Abstract
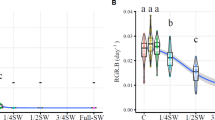
In an estuary of a neotropical region located in Brazil, Urochloa arrecta occurs only in the area of freshwater. Nonetheless, information about the capacity of this population to invade areas considered inhospitable, i.e., saltwater, is scarce. In this sense, the aim of this study was to evaluate the effect of intermediate (20 ppt) and high (30 ppt) salinity on individuals from a population of U. arrecta located in the freshwater region of an estuarine ecosystem. Analyses of plant biomass, nitrogen, phosphorus, malondialdehyde (MDA) and hydrogen peroxide content were evaluated as possible indicators of tolerance to salt stress. Our results showed that salinity reduced growth and increased oxidative stress. However, under conditions of intermediate salinity, U. arrecta individuals showed a biomass gain greater than 60%, MDA content similar to that in freshwater, and higher nitrogen absorption and assimilation. We conclude that U. arrecta probably presents physiological adjustments that allow its survival at intermediate salinity. Thus, the ability of this species to expand in this area alerts to the importance of studies that seek to adopt policies for the control or management of the species in saline ecosystems.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahanger, M. A., N. S. Tomar, M. Tittal, S. Argal & R. M. Agarwal, 2017. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiology and Molecular Biology of Plants 23: 731–744.
Alexieva, V., I. Sergiev, S. Mapelli & E. Karanov, 2001. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell and Environment 24: 1337–1344.
Allen, S. E., H. M. Grimshaw, J. A. Parkinson & C. Quarmby, 1974. Chemical Analysis of Ecological Materials, Blackwell, Oxford:
Alves, R. M. A., M. B. Albuquerque & L. G. Barbosa, 2017. Status of the invasion of a Poaceae species in tropical semiarid reservoirs. Planta Daninha 35: 1–8.
Amorim, S. R., C. A. Umetsu, D. Toledo & A. F. M. Camargo, 2015. Effects of a non native species of Poaceae on aquatic macrophyte community composition: a comparison with a native species. Journal of Aquatic Plant Management 53: 191–196.
Atia, A., A. Debez, M. Rabhi, Z. Barhoumi, C. C. Haouari, H. Gouia, C. Abdelly & A. Smaoui, 2019. Salt Tolerance and Potential Uses for Saline Agriculture of Halophytes from the Poaceae. 223–237.
Ball, M. C., 1996. Comparative ecophysiology of mangrove forest and tropical lowland moist rainforest. Tropical Forest Plant Ecophysiology 461–462.
Barreiros, A. L. B. S., J. M. David & J. P. David, 2006. Estresse oxidativo: Relação entre geração de espécies reativas e defesa do organismo. Quimica Nova 29: 113–123.
Bora, L. S., S. M. Thomaz & A. A. Padial, 2020. Evidence of rapid evolution of an invasive poaceae in response to salinity. Aquatic Sciences 82: 1–16.
Callaway, J. C., V. T. Parker, M. C. Vasey & L. M. Schile, 2007. Emerging issues for the restoration of tidal marsh ecosystems in the context of predicted climate change. BioOne 54: 234–248.
Carniatto, N., S. M. Thomaz, E. R. Cunha, R. Fugi & R. R. Ota, 2013. Effects of an invasive alien Poaceae on aquatic macrophytes and fish communities in a neotropical reservoir. Biotropica 45: 747–754.
Carvalho, R. F., V. Quecini & L. E. P. Peres, 2010. Hormonal modulation of photomorphogenesis-controlled anthocyanin accumulation in tomato (Solanum lycopersicum L. cv Micro-Tom) hypocotyls: physiological and genetic studies. Plant Science 178: 258–264.
Céccoli, G., J. Ramos, V. Pilatti, I. Dellaferrera & J. C. Tivano, 2015. Salt glands in the Poaceae family and their relationship to salinity tolerance. The Botanical Review 81: 162–178.
Coetzee, J. A., 2014. Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodiversity and Conservation 23: 1319–1330.
Crain, C. M., 2007. Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients. Estuaries and Coasts 30: 26–34.
Crain, C. M., B. R. Silliman, S. L. Bertness & M. D. Bertness, 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85: 2539–2549.
Cram, J. W., P. G. Torr & D. A. Rose, 2002. Salt allocation during leaf development and leaf fall in mangroves. Trees - Structure and Function 16: 112–119.
Deegan, B. M., S. D. White & G. G. Ganf, 2007. The influence of water level fluctuations on the growth of four emergent macrophyte species. Aquatic Botany 86: 309–315.
Engels, J. G. & K. Jensen, 2010. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 119: 679–685.
Esteves, B. S. & M. S. Suzuki, 2009. Efeito da salinidade sobre as plantas. Oecologia Australis 12: 662–679.
Ferreira, J. P. R., G. Hassemer & R. Trevisan, 2017. Aquatic macrophyte flora of coastal lakes in Santa Catarina, southern Brazil. Iheringia - Serie Botanica 72: 409–419.
Flora do Brasil, 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/
Flowers, T. J. & T. D. Colmer, 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963.
Flowers, T. J., R. Munns & T. D. Colmer, 2015. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Annals of Botany 115: 419–431.
Foyer, C. H. & G. Noctor, 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875.
Foyer, C. H. & G. Noctor, 2013. Redox signaling in plants. Antioxidants & Redox Signaling 18: 2087–2090.
Foyer, C. H., A. V. Ruban & G. Noctor, 2017. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochemical Journal 474: 877–883.
Garg, N. & G. Manchanda, 2009. ROS generation in plants: boon or bane? Plant Biosystems 143: 81–96.
Gil, L., X. Capó, S. Tejada, G. Mateu-Vicens, P. Ferriol, S. Pinya & A. Sureda, 2020. Salt variation induces oxidative stress response in aquatic macrophytes: the case of the Eurasian water-milfoil Myriophyllum spicatum L. (Saxifragales: Haloragaceae). Estuarine, Coastal and Shelf Science 239: 1–6.
Gill, S. S. & N. Tuteja, 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930.
Gratão, P. L., A. Polle, P. J. Lea & R. A. Azevedo, 2005. Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology 32: 481.
Gratão, P. L., C. C. Monteiro, R. F. Carvalho, T. Tezotto, F. A. Piotto, L. E. P. Peres & R. A. Azevedo, 2012. Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiology and Biochemistry 56: 79–96.
Hafsi, C., M. C. Romero-Puertas, D. K. Gupta, L. A. del Río, L. M. Sandalio & C. Abdelly, 2010. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environmental and Experimental Botany 69: 129–136.
Halliwel, B. & J. M. Gutteridge, 2015. No Free Radicals in Biology and Medicine, Oxford University Press, Oxford:
Heath, R. L. & L. Packe, 1968. Photoperoxidation in isolated chloro- plasts. I. Kinetics and stochiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125: 189–198.
Hoagland, D. C. & D. I. Arnon, 1950. The water culture method for growing plant without soil. California Agricultural Experiment. Circular 337. The College of Agriculture University of California, Berkeley.
Houle, G., L. Morel, C. E. Reynolds & J. Siegel, 2001. The effect of salinity on different developmental stages of an endemic annual plant, Aster laurentianus (Asteraceae). Botanical Society of America 88: 62–67.
Houston, W. A. & L. J. Duivenvoorden, 2002. Replacement of littoral native vegetation with the ponded pasture grass Hymenachne amplexicaulis: effects on plant, macroinvertebrate and fish biodiversity of backwaters in the Fitzroy River, Central Queensland, Australia. Marine and Freshwater Research 53: 1235–1244.
Hussain, T., H. W. Koyro, B. Huchzermeyer & M. A. Khan, 2015. Eco-physiological adaptations of Panicum antidotale to hyperosmotic salinity: water and ion relations and anti-oxidant feedback. Flora: Morphology, Distribution, Functional Ecology of Plants 212: 30–37.
IPCC, 2021. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds)]. Cambridge University Press. In Press.
Koyro, H. W., T. Hussain, B. Huchzermeyer & M. A. Khan, 2013. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environmental and Experimental Botany 91: 22–29.
Krauss, K. W., C. E. Lovelock, K. L. McKee, L. López-Hoffman, S. M. L. Ewe & W. P. Sousa, 2008. Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany 89: 105–127.
Larcher., 2000. Ecofisiologia vegetal. São Carlos. Rima.
Levin, L. A., D. F. Boesch, A. Covich, C. Dahm, C. Erséus, K. C. Ewel, R. T. Kneib, A. Moldenke, M. A. Palmer, P. Snelgrove, D. Strayer & J. M. Weslawski, 2001. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4: 430–451.
Li, Y., W. Niu, X. Cao, J. Wang, M. Zhang, X. Duan & Z. Zhang, 2019. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biology 19: 1–15.
Lüttge, U., 2007. Physiological Ecology of Tropical Plants, Springer, Germany:
Manousaki, E. & N. Kalogerakis, 2011. Halophytes-an emerging trend in phytoremediation. International Journal of Phytoremediation 13: 959–969.
Mendelssohn, I. A., K. L. McKee & T. Kong, 2001. A comparison of physiological indicators of sublethal cadmium stress in wetland plants. Environmental and Experimental Botany 46: 263–275.
Michelan, T. S., S. M. Thomaz, P. Carvalho, R. B. Rodrigues & M. J. Silveira, 2010a. Regeneration and colonization of an invasive macrophyte grass in response to desiccation. Natureza & Conservação 8: 133–139.
Michelan, T. S., S. M. Thomaz, R. P. Mormul & P. Carvalho, 2010b. Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshwater Biology 55: 1315–1326.
Michelan, T., M. Dainez Filho & S. Thomaz, 2017. Aquatic macrophyte mats as dispersers of one invasive plant species. Brazilian Journal of Biology 78: 5–8.
Midgley, J. M., M. P. Hill & M. H. Villet, 2006. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. African Journal of Aquatic Science 31: 25–30.
Morard, P. & J. Silvestre, 1996. Plant injury due to oxygen deficiency in the root environment of soilless culture: a review. Plant and Soil 184: 243–254.
Mormul, R. P., S. M. Thomaz, J. Higuti & K. Martens, 2010. Ostracod (Crustacea) colonization of a native and a non-native macrophyte species of Hydrocharitaceae in the Upper Paraná floodplain (Brazil): an experimental evaluation. Hydrobiologia 644: 185–193.
Munns, R. & M. Tester, 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59: 651–681.
Nunes, L. S. C. & A. F. M. Camargo, 2017. A simple non-destructive method for estimating aboveground biomass of emergent aquatic macrophytes. Acta Limnologica Brasiliensia 49: 1–9.
Nunes, L. S. C. & A. F. M. Camargo, 2018. Do interspecific competition and salinity explain plant zonation in a tropical estuary? Hydrobiologia 812: 67–77.
Nunes, L. S. C. & A. F. M. Camargo, 2020. Effects of salinity on growth, competitive interaction and total nitrogen content of two estuarine macrophyte species cultivated on artificial substrate. Aquatic Ecology 54: 973–983.
Nunes, L. S. C., C. A. Umetsu, M. E. F. Rodrigues, V. J. Pott & A. F. M. Camargo, 2019. Inventory of aquatic macrophyte species in coastal rivers of the São Paulo state, Brazil. Oecologia Australis 23: 829–845.
Odum, W. E., 1988. Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics 19: 147–176.
Parihar, P., S. Singh, R. Singh, V. P. Singh & S. M. Prasad, 2015. Effect of salinity stress on plants and its tolerance strategies: a review. Environmental Science and Pollution Research 22: 4056–4075.
Pott, V. J., A. Pott, L. C. P. Lima, S. N. Moreira & A. K. M. Oliveira, 2011. Aquatic macrophyte diversity of the Pantanal wetland and upper basin. Brazilian Journal of Biology 71: 255–263.
Reinert, B. L., M. R. Bornschein & C. Firkowski, 2007. Distribuição, tamanho populacional, hábitat e conservação do bicudinho-do-brejo Stymphalornis acutirostris Bornschein, Reinert e Teixeira, 1995 (Thamnophilidae). Revista Brasileira De Ornitologia 15: 493–519.
Ribeiro, J. P. N., R. S. Matsumoto, L. K. Takao, A. C. Peret & M. I. S. Lima, 2011. Spatial distribution of Crinum americanum L. in tropical blind estuary: hydrologic, edaphic and biotic drivers. Environmental and Experimental Botany 71: 287–291.
Rodrigues, M. E. F., V. C. Souza & M. L. M. Pompêo, 2017. Levantamento florístico de plantas aquáticas e palustres na Represa Guarapiranga, São Paulo, Brasil. Boletim De Botânica 35: 1–64.
Short, F. T., S. Kosten, P. A. Morgan, S. Malone & G. E. Moore, 2016. Impacts of climate change on submerged and emergent wetland plants. Aquatic Botany 135: 3–17.
Suárez, N. & E. Medina, 2005. Salinity effect on plant growth and leaf demography of the mangrove, Avicennia germinans L. Trees 19: 721–727.
Thomaz, S. M., P. Carvalho, A. A. Padial & J. T. Kobayashi, 2009. Temporal and spatial patterns of aquatic macrophyte diversity in the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 617–625.
Thomaz, S. M., R. P. Mormul & T. S. Michelan, 2015. Propagule pressure, invasibility of freshwater ecosystems by macrophytes and their ecological impacts: a review of tropical freshwater ecosystems. Hydrobiologia 746: 39–59.
Thouvenot, L. & G. Thiébaut, 2018. Regeneration and colonization abilities of the invasive species Elodea canadensis and Elodea nuttallii under a salt gradient: implications for freshwater invasibility. Hydrobiologia 817: 193–203.
Umetsu, C. A., F. C. Aguiar, M. T. Ferreira, L. F. Cancian & A. F. M. Camargo, 2018. Addressing bioassessment of tropical rivers using macrophytes: the case of Itanhaém Basin, São Paulo, Brazil. Aquatic Botany 150: 53–63.
Villamagna, A. M. & B. R. Murphy, 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biology 55: 282–298.
Xue, L., X. Li, Z. Yan, Q. Zhang, W. Ding, X. Huang, B. Tian, Z. Ge & Q. Yin, 2018. Native and non-native halophytes resiliency against sea-level rise and saltwater intrusion. Hydrobiologia 806: 47–65.
Acknowledgments
We thank Carlos Fernando Sanches, Sonia Maria R. Carregari and Baltasar Fernandes Garcia Neto for all assistance with the experiment and analysis.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Andre Andrian Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santini, R., de Lima, J.P., Gratão, P.L. et al. Evaluation of growth and oxidative stress as indicative of salinity tolerance by the invasive tropical aquatic macrophyte tanner grass. Hydrobiologia 849, 1261–1271 (2022). https://doi.org/10.1007/s10750-021-04787-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04787-4