Abstract
In this study, polymorphism in behaviour, morphology, and stable isotope signatures of burbot from Lake Southern Konnevesi, Finland, Europe, was examined. First, local knowledge was collected on exceptional polymorphism of the spawning behaviour and morphology of burbot. These phenomena were then studied based on catch samples. Interviews and catch sample analyses suggested two morphs of burbot: one morph spawning in late February in the littoral zone and other in late March, in deep profundal, depths of about 30 m. Fish caught from the profundal zone had higher average proportional somatic body weights and wider heads than those caught from the littoral spawning sites in February. The length-at-age of the individuals from the littoral catch was longer than that of the profundal catch. Stable isotope analysis revealed differences in the mean carbon isotope ratio between sampling sites, suggesting differences in diet. Variability in several of the aforementioned variables was correlated. This study is the first to imply polymorphism in burbot populations from a lake in Eurasia, where burbot are considered as near-threatened species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymorphism refers to the presence of individuals of two or more phenotypic forms in a biological population, which are potentially able to mate randomly, at a given time and within an area. It can occur, e.g. in anatomical, behavioural, and life-history traits. It has been found in many freshwater fish populations, for example, several morphs of Arctic charr and whitefish within a postglacial lake (Klemetsen, 2013; Thomas et al., 2019). Phenotypic polymorphism may be due to hereditable genetic variability or environmental factors, and the effect of these factors may be difficult to distinguish. For example, feeding differences may be due to intra-lake differences in food resources or inherited behavioural traits. Thus, differences in behaviour may or may not be genetically determined (Magnhagen, 2012). Eventually, polymorphism of traits related to the probability of mating between individuals can lead to sympatric speciation.
Polymorphism is an important phenomenon that needs to be understood to better protect the species at the population level. Several advantageous consequences have been suggested to stem from polymorphism in traits within a population. Occupation of several different niches enables the utilization of a greater diversity of resources and reduces intraspecific competition. Polymorphism may also enhance population stability and persistence, colonization success, range expansions, evolutionary potential, and speciation (Forsman et al., 2008; Schindler et al., 2010; Wennersten & Forsman, 2012; Bernatchez, 2016; Forsman, 2016).
Burbot [Lota lota (Linnaeus, 1758)] is a freshwater gadoid fish with circumpolar distribution. It resides in lakes, rivers, and coastal brackish waters with well-oxygenated cold-water habitats available all year round. Interestingly, in North American lakes, this species exhibits considerable polymorphism, from two parapatric subspecies L. l. lota and L. l. maculosa (Lesueur, 1817) on a continental scale to within-lake variability in reproduction, with individuals spawning in shallow, deep profundal, and riverine sites. The spawning time also varies considerably from mid-winter, under the ice, to the open water season (Elmer et al., 2008, 2012; Underwood et al., 2016; Blumstein et al., 2018). Such variability can be important for resilience during global warming, as ice conditions, and consequently, the phenology of events are changing rapidly (Sharma et al., 2016; Korhonen, 2019).
In Eurasia, intra-lake polymorphism in burbot behaviour has not been reported. However, burbot are known to spawn in the deep profundal zone, to depths of more than 40 m in alpine lakes, at the southern edge of their distribution (Hirning, 2006; ref. Probst, 2008). Conversely, shallow littoral spawning has been documented in regularly frozen lakes, in the northern part of their distribution, and only in mid-winter (e.g. Lehtonen, 1998). Exceptionally, in Lake Southern Konnevesi in Central Finland, fishermen have traditional and experience-based local knowledge of two different types of burbot in terms of spawning time and location. Additional information on the polymorphism of burbot populations may prove significant for the protection of its populations as burbot abundance has shown a decreasing trend worldwide (Stapanian et al., 2009), including Finland, where they have been recently classified as a near-threatened species in the IUCN Red List (Hyvärinen et al. 2019). Currently, it is routinely assumed that individuals of burbot within one lake form one population; however, genetically distinct subpopulations with different phenological characteristics worth preserving may exist in reality.
The aim of this study was (i) to collect local knowledge on burbot polymorphism by interviewing experienced fishermen (ii) to assess polymorphism by comparing the timing of gonadal development, morphometrics, and stable isotope ratios from catch samples collected from traditionally known littoral and profundal fishing grounds.
Materials and methods
Study area
Lake Southern Konnevesi (hereafter Konnevesi) is located in central Finland (62° 40ʹ N, 26° 30ʹ E). It is the central lake of the Rautalampi watercourse, which is the north-eastern tributary of the Kemijoki catchment area. The lake is geologically young and glacial in origin (a more detailed description in Karjalainen et al., 2022). Konnevesi covers an area of 120 km2 with mean and maximum depths of 12.5 m and 56 m, respectively. The lake is oligotrophic and has a low colour content (15–25 mg Pt l–1). The total phosphorus concentration in winter is 6 µg l–1 (data from the observation station Konnevesi 64, March, depth 25 m, open data, www.syke.fi/avointieto). As far as the records show, Konnevesi is completely frozen every winter, typically in December, but varying from November to January in different years. Ice break-up typically occurs in the first half of May, and sometimes at the end of April. In recent decades, the period of ice cover has been decreasing.
Local knowledge
The survey sought to find Konnevesi fishermen who were experienced in specifically targeting burbot from deep profundal waters during winter. Four fishermen—two individual fishermen and two that were fishing together—were found and interviewed to obtain information on the winter behaviour of burbot from Lake Southern Konnevesi. Each individual has been fishing in Konnevesi for several decades, and their traditional knowledge of fish and fisheries is dated to the 1930s and beyond. The interviews were not structured; however, the fishermen were encouraged to share their experiences on burbot spawning times and locations and any specific features related to burbot appearance and behaviour.
Sampling
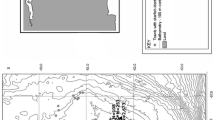
Burbot were sampled from four locations on Konnevesi, from the catch of one of the fishermen interviewed. He used self-designed two-throated wire trap nets at each site. Catches from the littoral zone (bottom depth < 10 m) were collected from three sites: L1 Lanstunlahti, L2 Pienilahti, and L3 Hömpänlahti (Fig. 1). These sites were known to be shallow water spawning grounds in February. Samples were collected on 15 and 22 February, 2015.
Samples from the catch in the deep profundal zone (depth of approximately 30 m) were taken at site P1: Häntiäisselkä on February 26 and March 8, 2015. From this site, ripe burbot are caught up to the end of March. The fisherman also recorded the spawning peak (mainly assessed by the highest proportion of catch with eggs and milt running) in the years 2015–2019. Littoral sites were located approximately 1 km (L1) to 3 km (L2, L3) from the profundal site.
The sampled individuals (Table 1) were selected visually from the catch to cover the length distribution of the catch as evenly as possible. At every site, most of the catch consisted of males. The sample from the profundal site P1 consisted of somewhat older fish than those from littoral samples.
Preparation, measurements, and analyses
Samples were stored in a freezer (– 20 °C) and thawed for 12–15 h in a cold-water tank prior to laboratory examination. The following morphometric variables were recorded:
-
Total length (TL), accuracy of 1 mm
-
Standard length (TL—caudal fin length), accuracy of 1 mm
-
Length of the dorsal fin, accuracy of 1 mm
-
The shortest distance between upper edges of eyes, accuracy of 0.1 mm
-
Length from the mid-edge of the snout to the right eye, accuracy of 0.1 mm
-
Length from the snout to the first spine of the dorsal fin, accuracy of 0.1 mm
-
Length from the snout to the pelvic spine, accuracy of 1 mm
-
Wet weight, accuracy of 0.1 g
-
Somatic weight (weight without internal organs)
-
Gonad weight, accuracy of 0.1 g
-
Liver weight, accuracy of 0.1 g.
The fish were photographed prior to examinations for potential further scrutiny.
The body cavity was opened, the internal organs (intestine, heart, liver, pancreas, and gonads) were removed, and the fish was weighed. Sex was determined macroscopically from the gonads, and the state of maturity was assessed using the Kesteven (1960) scale, modified for burbot:
-
Immature (Kesteven 1–2) gonads not fully developed for spawning during this season
-
Developing (Kesteven 3–4) gonads, eggs visible, milt or eggs do not run when pressed gently
-
Ripe/spawning (Kesteven 5–7) gonads, fully developed, milt or eggs do not run when gonads are pressed gently, milt or eggs run when pressed gently
-
Spent (Kesteven 8) gonads emptied, remains of milt or eggs visible.
Age was determined from the otoliths (sagittae). Otoliths were cut in half by pressing them from the centre with the blunt side of a knife blade against the palm. The cutting surface was sanded with very fine sandpaper to reveal the middle cross-section. Otoliths were then stained for 15 min in a neutral red solution (e.g. Raitaniemi et al., 2000; Easey & Millner, 2008). Age was determined by counting annual rings from the prepared otolith cross-section with a stereomicroscope. Two people independently determined and compared the age of the fish. If they differed, counting was repeated until a consensus was reached. Age was coded with the assumption that the fish was born on the first of January. Thus, for example, a 5 + fish in its sixth year was coded as 6 years old.
The stable isotope ratios of carbon (13C/12C) and nitrogen (15N/14N) were measured. A small sample of white muscle tissue, from a depth of 1 cm, was cut from the side of the fish, just below the anterior dorsal fin. Samples were stored frozen (– 20 °C) in sealed glass tubes and later dried with Christ, alpha 1–4 LD plus freeze-dryer for approximately 14 h. Samples were pulverized and a 0.55–0.65 mg sample was weighed into a tin foil cup. Samples were analysed using a stable isotope mass spectrometer (Thermo Finnigan DeltaPlus Advantage) connected to an elemental analyser (Flash EA 1112). Results were expressed using the standard delta (δ) notation as parts per thousand (‰) difference from the international standard. Internal standards of known relation to the international standards of Vienna Pee Dee belemnite (for carbon) and atmospheric N2 (for nitrogen) were used as reference material. The precision was always better than 0.2‰ based on the standard deviation of the replicates of the internal standards. Sample analysis also yielded the percentage of carbon and nitrogen from which C/N ratios (by weight) were derived.
Statistical analyses
Data were sorted by sample site and zone (littoral/profundal). The differences in distributions of the state of maturity between littoral profundal samples were analysed using a χ2-test.
For different morphometric variables, comparisons were first made between sites using analysis of covariance (ANCOVA) or analysis of variance (ANOVA). Site was used as a factor, total length as a covariate, and sex as factor if the effect was statistically significant (P < 0.05). If the factor sample site had a significant effect on the dependent variable, pairwise comparisons between the sites were made using the least significant difference (LSD) test. Second, nested ANOVA/ANCOVA with the zone (littoral or profundal) as a fixed factor and site within a zone as a random factor was applied to assess whether the differences between sites were associated significantly to these zones.
Effects of the variables on stable isotope ratios (δ) were analysed using the same principle as the morphometric data.
In all comparisons, the effects of length and sex on the dependent variable was controlled, if significant. Importantly, Log10-transformation was applied to both the covariate length and, where appropriate, the dependent variable (mentioned in the results) to ensure a linear relationship between the covariate and the dependent variable and the to meet the requirements for the distribution of the residuals. The relationship between the covariate length (L) and any weight-dimension-dependent variables, W (fish weight or liver weight), was routinely assumed to be a power function, and thus linearized by the log-transformation of both variables as:
Ratio-based indices (e.g. Fulton’s condition index and hepatosomatic index) were not used in the analysis because the relationship between the weight-dimension-dependent variable and total length as a covariate cannot be routinely assumed to be isometric, i.e. to obey the cube law, which is: b = 3 (e.g. Froese, 2006).
Differences in length-at-age were analysed using the von Bertalanffy (1938) growth model with indicator (dummy) variables in two phases, due to the high number of parameters to be estimated vs. number of observations. Differences between pooled littoral (sites L1–L3 combined) and the profundal samples were analysed using the following model:
where La = total length (mm); L∞ = mean asymptotic length-at-age ∞; ILP = parameter for the profundal site effect on L∞; Prof = dichotomous indicator variable, 0 for littoral and 1 for profundal fish; ILS = parameter for the effect of sex on L∞; Sex = dichotomous indicator variable, 0 = female, 1 = male; K = growth coefficient; IKP = parameter for the effect of profundal on K; IKS = parameter for the effect of sex on K; Age = age in years, coded as mentioned above; t0 = imaginary age when L = 0. For simplicity, the effect of the profundal site and sex was assumed to be independent (no interaction).
Parameters of the growth model and their confidence intervals were estimated using an iterative least squares regression. Both the dependent variable La and the model were log-transformed to correspond to the general requirements for the residuals. The fitting of the model was performed in a way where the parameters L∞, K, and t0 were always included in the model; however, additional parameters were added stepwise by forward selection starting from the one where the fit of the model improved the most significantly. The next most significant was then added if the addition had a significant effect on fit. The significance of the fit improvement was assessed by the F-test (P < 0.05) between models with and without the new variable. Additionally, when the parsimonious model was reached, residuals (predicted—observed values) were calculated for all littoral site fish. Residuals were then compared between littoral sites (ANOVA) to assess whether size-at-age differed between different littoral sites.
The association of variability, the covariation between two variables that showed significant differences between sites, was robustly analysed using the Spearman correlation coefficient (ρ). First, the effect of length and sex was modelled if significant (ANCOVA), and the model residuals were calculated. These residuals contain variability that was not dependent on length or sex. The ρ and its P-value for all pairs of residual variables were then calculated.
Results
Local knowledge
Burbot polymorphism was first brought to the attention of researchers by a local fisherman. This survey found four fishermen that actively targeted burbot from deep profundal areas, while fishing in the winter. Interviews with them established the unanimous view that early- and shallow-spawning, mature burbot occurred in nearshore littoral trap nets at depths of approximately one to a few metres in February. The catch per unit effort typically peaked in the second half of February, when spawning occurred, i.e. eggs and milt run when the belly of the fish was lightly pressed. About a week later, the catch per unit effort in the littoral zone dropped to virtually zero.
The interviewed fishermen fished for burbot in the profundal zone with special vertically set yarn or wire trap nets, typically one month later. Some of the fishermen also used gillnets. Catches of ripe/spawning individuals peaked in the second half of March. They fished in three different profundal ‘hot spots’ within different basins. These sites were located on steep slopes at a depth of approximately 30 m in the basins with the maximum depth of more than 40 m, and were characterized by solid clayish substrates. According to one of the fishermen, the ‘hot spots’ were small and narrow in depth. If a trap net was set in a position 5 m shallower or deeper than 30 m, horizontally by only a few tens of metres from the ‘hot spot’, the catch was practically zero. To their knowledge, local people were already aware of the profundal ‘hot spots’ of the late winter spawning burbot, at least in the 1930s or perhaps before.
One of the fishermen had noticed that the late profundal-spawning individuals were different in shape from the littoral fish, i.e. the profundal fish were more ‘robust’, more reminiscent of ‘Baltic Sea burbot’ than the shallow-spawning fish (Baltic Sea burbot are known to be heavier relative to their length than lake burbot, Gottberg, 1910). He further noted that fish caught in the profundal site in March were less afflicted with parasites than those caught in the littoral sites in February. They had a much lower prevalence of cataracts, caused by eyeflukes (Diplostomum sp.) and lower numbers of liver cysts (Triaenophorus, Cestoda).
Immediately after the break-up of ice and before the impact of waves, he observed car tire-sized round dips at the depth of 0.5–1 m on sandy beaches near his littoral fishing sites. He regarded them as ‘burbot nests’ where littoral spawners released their eggs.
Catch samples
Phenology of the stage of maturity
Ripe/spawning fish were observed at all sites (Fig. 2). The timing of gonadal maturation between the littoral and profundal sites differed. At profundal site P1 on February 26, 2015, the fish were typically at an earlier stage of maturity than the fish that were sampled earlier at the littoral site, despite a later sampling time (littoral samples combined, immatures excluded, χ2-test, P < 0.05).
Distribution of sexual maturity of burbot in different sampling sites in Lake Southern Konnevesi, using the modified Kesteven (1960) scale. L littoral, P profundal
In the later profundal sample taken on March 8, 2015 a significantly higher proportion of the fish were ripe/spawning than in the earlier (difference between profundal samples, χ2-test, P < 0.03), approximately at the same stage as that from the littoral catch 2–3 weeks before. According to the catch monitoring data by a local fisherman, the peak in the proportion of spawning fish, eggs and milt running easily, occurred at profundal site P1 in the last third of March 2015, and in later years also, about a month later than that in littoral sites. The peak at littoral sites occurred in late February.
Morphology
Statistically significant differences in morphological variables were found between sites when effects of total length and sex were controlled, when necessary (Table 2). First, the logarithmic somatic body weight at the profundal site was higher than that at any littoral site (Fig. 3A, Table 2). No significant differences were found between littoral sites. In the nested analysis, the difference between the littoral and profundal zones was significant. Second, the distance between the eyes was longer for individuals at the profundal site. (Fig. 3B, Table 2). In pairwise comparisons between sites, all P < 0.064 for the profundal site P1 compared to any littoral site. No significant differences were found between the littoral sites. In the nested model, the difference between the zones was significant. Third, the logarithmic distance from the snout to the right eye differed between fishes from different sites (Fig. 3C, Table 2) and was found to be significantly longer for those at the profundal site P1 than those at L2 and L3. In the nested model, the differences were, however, not significantly associated with the zones. Finally, the logarithmic weight of the liver of fishes at the profundal site P1 was significantly higher than those at L2 and L3 (Fig. 3D, Table 2). Sex influenced liver weight, with the geometric mean liver weight being 24% smaller for females than that for males. No significant differences in the liver weight were found between the littoral sites. The zone had a marginally (P < 0.1) significant effect on the liver weight in the nested model.
Phenotypic characteristics of selected Lake Konnevesi burbot samples from different sites. A The geometric mean of somatic body weight, the weight without internal organs, standardized for the total length of 370 mm; B the distance between the eyes, standardized for the total length of 379 mm; C the geometric mean length, from the mid snout to the right eye, standardized for 379 mm total length; and D the geometric mean liver weight, standardized for the total length of 370 mm. P profundal site, L littoral site. Vertical lines = standard error, numbers above x-axis indicate P-value for differences between the littoral sites and the profundal site
No significant differences between sites (all P > 0.2) were found in the other morphological variables tested (see list of all variables in the Materials and Methods).
Stable isotope and elemental ratios
Significant differences in the carbon isotope ratio (δ13C) were found between the sites (Fig. 4, Table 2). The δ13C in the profundal site was significantly lower than that in any littoral site. Littoral site L3 differed significantly from the other two littoral sites. However, according to the nested model, the differences were not significant between the profundal and littoral zones.
Observed and mean (vertical and horizontal lines = s.e.) C and N isotope ratios. For C, the geometric mean, s.e., assumed of log-normal distribution, not standardized for length. For N, the arithmetic mean, standardized for the length of 373 cm. The numbers above the x-axis indicate the P-value for differences between the littoral sites and the profundal site for the δ13C values, and the numbers on the y-axis denote the P-values for the δ15N values
Between-site differences were also found in the nitrogen isotope ratio (δ14N) (Fig. 4, Table 2) with the profundal site differing from the littoral site L3 only, but not from other littoral sites. Littoral site L3 also differed from L2. There was no difference between zones.
No significant differences were found in the carbon-to-nitrogen ratio (C/N) between the sites.
Size-at-age
Growth analysis using the von Bertalanffy Model (Fig. 5, Table 3) revealed a significant difference in size-at-age between the pooled littoral site samples and the profundal site (F-test, P = 0.003), compared to the model without the indicator variable for the effect of profundal site on L∞ (indicator variable Prof: 0 = littoral, 1 = profundal; parameter ILP). The estimate of the parameter ILP was negative, and the asymptotic length was smaller at the profundal site. No significant differences (ANOVA, P = 0.31) were found between littoral sites in the residuals from this model.
Association between variables
A significant association of residual variability was found between several variables after the removal of any significant effect of length and/or sex, but not those of the sampling site or the zone (Table 4). For example, the residual of high somatic weight was associated with that of a large head, high liver weight, low δ13C, and small size-at-age, thus indicating slow growth.
Discussion
The results of the catch sample analysis suggested that behavioural polymorphism occurred in Lake Konnevesi burbot between different sampling sites. This conclusion was consistent with the local knowledge of the fishermen about at least two distinct burbot spawning peaks—the first in late February and the second in late March.
Four fishermen from Konnevesi deliberately targeted burbot at the deep (about 30 m) profundal sites during the ice-covered seasons. However, there may well be more, as the total number of people engaged in fishing in Konnevesi is at least several hundred, and burbot is their common catch. In 2003–2004, the annual burbot yield of Konnevesi was approximately four tonnes (Valkeajärvi & Salo, 2006). Winter fishing for spawning burbot in littoral areas by angling, baited hooks, and trap nets was common in Konnevesi, as in other Finnish lakes. Gillnetting (for recreational, household, and commercial use) is allowed and common in Finland. However, gillnetting often targets pike and pikeperch during winters, and therefore focuses on their overwintering areas, with the highest catch per unit effort typically occurring at depths of less than 20 m. Therefore, any kind of recreational fishing in deeper areas is quite rare during the winter. This traditional Finnish fishing behaviour makes it understandable that previously documented knowledge (Lehtonen, 1973a, 1998 and references therein) on the spawning of burbot in Finnish lakes and Baltic Sea coastal waters mentions only littoral spawning. It occurs typically in January in southernmost Finland, in February in most parts of Finland, the latitudes of Lake Konnevesi included, or in early March in northern Finland. Globally, we did not find any literature regarding several spawning peaks within a single lake anywhere outside of North America. In the Laurentian Great Lakes of North America, considerable intra-lake polymorphism in spawning time from winter to spring and summer spawning occurs (Jude et al., 2013) indicating high flexibility in the reproduction behaviour of this species. The Great Lakes are of the same age as that of the Northern European lakes but have a completely different size and are more habitually diverse. This may explain why several morphs have evolved in the Great Lakes. On the other hand, European burbot may be more polymorphic than that reported because local knowledge has not been systematically collected so far. Burbot sampling in winter has also not been comprehensive in different habitats.
Notably, actual spawning in the deep profundal slopes of Konnevesi has not yet been confirmed by visual observations or by the discovery of egg deposits, despite attempts to use underwater cameras with infrared light and sediment sampling by the authors, in recent years. In principle, late maturing, profundal-residing fish can migrate to other locations for spawning, even to the littoral zone. In Northern Europe, the hatching of burbot larvae occurs around the break-up of ice, typically in early May (Lehtonen, 1998). In larval sampling for coregonid fishes, burbot larvae are regularly obtained as by-catch, in both littoral and pelagic areas in Konnevesi and other Finnish lakes (Karjalainen et al., 1998 and own unpublished data). The embryonic development of burbot requires approximately 100–120 degree days (Műller, 1960). In winter, under ice, the surface water temperature in both pelagic and littoral areas is typically below 1 °C; however, it is 1–3 °C warmer in the deep profundal bottom waters (Valkeajärvi, 1988; own observations; and for more detailed under-ice temperature dynamics see Pulkkanen, 2013). Spawning burbot should lay their eggs in warmer profundal waters in late winter to obtain the required degree days for hatching, assuming that the timing of hatching also matches with the break-up of ice. Interestingly, in large pre-alpine lakes that typically do not have ice cover in winter, e.g. at Lake Constance in central Europe (Franssen & Scherrer, 2008), burbot do indeed spawn in the deep profundal zone to depths of 40–120 m (Hirning, 2006; ref. Probst, 2008) instead of the littoral zone. On the other hand, considerable intra-lake polymorphism has been found at spawning site selection in the Laurentian Great Lakes. There the sites range from the littoral to deep profundal (Jude et al., 2013).
Our morphological measurements implied morphological polymorphism between the sampling sites. Some of the polymorphism was clearly associated with the zones (littoral and profundal) from where the samples were caught. As suggested, individuals caught in the profundal zone proved, on average, to be stouter, relatively more muscular, with relatively wider heads than those from the littoral catch. Marginally significant polymorphism was also observed in proportional liver weight, which is known to remain fairly constant or even diminish during the winter period (Lehtonen, 1973a; Pulliainen & Korhonen, 1990). Contrary to this expectation, fish caught later from the profundal site had heavier livers. Differences in some variables were found not only between littoral and profundal sites, but also between different littoral sites. Polymorphism may be multi-dimensional, in addition to the dimension littoral–profundal. There is also a considerable probability that spuriously significant differences emerge in the ANOVA/ANCOVA comparing each site to all others. These differences should be considered preliminary and should be confirmed/rejected with more extensive sampling.
Notably, all morphological variables were controlled based on fish size and sex, if necessary. Therefore, these results were not artefacts due to differences in size and sex distribution between sites. We are not aware of any previous observations of anatomical/morphological polymorphism, other than sexual, in burbot within a lake, or between lakes outside North America (Fisher et al., 1996; McPhail & Paragamian, 2000; Elmer et al., 2008; Recknagel et al., 2014). With respect to sexual dimorphism, our observations were inconsistent with those reported by Cott et al. (2013), who found that males had smaller livers than those of females.
The ratio of stable carbon isotopes differed between sites but not significantly between zones. The stable isotope signatures do not only depend on feeding but also on the geographic locations that determine the base-line level. Both the δ13C and δ15N values in the burbot, from site L3, were clearly different from those at other sites, which may be due to the fact that this site is within a different basin. This renders it difficult to assess the genuine effect of zone on the results.
Overall, the lower average δ13C value in the profundal site individuals compared to those in any littoral site, suggest that the profundal-residing fish relied more on the pelagic autochthonous food web in their feeding than the burbot sampled from the littoral sites (Vander Zanden & Rasmussen, 1999; Syväranta et al., 2006). The differences in spawning times or sites, within the basin do not imply differences in feeding habitats and habits; however, the carbon isotope ratio contains information on long-term feeding. In addition, differences in size-at-age between littoral and profundal-residing fish, supports the conclusion that the Konnevesi burbot are polymorphic in terms of food and/or habitat temperature.
The nitrogen isotope ratio is known to increase by approximately 3 ‰ between trophic levels in the food web (DeNiro & Epstein, 1981; Minagawa & Wada, 1984; Peterson & Fry, 1987a, b). No differences in δ15N within the same sub-basin (P1, L1, and L2) were observed between the profundal and littoral samples, suggesting that the fish were fed at the same trophic level.
Burbot in Finnish lakes have been suggested to change their feeding habitat and food between the seasons (e.g. Lehtonen, 1998; Pääkkönen, 2000), with adult individuals residing in cool deep profundal waters during the warmest months, and more widely at different depths during the cooler season. However, our understanding of the feeding niche of burbot may be biased. Sampling in feeding studies has usually been targeted at habitats where burbot are assumed to reside most likely in different seasons. Therefore, sampling during the open water season has concentrated mostly on deep profundal. A high critical maximum temperature, a high optimum temperature for metabolism, and the ability to feed at higher temperatures, however, enables burbot to succeed in a variety of environments in the summer (Pääkkönen et al., 2003). A more comprehensive and detailed understanding of burbot feeding biology, including habitat and food selection, requires a spatiotemporally more systematic stomach sampling approach in the future.
One issue with the data from this study is that some of the fish residing in the littoral zone in February, might have migrated into the deep profundal zone later in the spring, and vice versa. Thus, we must not assume that littoral and profundal samples originated from completely separate subpopulations. Some sample fish collected from the profundal site may have recently resided in the littoral zone. However, in this preliminary study, we did not select the sampled fish on the basis of sexual maturity before analysis, but analysed samples in their original state. Burbot disappear from the littoral zone by the beginning of March at the latest (own observations; Gottberg, 1910; Lehtonen, 1973b) to depths of more than 10–15 m (Lehtonen, 1973b).
Valkeajärvi (1983) found that Carlin-tagged burbot in Konnevesi moved several kilometres across the basin and into other basins from the original tagging and release sites (tagged at a littoral spawning site in February). Other studies (e.g. Koops, 1959; Tesch, 1967) have confirmed a wide range of movement in burbot. Harrison et al. (2015) showed spatiotemporally consistent personality-dependent space use in burbot, from ‘resident’ to ‘mobile’, and including seasonal plasticity in spatial behaviour. Hardy et al. (2015) demonstrated adaptive plasticity in habitat selection. Despite uncontrolled and partial spatial mixing, clear differences in the average characteristics of the fish between the littoral and profundal sites were observed in Konnevesi. Therefore, the results can be considered reliable and robust. In addition, several traits of fish were associated with each other, which also suggests that the Konnevesi burbot do not form a single population in which different traits are randomly distributed. Both behaviour and morphological traits have genuine polymorphism that manifests as a correlated suite, at the individual level. The individual-level association should be further investigated in more detail, using advanced multivariate methods with larger datasets.
In principle, plasticity associated with ontogenic development of burbot could explain the phenomenon of older, late spawning fish residing in the profundal zone in late winter: young fish could tend to spawn in early winter in the littoral zone and when older, in late winter in the profundal zone. According to Gottberg (1910), the spawning time of burbot in Finland depends on size; larger individuals spawn later, but this has not been reported by recent studies (Lehtonen 1973a, b, 1998; Vainikka & Heikkilä, 2005). In Konnevesi, the fact that profundal-sampled fish were, on average, slower growing and morphometrically different than littoral-sampled fish, suggests that typical profundal fish have different life-cycle tactics and behaviours than those of littoral fish for a considerably long period of their life. These differences cannot emerge simply because of an ontogenic niche shift within a homogenous population. Analysis of parasites living in fish for several years could provide additional information on multi-year isolation of fish (e.g. Huxham et al., 1995). One fisherman noticed a difference in cataracts between littoral and profundal fish. The cataract causing Diplostomum eyeflukes accumulate for many years and are transmitted to the fish from littoral snails (Chappell, 1995). Their numbers thus correlate with the tendency of fish to reside in the littoral zone. Therefore, parasites in burbot should be studied further. Mark-recapture and telemetry studies can also provide additional information on the seasonal movements of burbot individuals.
Phenotypic polymorphism may arise within a genotype due to environmental factors, or it may be due to genetic polymorphism. If individuals are not plastic in their spawning behaviour from year to year but have spawning site fidelity and fixed phenology, genetic isolation between morphs may develop rapidly, leading to even rapid speciation, despite the fact that in other life stages i.e. newly hatched larvae, they would reside in the same area. Speciation due to spawning site segregation has recently been observed, for example, in flounder from the Baltic Sea (Momigliano et al., 2017). Despite its wide home range (Valkeajärvi, 1983), the burbot of Konnevesi may very well be philopatric and tend to be faithful to their spawning site. Hedin (1983) has shown that along the Baltic Sea coast burbot return to their home rivers to spawn. Significant genetic diversity in burbot has recently been observed to occur at different spatial scales from different water bodies to within a water body (Barluenga et al., 2006; Elmer et al., 2008; Underwood et al., 2016; Blumstein et al., 2018; Wetjen et al., 2019).
To conclude, both local knowledge and data suggest behavioural and morphological polymorphism in burbot from Konnevesi. It appears that there are at least two burbot morphs with different spawning behaviour, both temporally and likely spatially, associated with differences in morphology and perhaps feeding. The protection of harvested species requires an understanding of polymorphism, particularly for the protection of genetic diversity of populations (e.g. Blumstein et al., 2018). A deeper understanding of polymorphism in spatio-temporal traits of reproductive behaviour is required for biologically sustainable fisheries management. The protection of habitats against effects of eutrophication and consequently, reduced oxygen levels, for example, are necessary to fully preserve the diversity within fish populations. Spatio-temporal polymorphism in reproduction may prove crucial for resilience against environmental stressors (sensu portfolio effect by Schindler et al. (2010)), and the consequences of climate change, which is rapidly changing the length of winters and the timing of ice phenomena in boreal lakes. Therefore, further studies of genetic polymorphism in burbot within lakes are needed. The potential occurrence of reproductive polymorphism also needs to be studies on a wider geographical scale. To this end, the systematic collection of local knowledge should not be ignored, as traditional local knowledge regarding spawning sites and times is abundantly available and can cover much wider spatial and temporal scales than any scientific assessments can ever.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Barluenga, M., M. Sanetra & A. Meyer, 2006. Genetic admixture of burbot (Teleostei: Lota lota) in Lake Constance from two European glacial refugia. Molecular Ecology 12: 3583–3600.
Bernatchez, L., 2016. On the maintenance of genetic variation and adaptation to environmental change: considerations from population genomics in fishes. Journal of Fish Biology 89: 2519–2556.
Blumstein, D. M., D. Mays & K. T. Scribner, 2018. Spatial genetic structure and recruitment dynamics of burbot (Lota lota) in Eastern Lake Michigan and Michigan tributaries. Journal of Great Lakes Research 44: 149–1656.
Chappell, L. H., 1995. The biology of diplostomatid eyeflukes of fishes. Journal of Helminthology 69: 97–101.
Cott, P. A., T. A. Johnston & J. M. Gunn, 2013. Sexual dimorphism in an under-ice spawning fish: The burbot (Lota lota). Canadian Journal of Zoology 91: 732–740.
DeNiro, M. J. & S. Epstein, 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica Et Cosmochimica Acta 45: 341–353.
Easey, M. W. & R. S. Millner, 2008. Improved methods for the preparation and staining of thin sections of fish otoliths for age determination. Cefas Science Serie Technical Report 143: 1–14.
Elmer, K. R., H. Recknagel, A. Thompson & A. Meyer, 2012. Asymmetric admixture and morphological variability at a suture zone: parapatric burbot subspecies (Pisces) in the Mackenzie River basin, Canada. Hydrobiologia 683: 217–229.
Elmer, K. R., J. K. J. Van Houdt, A. Meyer & F. A. M. Volcklaert, 2008. Population genetic structure of North American burbot (Lota lota maculosa) across the Nearctic and its contact zone with Eurasian burbot (Lota lota lota). Canadian Journal of Fisheries and Aquatic Sciences 65: 2412–2426.
Fisher, S. J., D. W. Willis & K. L. Pope, 1996. An assessment of burbot (Lota lota) weight–length data from North American populations. Canadian Journal of Zoology 74: 570–575.
Franssen, H. J. & S. C. Scherrer, 2008. Freesing of lakes on the Swiss plateau in the period 1901–2006. International Journal of Climatology 28: 421–433.
Forsman, A., 2016. Is colour polymorphism advantageous to populations and species? Molecular Ecology 25: 2693–2698.
Forsman, A., J. Ahnesjö, S. Caesar & M. Karlsson, 2008. A model of ecological and evolutionary consequences of color polymorphism. Ecology 89: 34–40.
Froese, R., 2006. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. Journal of Applied Ichthyology 22: 241–253.
Gottberg, G., 1910. Havaintoja mateen kasvusta, kudusta ja ravinnosta vesissämme. Suomen Kalastuslehti 19: 113–120 (in Finnish).
Hardy, R. S., S. M. Stephenson, M. D. Neufeld & S. P. Young, 2015. Adaptation of lake-origin burbot stocked into a large river environment. Hydrobiologia 757: 35–47.
Harrison, P. M., L. F. G. Gutowsky, E. G. Martins, D. A. Patterson, S. J. Cooke & M. Power, 2015. Personality-dependent spatial ecology occurs independently from dispersal in wild burbot (Lota lota). Behavioral Ecology 26: 483–492.
Hedin, J., 1983. Seasonal spawning migrations of the burbot (Lota lota L.) in a coastal stream of the northern Bothnian Sea. Fauna Norrlandica 6: 1–9.
Hirning, M., 2006. Laichgebiete und Laichwanderverhalten von Trüschen (Lota lota) im Bodensee. Magisterarbeit, Universität Konstanz. Ref. Probst 2008.
Huxham, M., D. Raffaelli & A. Pike, 1995. Parasites and food web patterns. Journal of Animal Ecology 64: 168–176.
Hyvärinen, E., A. Juslén, E. Kemppainen, A. Uddström & U.-M. Liukko (eds), 2019. The 2019 red list of Finnish species. Ympäristöministeriö & Suomen ympäristökeskus. Helsinki.
Jude, D. J., Y. Wang, S. Hensler & J. Janssen, 2013. Burbot early life history strategies in the Great Lakes. Transactions of the American Fisheries Society 142: 1733–1745.
Karjalainen, J., R. Sjövik, T. Väänänen, T. Sävilammi, L.-R. Sundberg, S. Uusi-Heikkilä & T. J. Marjomäki, 2022. Genetic-based evaluation of management units for sustainable vendace (Coregonus albula) fisheries in a large lake system. Fisheries Research 246: 106173.
Karjalainen, J., S. Ollikainen, S. Staff, M. Viljanen & P. Väisänen, 1998. Puruveden kalanpoikasyhteisöt: koostumus ja ravinnonkäyttö [Larval fish communities in Lake Puruvesi: species composition and diet]. Karjalan Tutkimuslaitoksen Julkaisuja - University of Joensuu, Publications of Karelian Institute 122: 52–55 (in Finnish).
Kesteven, G. L. (ed.), 1960. Manual of field methods in fisheries biology. FAO Manuals in Fisheries Science 1: 1–152.
Klemetsen, A., 2013. The most variable vertebrate on Earth. Journal of Ichthyology 53: 781–791.
Koops, H., 1959. The burbot population of the Elbe. Investigation of the commercial significance of the burbot (Lote lota L.) with reference to the dam at Geesthacht, now in progress. Kurze Mitteilungen aus dem Institut fűr Fischereibiologie der Universität Hamburg 9: 2–61.
Korhonen, J., 2019. Long-term changes and variability of the winter and spring season hydrological regime in Finland. University of Helsinki Report Series in Geophysics 679: 1–82.
Lehtonen, H., 1973a. Mateen biologiasta Suonteenjärvessä ja Tvärminnessä. Luonnon Tutkija 77(3–4): 91–100 (in Finnish).
Lehtonen, H., 1973b. Lämpötilan ja syvyyden vaikutus mateen olinpaikkoihin. Kalamies 1973(9): 3 (in Finnish).
Lehtonen, H., 1998. Winter biology of burbot (Lota lota L.). Memoranda Societatis pro Fauna et Flora Fennica 7: 45–52.
McPhail, J. D. & V. L. Paragamian, 2000. Burbot biology and life history. Transactions of the American Fisheries Society 128: 11–23.
Magnhagen, C., 2012. Personalities in a crowd: what shapes the behavior of Eurasian perch and other shoaling fishes. Current Zoology 58: 35–44.
Minagawa, M. & E. Wada, 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48: 1135–1140.
Momigliano, P., H. Jokinen, A. Fraimout, A.-B. Florin, A. Norkko & J. Merilä, 2017. Extraordinarily rapid speciation in a marine fish. Proceedings of the National Academy of Sciences of the United States of America 114: 6074–6079.
Műller, W., 1960. Beiträge zur Biologie der Quappe (Lota lota L.) nach Untersuchungen in den Gewässern zwischen Elbe und Oder. Zeitschrift fűr Fischerei 9: 1–72 (in German).
Pääkkönen, J.-P., 2000. Feeding biology of burbot: adaptation to profundal lifestyle? Jyväskylä Studies in Biological and Environmental Science 87: 1–33.
Pääkkönen, J.-P., O. Tikkanen & J. Karjalainen, 2003. Development and validation of a bioenergetics model for juvenile and adult burbot. Journal of Fish Biology 63: 956–969.
Peterson, B. J. & B. Fry, 1987a. Stable isotopes in ecosystem studies. Annual Review of Ecology, Evolution and Systematics 18: 293–320.
Peterson, B. J. & B. Fry, 1987b. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Probst, W. N., 2008. New insight into the ecology of perch Perca fluviatilis L. and Lota lota (L.) with special focus on their pelagic life-history. Doctoral dissertation. Universität Konstantz.
Pulkkanen, M., 2013. Under-ice temperature and oxygen conditions in boreal lakes. Jyväskylä Studies in Biological and Environmental Science 256: 1–39.
Pulliainen, E. & K. Korhonen, 1990. Seasonal changes in condition indices in adult mature and non-maturing burbot, Lota lota (L.), in the north-eastern Bothnian Bay, northern Finland. Journal of Fish Biology 36: 251–259.
Raitaniemi, J, K. Nyberg & I. Torvi, 2000. Kalojen iän ja kasvun määritys. Riistan- ja kalantutkimus, Helsinki. http://urn.fi/URN:NBN:fi-fe2017111550717
Recknagel, H., A. Amos & K. R. Elmer, 2014. Morphological and ecological variation among populations and subspecies of burbot (Lota lota [L, 1758]) from the Mackenzie River Delta, Canada. Canadian Field-Naturalist 128: 377–384.
Schindler, D., R. Hilborn, B. Chasco, C. P. Boatright, T. P. Quinn, L. A. Rogers & M. S. Webster, 2010. Population diversity and the portfolio effect in an exploited species. Nature 465: 609–612.
Sharma, S., J. J. Magnuson, R. D. Batt, L. A. Winslow, J. Korhonen & Y. Aono, 2016. Direct observations of ice seasonality reveal changes in climate over the past 320–570 years. Scientific Reports 6: 25061.
Stapanian, M. A., V. L. Paragamian, C. P. Madenijan, J. R. Jackson, J. Lappalainen, M. J. Evenson & M. D. Neufeld, 2009. Worldwide status of burbot and conservation measures. Fish and Fisheries 11: 34–56.
Syväranta, J., H. Hämäläinen & R. J. Jones, 2006. Within-lake variability in carbon and nitrogen stable isotope signatures. Freshwater Biology 51: 1090–1102.
Tesch, F. W., 1967. Activity and behaviour of migrating Lampetra fluviatilis, Lota lota and Anguilla Anguilla in tidel area of river Elbe. Helgoländer Wissenschaftliche Meeresuntersuchungen 16: 92–111.
Thomas, S., M. J. Kaintz, P.-A. Amundsen, B. Hayden, S. Taipale & K. K. Kahilainen, 2019. Resource polymorphism in European whitefish: Analysis of fatty acid profiles provides more detailed evidence than traditional methods alone. PLOS ONE 14(8): e0221338.
Underwood, Z. E., E. G. Mandeville & A. W. Walters, 2016. Population connectivity and genetic structure of burbot (Lota lota) populations in the Wind River Basin, Wyoming. Hydrobiologia 765: 329–342.
Vainikka, A. & J. J. Heikkilä, 2005. Mateen pilkintää Pohjois-Päijänteellä 1999–2005. Suomen Kalastuslehti 3: 16–18.
Valkeajärvi, P., 1983. Ahvenen (Perca fluviatilis (L.)), hauen (Esox lucius L.), mateen (Lota lota (L.)), siian (Coregonus lavaretus L. s.1.) ja särjen (Rutilus rutilus (L.)) liikkuvuus, kasvu ja kuolevuus Konnevedessä merkintöjen mukaan. Jyväskylän Yliopiston Biologian Laitoksen Tiedonantoja 33: 83–108 (in Finnish).
Valkeajärvi, P., 1988. Stock fluctuations in the vendace (Coregonus albula L.) in relation to water temperature at egg incubation time. Finnish Fisheries Research 9: 255–265.
Valkeajärvi, P. & H. Salo, 2006. Kalastus Konnevedessä 2003–2004. Finnish Game and Fisheries Reseach Institute, Jyväskylä. Manuscript, 28 p. (in Finnish).
Vander Zanden, M. J. & J. B. Rasmussen, 1999. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80: 1395–1404.
Wennersten, L. & A. Forsman, 2012. Population-level consequences of polymorphism, plasticity and randomized phenotype switching: a review of predictions. Biological Reviews 87: 756–767.
Wetjen, M., T. Schmidt, A. Schrimpf & R. Schultz, 2019. Genetic diversity and population structure of burbot Lota lota in Germany: Implications for conservation and management. Fisheries Management and Ecology 27: 170–184.
von Bertalanffy, L., 1938. A quantitative theory of organic growth. Human Biology 10: 181–213.
Acknowledgements
We thank the fishermen of Konnevesi for sharing valuable information based on their local tradition and experiences and especially Onni Pakarinen for his crucial assistance in collecting fish samples. Laboratory technician Janne Koskinen assisted in interviews with fishermen.
Funding
Open Access funding provided by University of Jyväskylä (JYU). No external to JYU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Handling Editor: Michael Power
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marjomäki, T.J., Mustajärvi, L., Mänttäri, J. et al. Indications of polymorphism in the behaviour and morphology of burbot (Lota lota) in a European lake. Hydrobiologia 849, 1839–1853 (2022). https://doi.org/10.1007/s10750-022-04830-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04830-y