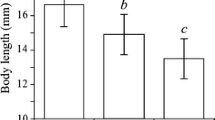
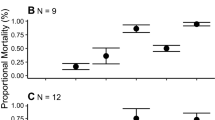
Abstract
Outcomes of predator–prey interactions depend on biotic and abiotic factors. Habitat complexity, for example, mediates predator functional response type and parameters (attack rate and handling time). However, the relationship between habitat complexity and functional response varies across ecosystems. We assessed interactions between the giant water bug, Belostoma sp., and its prey, rams-horn snails, Helisoma trivolvis, across four prey densities (N = 2, 4, 8, 16 snails) and three habitat complexity levels (No-complexity, Low-complexity, High-complexity) to understand how complexity affected the functional response. We also tested effects of predator and prey body size on number of prey killed. Belostoma exhibited a Type III functional response in all habitat complexity treatments. Attack rate tended to increase with increasing complexity. Handling time was different among treatments, being lowest in the No-complexity treatment and highest in the Low-complexity treatment. Belostoma body size was positively related, while Helisoma body size was inversely related, to the number of Helisoma killed. We show habitat complexity does not affect the shape of predator functional response but impacts response parameters in the Belostoma–Helisoma system. We reaffirm that attack rate, handling time, and mortality outcomes for prey within predator–prey interactions are affected by abiotic factors through habitat complexity.



Similar content being viewed by others
Availability of data and material
Data and figures will be made available in the Figshare repository.
Code availability
R code to recreate the analyses will be made available in a Figshare repository as well as the corresponding author’s Github webpage.
References
Agrawal, A. A., D. D. Ackerly, F. Adler, A. E. Arnold, C. Cáceres, D. F. Doak, E. Post, P. J. Hudson, J. Maron, K. A. Mooney, M. Power, D. Schemske, J. Stachowicz, S. Strauss, M. G. Turner & E. Werner, 2007. Filling key gaps in population and community ecology. Frontiers in Ecology and the Environment 5: 145–152.
Alexander, M., J. Dick, N. O’Connor, N. Haddaway & K. Farnsworth, 2012. Functional responses of the intertidal amphipod Echinogammarus marinus: effects of prey supply, model selection and habitat complexity. Marine Ecology Progress Series 468: 191–202.
Aljetlawi, A. A., E. Sparrevik & K. Leonardsson, 2004. Prey-predator size-dependent functional response: derivation and rescaling to the real world. Journal of Animal Ecology 73: 239–252.
Anderson, T. L., 2016. Predation risk between cannibalistic aeshnid dragonflies influences their functional response on a larval salamander prey. Journal of Zoology 300: 221–227.
Anderson, T. L., J. L. Heemeyer, W. E. Peterman, M. J. Everson, B. H. Ousterhout, D. L. Drake & R. D. Semlitsch, 2015. Automated analysis of temperature variance to determine inundation state of wetlands. Wetlands Ecology and Management 23: 1039–1047.
Avery, R. A., 1971. Estimates of food consumption by the lizard Lacerta vivipara Jacquin. Journal of Animal Ecology 40: 351–365.
Barrios-O’Neill, D., R. Kelly, J. T. A. Dick, A. Ricciardi, H. J. MacIsaac & M. C. Emmerson, 2016. On the context-dependent scaling of consumer feeding rates. Ecology Letters 19: 668–678.
Bolker, B. M., 2008. Ecological models and data in R. Princeton University Press, Princeton, NJ.
Bolker, B. M., & R Core Team, 2020. bbmle: Tools for general maximum likelihood estimation.
Brose, U., 2010. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Functional Ecology 24: 28–34.
Buck, T. L., G. A. Breed, S. C. Pennings, M. E. Chase, M. Zimmer & T. H. Carefoot, 2003. Diet choice in an omnivorous salt-marsh crab: different food types, body size, and habitat complexity. Journal of Experimental Marine Biology and Ecology 292: 103–116.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Inference: A Practical Information-Theoretic Approach. Springer, New York.
Cammen, L. M., 1979. Ingestion rate: an empirical model for aquatic deposit feeders and detritivores. Oecologia 44: 303–310.
Chamberlain, S. A., J. L. Bronstein & J. A. Rudgers, 2014. How context dependent are species interactions? Ecology Letters 17: 881–890.
Clark, T. L. & F. J. Messina, 1998. Foraging behavior of lacewing larvae (Neuroptera: Chrysopidae) on plants with divergent architectures. Journal of Insect Behavior 11: 303–317.
Colton, T. F., 1987. Extending functional response models to include a second prey type: an experimental test. Ecology 68: 900–912.
Crowder, L. B. & W. E. Cooper, 1982. Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813.
Cuthbert, R. N., T. Dalu, R. J. Wasserman, A. Callaghan, O. L. F. Weyl & J. T. A. Dick, 2019. Using functional responses to quantify notonectid predatory impacts across increasingly complex environments. Acta Oecologica 95: 116–119.
Dewitt, T. J., B. W. Robinson & D. S. Wilson, 2000. Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evolutionary Ecology Research 2: 129–148.
Diehl, S., 1988. Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53: 207–214.
Drake, D. L., T. L. Anderson, L. M. Smith, K. M. Lohraff & R. D. Semlitsch, 2014. Predation of eggs and recently hatched larvae of endemic ringed salamanders (Ambystoma annulatum) by native and introduced aquatic predators. Herpetologica 70: 378–387.
Eggleston, D. B., R. N. Lipcius & A. H. Hines, 1992. Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Marine Ecology Progress Series 85: 55–68.
Englund, G., G. Ohluynd, C. L. Hein & S. Diehl, 2011. Temperature dependence of the functional response. Ecology Letters 14: 914–921.
Gergs, A. & H. T. Ratte, 2009. Predicting functional response and size selectivity of juvenile Notonecta maculata foraging on Daphnia magna. Ecological Modelling 220: 3331–3341.
Gingras, D., P. Dutilleul & G. Boivin, 2003. Effect of plant structure on host finding capacity of lepidopterous pests of crucifers by two Trichogramma parasitoids. Biological Control 27: 25–31.
González-Suárez, M., M. Mugabo, B. Decencière, S. Perret, D. Claessen & J. F. Le Galliard, 2011. Disentangling the effects of predator body size and prey density on prey consumption in a lizard. Functional Ecology 25: 158–165.
Gotceitas, V., 1990. Variation in plant stem density and its effects on foraging success of juvenile bluegill sunfish. Environmental Biology of Fishes 27: 63–70.
Gotceitas, V. & P. Colgan, 1989. Predator foraging success and habitat complexity: quantitative test of the threshold hypothesis. Oecologia 80: 158–166.
Green, S. J. & I. M. Cote, 2014. Trait-based diet selection: prey behavior and morphology predict vulnerability to predation in reef fish communities. Journal of Animal Ecology 83: 1451–1460.
Hassell, M.P., J.H. Lawton, & J.R. Beddington, 1977. Sigmoid functional responses by invertebrate predators and parasitoids. Journal of Animal Ecology 46: 249–262.
Heck, K. L. & L. B. Crowder, 1991. Habitat structure and predator-prey interactions in vegetated aquatic systems. In Bell, S., E. McCoy & H. Mushinsky (eds.), Habitat Structure: The Physical Arrangement of Objects in Space. Chapman and Hall, London, UK: 281–299.
Holling, C. S., 1959. The components of predation as revealed by a study of small-mammal predation of the european pine sawfly. The Canadian Entomologist 91: 293–320.
Hossie, T. J. & D. L. Murray, 2010. You can’t run but you can hide: refuge use in frog tadpoles elicits density-dependent predation by dragonfly larvae. Oecologia 163: 395–404.
Hossie, T. J., K. C. Chan, & D. L. Murray, 2021. Increasing availability of palatable prey induces predator-dependence and increases predation on unpalatable prey. Scientific Reports 11: 676.
Hoverman, J. T. & R. A. Relyea, 2007a. The rules of engagement: how to defend against combinations of predators. Oecologia 154: 551–560.
Hoverman, J. T. & R. A. Relyea, 2007b. How flexible is phenotypic plasticity? Developmental windows for trait induction and reversal. Ecology 88: 693–705.
Hoverman, J. T. & R. A. Relyea, 2008. Temporal environmental variation and phenotypic plasticity: a mechanism underlying priority effects. Oikos 117: 23–32.
Hoverman, J. T. & R. A. Relyea, 2009. Survival trade-offs associated with inducible defences in snails: the roles of multiple predators and developmental plasticity. Functional Ecology 23: 1179–1188.
Hoverman, J. T., J. R. Auld & R. A. Relyea, 2005. Putting prey back together again: integrating predator-induced behavior, morphology, and life history. Oecologia 144: 481–491.
Janssen, A., M. W. Sabelis, S. Magalhães, M. Montserrat & T. Van Der Hammen, 2007. Habitat structure affects intraguild predation. Ecology 88: 2713–2719.
Jara, F. G., 2016. Predator-prey body size relationship in temporary wetlands: effect of predatory insects on prey size spectra and survival. International Journal of Limnology 52: 205–216.
Jones, J., A. P. Thorpe & D. V. Obrecht, 2020. Limnological characteristics of Missouri reservoirs: synthesis of a long-term assessment. Lake and Reservoir Management 36: 412–422.
Juliano, S., 2001. Nonlinear curve fitting: predation and functional response curves. In Scheiner, S. & J. Gurevitch (eds.), Design and Analysis of Ecological Experiments. Oxford University Press, New York: 178–196.
Kalinkat, G., F. D. Schneider, C. Digel, C. Guill, B. C. Rall & U. Brose, 2013. Body masses, functional responses and predator-prey stability. Ecology Letters 16: 1126–1134.
Kareiva, P. & R. Sahakian, 1990. Tritrophic effects of a simple architectural mutation in pea plants. Nature 345: 433–434.
Kater, S. B., 1974. Feeding in Helisoma trivolvis: the morphological and physiological bases of a fixed action pattern. American Zoologist 14: 1017–1036.
Kesler, D. H. & W. R. Munns, 1989. Predation by Belostoma flumineum (Hemiptera): an important cause of mortality in freshwater snails. Journal of the North American Benthological Society 8: 342–350.
Klecka, J. & D. S. Boukal, 2013. Foraging and vulnerability traits modify predator-prey body mass allometry: freshwater macroinvertebrates as a case study. Journal of Animal Ecology 82: 1031–1041.
Klecka, J. & D. S. Boukal, 2014. The effect of habitat structure on prey mortality depends on predator and prey microhabitat use. Oecologia 176: 183–191.
Klug, H. & P. Hicks, 2014. The giant water bug, Belostoma lutarium (Stål): an ideal system for studies of ecology, evolution, and behavior. Journal of the Tennessee Academy of Science 89: 51–58.
Kotler, B., 2016. Fun and Games: predator-prey foraging games and related interactions. Israel Journal of Ecology and Evolution 62: 118–120.
Kovalenko, K. E., S. M. Thomaz & D. M. Warfe, 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685: 1–17.
Kratina, P., M. Vos, A. Bateman & B. R. Anholt, 2009. Functional responses modified by predator density. Oecologia 159: 425–433.
Langellotto, G. A. & R. F. Denno, 2004. Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139: 1–10.
Manatunge, J., T. Asaeda & T. Priyadarshana, 2000. The influence of structural complexity on fish-zooplankton interactions: a study using artificial submerged macrophytes. Environmental Biology of Fishes 58: 425–438.
Mccoy, M. W., A. C. Stier & C. W. Osenberg, 2012. Emergent effects of multiple predators on prey survival: the importance of depletion and the functional response. Ecology Letters 15: 1449–1456.
Mocq, J., P. R. Soukup, J. Naslund & D. S. Boukal, 2021. Disentangling the nonlinear effects of habitat complexity on functional responses. Journal of Animal Ecology 90: 1525–1537.
Moksnes, P. O., R. N. Lipcius, L. Pihl & J. Van Montfrans, 1997. Cannibal-prey dynamics in young juveniles and postlarvae of the blue crab. Journal of Experimental Marine Biology and Ecology 215: 157–187.
Nyström, P. & J. R. Pérez, 1998. Crayfish predation on the common pond snail (Lymnaea stagnalis): the effect of habitat complexity and snail size on foraging efficiency. Hydrobiologia 368: 201–208.
Paterson, R. A., J. T. A. Dick, D. W. Pritchard, M. Ennis, M. J. Hatcher & A. M. Dunn, 2015. Predicting invasive species impacts: a community module functional response approach reveals context dependencies. Journal of Animal Ecology 84: 453–463.
Pawar, S., A. I. Dell & V. M. Savage, 2012. Dimensionality of consumer search space drives trophic interaction strengths. Nature 486: 485–489.
Payton, M. E., M. H. Greenstone & N. Schenker, 2003. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? Journal of Insect Science 3: 34.
Peters, R., 1989. The Ecological Implications of Body Size. Cambridge University Press, Cambridge.
R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/.
Real, L., 1977. The kinetics of functional response. American Naturalist 111: 289–300.
Rennie, M. D. & L. J. Jackson, 2005. The influence of habitat complexity on littoral invertebrate distributions: patterns differ in shallow prairie lakes with and without fish. Canadian Journal of Fisheries and Aquatic Sciences 62: 2088–2099.
Rogers, D., 1972. Random search and insect population models. Journal of Animal Ecology 41: 369–383.
Rosenbaum, B. & B. Rall, 2018. Fitting functional responses: direct parameter estimation by simulating differential equations. Methods in Ecology and Evolution 9: 2076–2090.
Rossi, M. N., C. Reigada & W. A. C. Goday, 2006. The role of habitat heterogeneity for the functional response of the spider Nesticodes rufipes (Araneae: Theridiidae) to houseflies. Applied Entomology and Zoology 41: 419–427.
Runck, C. & D. W. Blinn, 1994. Role of Belostoma bakeri (Heteroptera) in the trophic ecology of a fishless desert spring. Limnology and Oceanography 39: 1800–1812.
Skalski, G. T. & J. F. Gilliam, 2001. Functional responses with predator interference: viable alternatives to the Holling type II model. Ecology 82: 3083–3092.
Smith, D. A., 1989. Tests of feeding selectivity in Helisoma trivolvis (Gastropoda: Pulmonata). Transactions of the American Microscopical Society 108: 402.
Smith, R. L., 1997. The evolution of paternal care in the giant water bugs (Heroptera: Belostomatidae). In Choe, J. C. & B. Crepsi (eds.), The Evolution of Social Behaviour in Insects and Arachnids. Cambridge University Press, London: 116–149.
Stoner, A. W., 2009. Habitat-mediated survival of newly settled red king crab in the presence of a predatory fish: role of habitat complexity and heterogeneity. Journal of Experimental Marine Biology and Ecology 382: 54–60.
Swart, C. C. & B. E. Felgenhauer, 2003. Structure and function of the mouthparts and salivary gland complex of the giant waterbug, Belostoma lutarium (Stål) (Hemiptera: Belostomatidae). Annals of the Entomological Society of America 96: 870–882.
Swart, C. C. & R. C. Taylor, 2004. Behavioral interactions between the giant water bug (Belostoma lutarium) and tadpoles of Bufo woodhousii. Southeastern Naturalist 3: 13–24.
Thompson, D. J., 1975. Towards a predator-prey model incorporating age structure: the effects of predator and prey size on the predation of Daphnia magna by Ischnura elegans. The Journal of Animal Ecology 44: 907.
Trexler, J., C. McCulloch & J. Travis, 1988. How can the functional response best be determined? Oecologia 76: 206–214.
Tripet, A. F. & N. Perrin, 1994. Size-dependent predation by Dugesia lugubris (Turbellaria) on Physa acuta (Gastropoda): experiments and Model. Ecology 8: 458–463.
Turesson, H. & C. Brönmark, 2007. Predator-prey encounter rates in freshwater piscivores: effects of prey density and water transparency. Oecologia 153: 281–290.
Uiterwaal, S. F. & J. P. DeLong, 2020. Functional responses are maximized at intermediate temperatures. Ecology 101: 1–10.
Uiterwaal, S. F., C. Mares & J. P. DeLong, 2017. Body size, body size ratio, and prey type influence the functional response of damselfly nymphs. Oecologia 185: 339–346.
Uszko, W., S. Diehl & J. Wickman, 2020. Fitting functional response surfaces to data: a best practice guide. Ecosphere 11:
Vucic-Pestic, O., B. C. Rall, G. Kalinkat & U. Brose, 2010. Allometric functional response model: body masses constrain interaction strengths. Journal of Animal Ecology 79: 249–256.
Wahlström, E., L. Persson, S. Diehl & P. Byström, 2000. Size-dependent foraging efficiency, cannibalism and zooplankton community structure. Oecologia 123: 138–148.
Wasserman, R. J., M. E. Alexander, O. L. F. Weyl, D. Barrios-O’Neill, P. W. Froneman & T. Dalu, 2016. Emergent effects of structural complexity and temperature on predator-prey interactions. Ecosphere 7: 1–11.
Winfield, I. J., 1986. The influence of simulated aquatic macrophytes on the zooplankton consumption rate of juvenile roach, Rutilus rutilus, rudd, Scardinius erythrophthalmus, and perch, Perca fluviatilis. Journal of Fish Biology 29: 37–48.
Acknowledgements
We thank the Division of Biological Sciences, University of Missouri, for greenhouse access; B. Knapp and the School of Natural Resources, University of Missouri, for access to Baskett; R. Abney, M. Clay, C. Crouch, T. Hessler, D. Hicks, J. Horne, E. Kinzinger, Z. Miller, E. Petty, A. Roistacher, D. Smith, J. Wilson, and R. Xu for assisting in experimental set-up, data collection, and discussions related to the project, and J. Hoverman for assisting with snail identification. All experiments conducted for this study are in compliance with the current laws of the United States of America.
Funding
JK was supported by AFRI EWD (2019-67011-29729) from the USDA National Institute of Food and Agriculture. SJC was supported by an NSF Graduate Research Fellowship.
Author information
Authors and Affiliations
Contributions
JCG aided in project design, collected data, conducted preliminary analysis, wrote and reviewed the original draft. SJC aided in project conceptualization, collected data, conducted preliminary analysis, wrote and edited the original draft. JK aided in project design, collected data, conducted preliminary analysis, wrote and edited the original draft. JACG aided in project design, collected data, conducted preliminary analysis, wrote and edited the original draft. TLA aided in project conception, collected data, conducted formal analysis, reviewed and edited the original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gunn, J.C., Clements, S.J., Kansman, J.T. et al. Effects of habitat complexity on giant water bug (Belostoma) functional response to rams-horn snail prey (Helisoma). Hydrobiologia 848, 4585–4597 (2021). https://doi.org/10.1007/s10750-021-04663-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04663-1