et al., 2018) may be worthy of investigation, in light of the wider allelopathic properties of cyanobacteria.
Data availability
The dataset supporting this study can be found at: https://doi.org/10.5281/zenodo.5841637.
References
Acevedo, M. A., F. P. Dillemuth, A. J. Flick, M. J. Faldyn & B. D. Elderd, 2019. Virulence-driven trade-offs in disease transmission: a meta-analysis. Evolution 73: 636–647.
Agha, R. & A. Quesada, 2014. Oligopeptides as biomarkers of cyanobacterial subpopulations. Toward an understanding of their biological role. Toxins 6: 1929–1950.
Agrawal, M. K., A. Zitt, D. Bagchi, J. Weckesser, S. N. Bagchi & E. von Elert, 2005. Characterization of proteases in guts of Daphnia magna and their inhibition by Microcystis aeruginosa PCC 7806. Environmental Toxicology: an International Journal 20: 314–322.
Anderson, R. & R. May, 1982. Coevolution of hosts and parasites. Parasitology 85: 411–426.
Benndorf, J. & M. Henning, 1989. Daphnia and toxic blooms of Microcystis aeruginosa in Bautzen Reservoir (GDR). Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 74: 233–248.
Berry, J. P., M. Gantar, M. H. Perez, G. Berry & F. G. Noriega, 2008. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Marine Drugs 6: 117–146.
Cáceres, C. E., S. R. Hall, M. A. Duffy, A. J. Tessier, C. Helmle & S. MacIntyre, 2006. Physical structure of lakes constrains epidemics in Daphnia populations. Ecology 87: 1438–1444.
Canter, H. M., 1950. Fungal parasites of the phytoplankton. I (Studies on British chytrids, X). Annals of Botany 14: 263–289. http://www.jstor.org/stable/42906609.
Carey, C. C., B. W. Ibelings, E. P. Hoffmann, D. P. Hamilton & J. D. Brookes, 2012. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Research 46: 1394–1407.
Carmichael, W. W., 1992. Cyanobacteria secondary metabolites: the cyanotoxins. Journal of Applied Bacteriology 72: 445–459.
Chislock, M. F., O. Sarnelle, L. M. Jernigan & A. E. Wilson, 2013. Do high concentrations of microcystin prevent Daphnia control of phytoplankton? Water Research 47: 1961–1970.
Chorus, I., 2012. Cyanotoxins: occurrence, causes, consequences. Springer Science & Business Media.
Czarnecki, O., M. Henning, I. Lippert & M. Welker, 2006. Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environmental Microbiology 8: 77–87.
Dallas, T., M. Holtackers & J. M. Drake, 2016. Costs of resistance and infection by a generalist pathogen. Ecology and Evolution 6: 1737–1744.
Decaestecker, E., C. Lefever, L. De Meester & D. Ebert, 2004. Haunted by the past: evidence for dormant stage banks of microparasites and epibionts of Daphnia. Limnology and Oceanography 49: 1355–1364.
DeMott, W. R., 1990. Retention efficiency, perceptual bias, and active choice as mechanisms of food selection by suspension-feeding zooplankton. In Behavioural Mechanisms of Food Selection (pp. 569–594). Springer, Berlin, Heidelberg.
DeMott, W. R. & F. Moxter, 1991. Foraging cyanobacteria by copepods: responses to chemical defense and resource abundance. Ecology 72: 1820–1834.
DeMott, W. R. & D. C. Müller-Navarra, 1997. The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshwater Biology 38: 649–664.
DeMott, W. R., Q. X. Zhang & W. W. Carmichael, 1991. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnology and Oceanography 36: 1346–1357.
Duffy, M. A. & K. K. Hunsberger, 2019. Infectivity is influenced by parasite spore age and exposure to freezing: do shallow waters provide Daphnia a refuge from some parasites? Journal of Plankton Research 41: 12–16.
Duffy, M. A., S. R. Hall, C. E. Cáceres & A. R. Ives, 2009. Rapid evolution, seasonality, and the termination of parasite epidemics. Ecology 90: 1441–1448.
Ebert, D., 2005. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. National Library of Medicine.
Ebert, D., P. Rainey, T. M. Embley & D. Scholz, 1996. Development, life cycle, ultrastructure and phylogenetic position of Pasteuria ramosa Metchnikoff 1888: rediscovery of an obligate endoparasite of Daphnia magna Straus. Philosophical Transactions of the Royal Society of London Series b: Biological Sciences 351: 1689–1701.
Ferrão-Filho, A. S., S. M. Azevedo & W. R. DeMott, 2000. Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshwater Biology 45: 1–19.
Fox, J., S. Weisberg, D. Adler, D. Bates, G. Baud-Bovy, S. Ellison, et al., 2012. Package ‘car,’ R Foundation for Statistical Computing, Vienna:
Francy, D. S., J. L. Graham, E. A. Stelzer, C. D. Ecker, A. M. G. Brady, P. Struffolino & K. A. Loftin, 2015. Water quality, cyanobacteria, and environmental factors and their relations to microcystin concentrations for use in predictive models at Ohio Lake Erie and inland lake recreational sites, 2013–14: U.S. Geological Survey Scientific Investigations Report 2015–5120, 58 p.
Garcia-Pichel, F., 1998. Solar ultraviolet and the evolutionary history of cyanobacteria. Origins of Life and Evolution of the Biosphere 28: 321–347.
Ger, K. A., L. A. Hansson & M. Lürling, 2014. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biology 59: 1783–1798.
Ghadouani, A., B. Pinel-Alloul, K. Plath, G. A. Codd & W. Lampert, 2004. Effects of Microcystis aeruginosa and purified microcystin-LR on the feeding behavior of Daphnia pulicaria. Limnology and Oceanography 49: 666–679.
Gobler, C. J., 2020. Climate change and harmful algal blooms: insights and perspective. Harmful Algae 91: 101731.
Grattan, L. M., S. Holobaugh & J. G. Morris Jr., 2016. Harmful algal blooms and public health. Harmful Algae 57: 2–8.
Griebel, J., S. Gießler, M. Poxleitner, A. N. Faria, M. Yin & J. Wolinska, 2015. Extreme environments facilitate hybrid superiority – the story of a successful Daphnia galeata × longispina hybrid clone. PLoS One 10: e0140275.
Ha, J. H., T. Hidaka & H. Tsuno, 2009. Quantification of toxic Microcystis and evaluation of its dominance ratio in blooms using real-time PCR. Environmental Science & Technology 43: 812–818.
Hall, S. R., L. Sivars-Becker, C. Becker, M. A. Duffy, A. J. Tessier & C. E. Cáceres, 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecology Letters 10: 207–218.
Hall, S. R., C. R. Becker, M. A. Duffy & C. E. Cáceres, 2011. Epidemic size determines population-level effects of fungal parasites on Daphnia hosts. Oecologia 166: 833–842.
Hallegraeff, G. M., 1993. A review of harmful algae blooms and their apparent global increase. Phycologia 32: 79–99.
Hanazato, T. & M. Yasuno, 1987. Evaluation of Microcystis as food for zooplankton in a eutrophic lake. Hydrobiologia 144: 251–259.
Harrell, F. E. Jr & M. F. E Jr Harrell, 2019. Package ‘Hmisc’. CRAN2018, 235–6.
Hégaret, H. & G. H. Wikfors, 2005. Effects of natural and field-simulated blooms of the dinoflagellate Prorocentrum minimum upon hemocytes of eastern oysters, Crassostrea virginica, from two different populations. Harmful Algae 4: 201–209.
Hegg, A., R. Radersma & T. Uller, 2022. Seasonal variation in the response to a toxin-producing cyanobacteria in Daphnia. Freshwater Biology. https://doi.org/10.1111/fwb.13899.
Hesse, O., W. Engelbrecht, C. Laforsch & J. Wolinska, 2012. Fighting parasites and predators: how to deal with multiple threats? BMC Ecology 12: 12.
Huang, I. S. & P. V. Zimba, 2019. Cyanobacterial bioactive metabolites – a review of their chemistry and biology. Harmful Algae 83: 42–94.
Ibelings, B. W., K. Bruning, J. De Jonge, K. Wolfstein, L. D. Pires, J. Postma & T. Burger, 2005. Distribution of microcystins in a lake foodweb: no evidence for biomagnification. Microbial Ecology 49: 487–500.
Ji, X., J. M. Verspagen, M. Stomp & J. Huisman, 2017. Competition between cyanobacteria and green algae at low versus elevated CO2: who will win, and why? Journal of Experimental Botany 68: 3815–3828.
Jones, G. J. & P. T. Orr, 1994. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Research 28: 871–876.
Kaebernick, M., B. A. Neilan, T. Börner & E. Dittmann, 2000. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Applied and Environmental Microbiology 66: 3387–3392.
Kehr, J. C., Y. Zilliges, A. Springer, M. D. Disney, D. D. Ratner, C. Bouchier, P. H. Seeberger, N. Tandeau De Marsac & E. Dittmann, 2006. A mannan binding lectin is involved in cell–cell attachment in a toxic strain of Microcystis aeruginosa. Molecular Microbiology 59: 893–906.
Keller, B., J. Wolinska, M. Manca & P. Spaak, 2008. Spatial, environmental and anthropogenic effects on the taxon composition of hybridizing Daphnia. Philosophical Transactions of the Royal Society b: Biological Sciences 363: 2943–2952.
Kirk, K. L. & J. J. Gilbert, 1992. Variation in herbivore response to chemical defenses: zooplankton foraging on toxic cyanobacteria. Ecology 73: 2208–2217.
Knoll, A. H., 2008. Cyanobacteria and earth history. In Herrero, A. & E. Flores (eds), The Cyanobacteria: Molecular Biology, Genomics, and Evolution Caister Academic Press, Norfolk: 484.
Kótai, J., 1972. Instructions for preparation of modified nutrient solution Z8 for algae. Norwegian Institute for Water Research, Oslo 11: 5.
Leão, P. N., A. R. Pereira, W. T. Liu, J. Ng, P. A. Pevzner, P. C. Dorrestein, G. M. König, V. M. Vasconselos & W. H. Gerwick, 2010. Synergistic allelochemicals from a freshwater cyanobacterium. Proceedings of the National Academy of Sciences 107: 11183–11188.
Little, T., J. Birch, P. Vale & M. Tseng, 2007. Parasite transgenerational effects on infection. Evolutionary Ecology Research 9: 459–469. https://www.evolutionary-ecology.com/abstracts/v09/2135.html
Lundstedt, L. & M. T. Brett, 1991. Differential growth rates of three cladoceran species in response to mono-and mixed-algal cultures. Limnology and Oceanography 36: 159–165.
Ma, X., W. Hu, P. Smilauer, M. Yin & J. Wolinska, 2018. Daphnia galeata and D. dentifera are geographically and ecologically separated whereas their hybrids occur in intermediate habitats: survey of 44 Chinese lakes. Molecular Ecology 28: 785–802.
Mantzouki, E., P. M. Visser, M. Bormans & B. W. Ibelings, 2016. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquatic Ecology 50: 333–350.
Manzi, F., R. Agha, Y. Lu, F. Ben-Ami & J. Wolinska, 2020. Temperature and host diet jointly influence the outcome of infection in a Daphnia-fungal parasite system. Freshwater Biology 65: 757–767.
Marrez, D. A. & Y. Y. Sultan, 2016. Antifungal activity of the cyanobacterium Microcystis aeruginosa against mycotoxigenic fungi. Journal of Applied Pharmaceutical Science 6: 191–198.
Metschnikoff, E., 1884. A disease of Daphnia caused by a yeast. A contribution to the theory of phagocytes as agents for attack on disease-causing organisms. Archiv Für Pathologische Anatomie Und Physiologie Und Für Klinische Medicin 96: 177–195.
Mitchell, S. E. & A. F. Read, 2005. Poor maternal environment enhances offspring disease resistance in an invertebrate. Proceedings of the Royal Society of London b: Biological Sciences 272: 2601–2607.
Mitchell, S. E., E. S. Rogers, T. J. Little & A. F. Read, 2005. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59: 70–80.
Moore, S. K., V. L. Trainer, N. J. Mantua, M. S. Parker, E. A. Laws, L. C. Backer & L. E. Fleming, 2008. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environmental Health 7(2): 1–12.
Morley, N. J., J. W. Lewis & D. Hoole, 2006. Pollutant-induced effects on immunological and physiological interactions in aquatic host–trematode systems: implications for parasite transmission. Journal of Helminthology 80: 137–149.
Nachev, M. & B. Sures, 2016. Environmental parasitology: Parasites as accumulation bioindicators in the marine environment. Journal of Sea Research 113: 45–50.
Namikoshi, M. & K. L. Rinehart, 1996. Bioactive compounds produced by cyanobacteria. Journal of Industrial Microbiology 17: 373–384.
Overholt, E. P., S. R. Hall, C. E. Williamson, C. K. Meikle, M. A. Duffy & C. E. Cáceres, 2012. Solar radiation decreases parasitism in Daphnia. Ecology Letters 15: 47–54.
Penczykowski, R. M., B. C. Lemanski, R. D. Sieg, S. R. Hall, J. Housley Ochs, J. Kubanek & M. A. Duffy, 2014. Poor resource quality lowers transmission potential by changing foraging behaviour. Functional Ecology 28: 1245–1255.
Pietrock, M. & D. J. Marcogliese, 2003. Free-living endohelminth stages: at the mercy of environmental conditions. Trends in Parasitology 19: 293–299.
R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ravet, J. L., M. T. Brett & D. C. Müller-Navarra, 2003. A test of the role of polyunsaturated fatty acids in phytoplankton food quality for Daphnia using liposome supplementation. Limnology and Oceanography 48: 1938–1947.
Reinl, K. L., J. D. Brookes, C. C. Carey, T. D. Harris, B. W. Ibelings, A. M. Morales-Williams, L. M. De Senerpont Domis, K. S. Atkins, P. D. F. Isles, J. P. Mesman, R. L. North, L. G. Rudstam, J. A. A. Stelzer, J. J. Venkiteswaran, K. Yokota & Q. Zhan, 2021. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshwater Biology 66: 1846–1859.
Reynolds, C. S. & A. E. Walsby, 1975. Water-blooms. Biological Reviews 50: 437–481.
Rohrlack, T., K. Christoffersen, M. Kaebernick & B. A. Neilan, 2004. Cyanobacterial protease inhibitor microviridin J causes a lethal molting disruption in Daphnia pulicaria. Applied and Environmental Microbiology 70: 5047–5050.
Rohrlack, T., G. Christiansen & R. Kurmayer, 2013. A putative anti-parasite defensive system in the cyanobacterium Planktothrix involving ribosomal and non-ribosomal oligopeptides. Applied and Environmental Microbiology 79: 2642–2647.
Saebelfeld, M., L. Minguez, J. Griebel, M. O. Gessner & J. Wolinska, 2017. Humic dissolved organic carbon drives oxidative stress and severe fitness impairments in Daphnia. Aquatic Toxicology 182: 31–38.
Sánchez, K. F., N. Huntley, M. A. Duffy & M. D. Hunter, 2019. Toxins or medicines? Phytoplankton diets mediate host and parasite fitness in a freshwater system. Proceedings of the Royal Society B 286: 20182231.
Sánchez-Baracaldo, P. & T. Cardona, 2020. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytologist 225: 1440–1446.
Schagerl, M., I. Unterrieder & D. G. Angeler, 2002. Allelopathy among cyanoprokaryota and other algae originating from Lake Neusiedlersee (Austria). International Review of Hydrobiology: A Journal Covering All Aspects of Limnology and Marine Biology 87: 365–374.
Schatz, D., Y. Keren, A. Vardi, A. Sukenik, S. Carmeli, T. Börner, E. Dittmann & A. Kaplan, 2007. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environmental Microbiology 9: 965–970.
Sergeev, V. N., L. M. Gerasimenko & G. A. Zavarzin, 2002. The proterozoic history and present state of cyanobacteria. Microbiology 71: 623–637.
Shishido, T. K., A. Humisto, J. Jokela, L. Liu, M. Wahlsten, A. Tamrakar, D. P. Fewer, P. Permi, A. P. D. Andreote, M. F. Fiore & K. Sivonen, 2015. Antifungal compounds from cyanobacteria. Marine Drugs 13: 2124–2140.
Shocket, M. S., A. Magnante, M. A. Duffy, C. E. Cáceres & S. R. Hall, 2019. Can hot temperatures limit disease transmission? A test of mechanisms in a zooplankton–fungus system. Functional Ecology 33: 2017–2029.
Smith, A. D. & J. J. Gilbert, 1995. Relative susceptibilities of rotifers and cladocerans to Microcystis aeruginosa. Archiv Für Hydrobiologie 132: 309–336.
Stewart Merrill, T. E. & C. E. Cáceres, 2018. Within‐host complexity of a plankton‐parasite interaction. Ecology 99: 2864–2867. https://www.jstor.org/stable/26627163
Sukenik, A. & A. Kaplan, 2021. Cyanobacterial harmful algal blooms in aquatic ecosystems: a comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 9: 1472.
Sukenik, A., R. Eshkol, A. Livne, O. Hadas, M. Rom, D. Tchernov, A. Vardi & A. Kaplan, 2002. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): a novel allelopathic mechanism. Limnology and Oceanography 47: 1656–1663.
Sures, B., 2003. Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology 126: S53–S60.
Sures, B., M. Nachev, C. Selbach & D. J. Marcogliese, 2017. Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology.’ Parasites & Vectors 10: 1–19.
Suttle, C. A., 2000. Cyanophages and their role in the ecology of cyanobacteria. In Whitton, B. A. & M. Potts (eds), The Ecology of Cyanobacteria Springer, Dordrecht: 563–589.
Tellenbach, C., N. Tardent, F. Pomati, B. Keller, N. G. Hairston, J. Wolinska & P. Spaak, 2016. Cyanobacteria facilitate parasite epidemics in Daphnia. Ecology 97: 3422–3432.
Teta, R., G. Della Sala, E. Glukhov, L. Gerwick, W. H. Gerwick, A. Mangoni & V. Costantino, 2015. Combined LC–MS/MS and molecular networking approach reveals new cyanotoxins from the 2014 cyanobacterial bloom in Green Lake, Seattle. Environmental Science & Technology 49: 14301–14310.
Toenshoff, E. R., P. D. Fields, Y. X. Bourgeois & D. Ebert, 2018. The end of a 60-year riddle: identification and genomic characterization of an iridovirus, the causative agent of white fat cell disease in zooplankton. G3: Genes. Genomes, Genetics 8: 1259–1272.
Urrutia-Cordero, P., M. K. Ekvall & L. A. Hansson, 2016. Controlling harmful cyanobacteria: taxa-specific responses of cyanobacteria to grazing by large-bodied Daphnia in a biomanipulation scenario. PLoS One 11: e0153032.
Utkilen, H. & N. Gjølme, 1995. Iron-stimulated toxin production in Microcystis aeruginosa. Applied and Environmental Microbiology 61: 797–800.
Van Dolah, F. M., D. Roelke & R. M. Greene, 2001. Health and ecological impacts of harmful algal blooms: risk assessment needs. Human and Ecological Risk Assessment: an International Journal 7: 1329–1345.
Volk, R. B. & F. H. Furkert, 2006. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiological Research 161: 180–186.
von Elert, E., A. Zitt & A. Schwarzenberger, 2012. Inducible tolerance to dietary protease inhibitors in Daphnia magna. Journal of Experimental Biology 215: 2051–2059.
Welker, M. & H. Von Döhren, 2006. Cyanobacterial peptides – nature’s own combinatorial biosynthesis. FEMS Microbiology Reviews 30: 530–563.
Wickham, H., 2016. ggplot2: Elegant graphics for data analysis, Springer-Verlag, New York, NY:
Wojtal-Frankiewicz, A., J. Bernasińska, T. Jurczak, K. Gwoździński, P. Frankiewicz & M. Wielanek, 2013. Microcystin assimilation and detoxification by Daphnia spp. in two ecosystems of different cyanotoxin concentrations. Journal of Limnology 72: 154–171.
Wolinska, J. & K. C. King, 2009. Environment can alter selection in host–parasite interactions. Trends in Parasitology 25: 236–244.
Wolinska, J., J. Seda, H. Koerner, P. Smilauer & A. Petrusek, 2011. Spatial variation of Daphnia parasite load within individual water bodies. Journal of Plankton Research 33: 1284–1294.
Zehnder, A. & P. R. Gorham, 1960. Factors influencing the growth of Microcystis aeruginosa Kütz. Emend. Elenkin. Canadian Journal of Microbiology 6: 645–660.
Zheng, H., J. Yin, Z. Gao, H. Huang, X. Ji & C. Dou, 2011. Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Applied Biochemistry and Biotechnology 164: 1215–1224.
Zilliges, Y., J. C. Kehr, S. Meissner, K. Ishida, S. Mikkat, M. Hagemann, A. Kaplan, T. Börner & E. Dittmann, 2011. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 6: e17615.
Acknowledgements
We would like to thank Ursula Newen for the maintenance of Daphnia cultures and Francesco Pomati for providing us with the Microcystis strain.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the German Science Foundation (WO 1587/8-1).
Author information
Authors and Affiliations
Contributions
FM, MM, JW and RA conceptualized the study. FM and MM performed the experiment, data analysis and results visualization. FM wrote the article, with help from RA, JW and MM. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Luigi Naselli-Flores
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manzi, F., Agha, R., Mühlenhaupt, M. et al. Prior exposure of a fungal parasite to cyanobacterial extracts does not impair infection of its Daphnia host. Hydrobiologia 849, 2731–2744 (2022). https://doi.org/10.1007/s10750-022-04889-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04889-7