Abstract
The introduction of non-indigenous organisms in new areas in the context of host-parasite interactions is still poorly understood. This study aimed at a parasitological and histopathological comparison of two phylogenetically distinct forms of the freshwater snail Theodoxus fluviatilis in the River Rhine system: the native Northern-European form, which showed a decline for unknown reasons and is nowadays extinct in the River Rhine, and the non-indigenous Danubian form, which was introduced via the Main–Danube canal. We histopathologically examined populations of Northern-European T. fluviatilis from three smaller rivers of the Rhine system and of Danubian T. fluviatilis from the River Rhine, after confirming the phylogenetic background of the respective population genetically. Results showed differences in the prevalence of trematodes and histopathologic organic alterations between the two snail forms. Both were infected with an opecoelid trematode Plagioporus cf. skrjabini, whereby its prevalence was significantly higher in the Northern-European than in the Danubian form. The parasitic trematode is, to our knowledge, a new trematode species in the River Rhine system, presumably co-introduced through the invasion of its second intermediate and final hosts, i.e. Ponto-Caspian amphipods and gobies. Its impact on native populations of Northern-European T. fluviatilis needs to be subject of future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquatic snail Theodoxus fluviatilis (Linné, 1758) (Gastropoda: Neritidae) is a characteristic species in freshwater ecosystems and has often been used as an indicator for river assessment and monitoring (e.g. Zettler, 2008). It is the most widespread species of the genus Theodoxus in Central Europe and native to all large rivers in Germany. With regard to the River Rhine and its tributaries, a decrease in the spatial distribution of T. fluviatilis is documented for the last decades, and nowadays only relict populations can be found in smaller tributaries of the River Rhine and the River Main system in Southwestern and Central Germany (Zettler, 2008). In the main stretch of the River Rhine, T. fluviatilis disappeared in the late 1990s for unknown reasons, until 2006, when morphologically different individuals were recorded in the River Rhine near the mouth of the River Main (Westermann et al., 2007). Genetic analysis of these specimens and comparisons with genetic material of native River Rhine populations revealed that the newly discovered snails phylogenetically belong to the Danubian form of T. fluviatilis, which is known from the Danube and Black Sea drainages (Bunje, 2005; Gergs et al., 2015). It is assumed that this originally Ponto-Caspian form of T. fluviatilis was introduced to the River Rhine by shipping through the Main–Danube canal that was opened in the year 1992 (Gergs et al., 2015). The Main–Danube canal connects the Danube to the River Rhine via the River Main and enables the spread of numerous non-indigenous species originating from the Ponto-Caspian system via the so-called southern corridor, e.g. by transport through hull fouling and ballast water of ships or active migration (e.g. Bij de Vaate et al., 2002). Currently, the Danubian form of T. fluviatilis in the River Rhine is observed to spread along the river since its discovery (IKSR/CIPR/ICBR, 2012) and to establish high population densities even in anthropogenically degraded habitats like harbours (Rothmeier & Martens, 2019).
The increasing occurrence of non-indigenous species in freshwater ecosystems in Central Europe, due to the spreading of Ponto-Caspian alien species, leads to various changes in ecological interactions including host-parasite relationships (e.g. Emde et al., 2014). For example, invasive North American crayfish species in Europe carry the parasitic oomycete Aphanomyces astaci Schikora, 1906, which is the causative agent of the crayfish plague affecting native crayfish populations (e.g. Söderhäll & Cerenius, 1999; Holdich et al., 2009). The co-introduction of generalist parasite species with low host specificity is observed to be important in invasions and can lead to their establishment if they are able to complete their life cycle in native hosts within the invaded area (Prenter et al., 2004; Alt et al., 2019). One of the better recognised groups of endoparasites in molluscan hosts are trematodes characterised by a complex life cycle (e.g. Cichy et al., 2011; Emde et al., 2014). Molluscs play an obligatory role of the first intermediate hosts for both the proliferation (e.g. sporocysts) and transmission (cercariae) stages (Esch et al., 2002). Trematodes affect the morphology, physiology, and behaviour of snails, which increases the chance of transmission to the next host (Herbison et al., 2018).
The influence of biological invasions on interspecies interactions, including parasitism, is still insufficiently recognised. Therefore, the aim of this study is a parasitological and histopathological comparison of populations of the newly introduced Danubian form and the native Northern-European form of T. fluviatilis in different rivers of the Rhine system in Central Europe, after confirming the phylogenetic difference of the respective population genetically. By analysing specimens from sites at the River Rhine on one hand and from three smaller rivers belonging to the River Rhine system (i.e. Jagst, Kocher and Tauber) on the other hand, we focus on detecting differences regarding infestations of trematodes and histopathological organic alterations.
Materials and methods
Sampling of Theodoxus fluviatilis and parasites
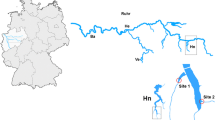
Sampling of individuals of T. fluviatilis was done in August and September 2018 at seven sites along the River Rhine, from Neuburg at river-km 355 to Mannheim at river-km 430, as well as at one site of the river Jagst, three sites of the river Kocher, and three sites of the river Tauber, i.e. a total of 14 sampling sites (Table 1). Two subsamples of snails were collected manually from riprap stones: 5 animals per sampling site were stored in pure 96% ethanol for genetic analysis (overall 70 individuals), and 12 to 46 snails per sampling site were transported to the lab in river water for histopathological analysis, whereby varying numbers of individuals per sampling site resulted from the availability of specimens with at least 5 mm shell length to conduct histological preparation. Overall, 212 specimens from the River Rhine and 150 specimens from the rivers Jagst, Kocher and Tauber, i.e. in total 362 individuals, were examined histopathologically. According to the Regional Council Karlsruhe, Baden-Württemberg, a sampling permission was not needed as T. fluviatilis is not a specially protected species according to the Federal Nature Conservation Act of Germany.
For identification of parasites found in T. fluviatilis, ten adult snails (shell length 9 ± 1 mm) from one site of the Kocher near Ingelfingen (Table 1; selection of this site due to the high trematode prevalence (90%), see results) were stored in pure 96% ethanol, dissected under an EZ4 microscope (Leica, Germany) and checked for parasites. Sporocysts and cercariae which were found in the visceral bag of the snails were mounted on glass slides, using a DM500 microscope (Leica, Germany) for morphological identification of the parasite species, which was carried out on the basis of morphological features of opecoelid cercariae (e.g. Chernogorenko et al., 1978; Cribb, 2002). Three subsamples of cercariae including approximately 20 specimens were stored in pure 96% ethanol for genetic analysis.
Genetic analysis of snails and trematodes
For genetic identification of the snails, animals were excerpted from their shell and muscle tissue was cut from the foot (slices of 1-2 mm) for DNA extraction using a modified high salt-extraction protocol (Koester & Gergs, 2017, 2014). Sequences of the mitochondrial cytochrome c oxidase subunit I gene (COI) were amplified by polymerase chain reaction (PCR) using the primers F4d (5′-TACTTTRTATATTATGTTTGGT-3′), and R1d (5′-TGRTAWARAATDGGRTCWCCHCCVCC-3′) (Bunje, 2005). Each 40 µl PCR reaction mixture contained 1 × reaction buffer S, 0.3 mM dNTPs, 2.75 mM MgCl2 (Peqlab Biotechnologie, Germany), 0.05 U Taq DNA Polymerase (Peqlab Biotechnologie, Germany), 0.5 µM of each primer (Eurofins Genomics, Germany), and approximately 4 ng genomic DNA of a single individual. The following PCR protocol was used to amplify the gene region: 95°C for 10 min, followed by 36 cycles of 95°C for 50 s, 54°C for 60 s, 72°C for 60 s, and a final extension at 72°C for 7 min. PCR products were sequenced by the company SeqIT (Kaiserslautern, Germany) in forward and reverse direction using a 3730 DNA Analyzer eight capillary sequencer (AppliedBiosystems, USA). We used Geneious 11.1.2 (Biomatters Ltd, available from www.geneious.com) to manually edit and align all sequences with known unique haplotypes of T. fluviatilis (GenBank accession nos. AY765306-AY765345, KJ493817, and MG969538; Bunje, 2005; Gergs et al., 2015; Richling & Groh, 2018) and one sequence of Theodoxus danubialis (Pfeiffer, 1828) (GenBank accession no. AY771303; Bunje, 2005).
Samples of cercariae (n = 3) found in T. fluviatilis collected from the river Kocher were genetically analysed by the company MBS Szkolenia Konferencje Usługi (Warsaw, Poland). Genomic DNA was extracted using a NucleoSpin Tissue Kit (Macherey–Nagel, Germany), following the manufacturer’s instructions (according to Behrens-Chapuis et al., 2018). DNA was eluted in 50 μl of elution buffer and stored at -20°C. Amplification of the COI gene was carried out using the primers Dice1F (5′-ATTAACCCTCACTAAATTWCNTTRGATCATAAG-3′) and Dice11R (5′-TAATACGACTCACTATAGCWGWACHAAATTTHCGATC-3′) (van Steenkiste et al., 2015). Each 40 µl PCR reaction mixtures contained 20 µl RedTaq ReadyMix (Sigma-Aldrich, Germany), 4 µl primer mix (concentration of each primer 5 µM), 2 µl DNA, and 16 µl double-distilled water (< 18.2 MΩ). The PCR profile included an initial denaturation at 94°C for 2 min, followed by 3 cycles of 94°C for 40 s, 51°C for 40 s, 72°C for 60 s; 5 touchdown cycles of 94°C for 40 s, 50°C to 46°C for 40 s (decreasing 1°C per cycle), 72°C for 60 s; 35 cycles of 94°C for 40 s, 45°C for 40 s, 72°C for 60 s, and a final extension at 72°C for 300 s (van Steenkiste et al., 2015). Sequencing was done using a 3500xl sequencer (AppliedBiosystems, USA) in forward and reverse direction. Sequences (n = 3) were edited and aligned using de novo assembly in the CAP3 program (Huang & Madan, 1999), resulting in a contig sequence. Species affiliation was determined using BOLDSYSTEM (available from www.boldsystems.org) and BLAST (National Center for Biotechnology Information, available from https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Parasitological and histopathological analysis
To ensure complete defaecation, snails collected for histopathological analysis were kept in river water in the laboratory for 3 days and were subsequently histologically prepared following the protocol of Watermann et al. (2008). Individuals were narcotized in magnesium chloride (3%) before fixation in Bouin’s fluid for 16 days, then washed and stored in 80% ethanol (p.a.). Afterwards, snails were embedded in paraffin and cut into 2–3 µm sections with a rotation microtome (Microm, Walldorf, Germany). Sections were mounted on glass slides and counter-stained with haematoxylin and eosin (e.g. Mulisch & Welsch, 2010). According to Rothmeier et al. (2020), the following snail’s tissues and organs were checked for the presence of trematodes and histopathological alterations: foot tissue, digestive gland, stomach, kidney, gill, and sexual organs (in females: gonads, receptaculum seminis, and accessory glands; in males: gonads, vesiculum seminis, and prostate gland). Alterations considered as pathologic were tissue dilatation or degeneration, black or brown pigment depositions, e.g. in stomach and digestive gland, and inhibited or arrested spermatogenesis or oogenesis in gonads. For further calculations, the percentage of affected snails per sampling site was calculated for each detected alteration. Trematode prevalence (% of infected snails) per sampling site was calculated according to Bush et al. (1997), i.e. the number of hosts infected with one or more individuals of a particular parasite species, i.e. miracidium, sporocyst or cercaria, was divided by the number of snail hosts examined. Sex-specific histopathological findings in male and female gonads were summed up as pathological findings in sexual organs.
Statistical analysis
All statistical analyses were done using the statistical software package R (version 3.6.3, R Core Team, 2020). Phylogenetic analysis of DNA-sequences of T. fluviatilis and cercariae and building of maximum likelihood (ML) trees was conducted using a ML analysis with the package phangorn (Schliep, 2011) according to the protocol of Gergs et al. (2015). For ML analysis, the substitution model TPM3u + I was determined for both, T. fluviatilis and cercariae, using jModelTest and the Akaike information criterion (Posada, 2008). By using the pml function of the package phangorn, the likelihood of a phylogenetic tree was computed and optimised with optim.pml. Bootstrapping was conducted with 1000 replicates to estimate the support for reconstructed branches of the ML tree.
To analyse differences in trematode prevalence and histopathological alterations between the two phylogenetically distinct snail forms (Danubian and Northern-European, see Results and Table 1), a Permutational Multivariate Analysis of Variance (PERMANOVA) was conducted using the adonis function of the R package vegan (version 2.5-7, Oksanen et al., 2020). Arcsine and square root transformed proportional data of trematode prevalence together with six histopathological findings (digestive gland dilatations, pigment depositions in digestive glands and in stomachs, gill dilatations, kidney dilatations, and pathological findings in sexual organs) were compared between the Danubian and Northern-European form using Bray–Curtis dissimilarity as a distance measure (999 permutations) and sampling sites as replicates. The percentual contributions of single parameters to overall dissimilarity were analysed by calculating similarity percentages using vegan’s function simper. The Mann–Whitney-U-test for independent samples was used to conduct a univariate comparison of trematode prevalence between the two phylogenetically distinct forms of T. fluviatilis.
Results
Genetic analysis of snails and trematodes
At the rivers Jagst and Tauber, all sampled individuals of T. fluviatilis were identified as the “Tauber”-haplotype (acc. no. MG969538; Table 1), and snails sampled from the three sites of the river Kocher belonged to the haplotype F1 (acc. no. AY765306; Table 1). Both the haplotypes “Tauber” and F1 are descendants from the basal haplotype F3, phylogenetically belonging to the Northern-European group of T. fluviatilis (Fig. 1). 31 out of 35 T. fluviatilis specimens analysed from the seven sampling sites of the River Rhine harbour the haplotype F31 (acc. no. AY765336; Table 1), belonging to the Danubian group (Fig. 1). Out of each five individuals per sampling site, one individual of the site near Neuburg and two individuals of the site near Leimersheim were identified as haplotype F34 (acc. no. AY765341; Table 1), which is assigned to the Ponto-Caspian “Theodoxus cf. velox”-group (Fig. 1). Additionally, one new haplotype (acc. no. MT563453) was found in a single individual from the River Rhine at the site near Altrip (Table 1). Based on the ML analysis for phylogenetic reconstruction, the newly described haplotype MT563453 also belongs to the Danubian group (Fig. 1). Resulting from genetic analyses, snails sampled for this study can be divided into two phylogenetically distinct forms: (i), individuals from sampling sites of the River Rhine are summarised as Danubian form, knowing that this group also harbours three presumably co-introduced individuals of the “Theodoxus cf. velox”-group from the Ponto-Caspian region, and (ii), snails from the rivers Jagst, Kocher and Tauber can be summarised and allocated to the Northern-European form of T. fluviatilis (Fig. 1).
Maximum likelihood tree of unique haplotypes of Theodoxus fluviatilis, rooted with an outgroup haplotype (T. danubialis). Only bootstrap values (n = 1000) of the main branches are displayed. For geographical classification of the haplotypes found in 2018 in the River Rhine and the rivers Jagst, Kocher and Tauber see Table 1. See Bunje (2005), Gergs et al. (2015), and Richling and Groh (2018) for further information about haplotypes, classification groups and phylogeographic clades of T. fluviatilis
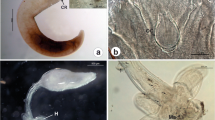
Genetic analyses of trematodes showed that the species found in T. fluviatilis represented the genus Plagioporus (Allocreadiata, Opecoelidae). The obtained COI gene sequence from cercariae (acc. no. MT756015.1; this study) showed 81% similarity to Plagioporus sinitsini Mueller, 1934 (acc. no. KM538106; van Steenkiste et al., 2015) and 76% similarity to Echinostoma revolutum (Fröhlich, 1802) (acc. no. NC_046395; Ran et al., 2020) (Fig. S1). Morphological features of cercariae correspond to the species Plagioporus skrjabini Kowal, 1951 (Fig. 2). Due to the morphological similarity to P. skrjabini and the genetic affiliation to the genus Plagioporus, but the lack of COI sequences in GenBank, the name Plagioporus cf. skrjabini will be used to emphasize uncertain species affiliation.
Microscope photographs of different life cycle stages of Plagioporus cf. skrjabini from the visceral bag of the freshwater snail Theodoxus fluviatilis (sampled at the river Kocher, Germany). a Cercaria of P. cf. skrjabini. Bar 50 µm. b Stylet of P. cf. skrjabini cercaria. Bar 25 µm. c Sporocyst of P. cf. skrjabini filled with cercariae. Bar 300 µm
Parasitological and histopathological analysis
Multivariate statistical analysis revealed a significant difference in the prevalence of P. cf. skrjabini, and histopathological parameters (digestive gland, gill and kidney dilatations, pigment depositions in digestive gland and stomachs and pathological findings in sexual organs) between the Northern-European and the Danubian form of T. fluviatilis (PERMANOVA, P = 0.02; see Fig. 3 for biplot of dissimilarity matrix based on Bray–Curtis dissimilarity). Of the analysed parameters, the prevalence of P. cf. skrjabini showed the highest contribution to overall dissimilarity between the two snail forms (simper, 28.7%; for percentual contributions of other variables see Table S1). The prevalence of P. cf. skrjabini was significantly higher in the Northern-European (52.5 ± 35.5%) than in the Danubian form (3.6 ± 3.5%) of analysed T. fluviatilis (Mann–Whitney-U-test, P = 0.004; Fig. 4).
Nonmetric Multidimensional Scaling (NMDS) biplot of the dissimilarity matrix (Bray–Curtis dissimilarity) of Plagioporus cf. skrjabini prevalence and histopathological alterations in two phylogenetic distinct forms of the snail Theodoxus fluviatilis from sampling sites at the River Rhine (Danubian form, n = 7) and the rivers Jagst, Kocher and Tauber (Northern European form, n = 7). Prevalence and histopathological variables were added using weighted averages. Site scores are not shown for clarity. Dig.gland.dilatation = pathologic dilatation of digestive gland tissue, Dig.gland.pigment = black or brown pigment depositions in digestive gland tissue, Gill.dilatation = pathologic dilatation of gill tissue, Kidney.dilatation = pathologic dilatation of kidney tissue, Prevalence = prevalence of trematode P. cf. skrjabini in T. fluviatilis, Sexual.organs.path = pathologic alterations in male or female gonads, Stomach.pigment = black or brown pigment depositions in stomach tissue
Prevalence (%) of the parasitic trematode Plagioporus cf. skrjabini in two phylogenetic distinct forms of the snail Theodoxus fluviatilis at the River Rhine (Danubian form, n = 7) and the rivers Jagst, Kocher and Tauber (Northern-European form, n = 7). Asterisks indicate statistically significant difference (Mann–Whitney-U-test, P = 0.004)
Different larval stages of P. cf. skrjabini were found in parasitised T. fluviatilis, i.e. miracidia, sporocysts and cercariae, which were located in various organs of the snails. At an early infestation stage, penetrating miracidia were found in the foot of the snails (Fig. S2a), from where they spread into the stomach developing sporocysts (Fig. S2b) and finally infect the digestive gland tissue (Fig. S2c) and gonads of the snails (Fig. S2d). Digestive glands and gonads of heavily parasitised snails were observed to be full of sporocysts (Fig. S2e), in which cercariae were in a developing stage or already mature and released into the host’s tissue, leaving extensive lacunas (Fig. S2e, f).
Discussion
Comparison of the native Northern-European and the newly introduced Danubian form of T. fluviatilis in Central Europe showed significant differences in the prevalence of P. cf. skrjabini and histopathological organic alterations. Though the parasite was found in both snail forms, it had a significantly higher prevalence in the Northern-European form of T. fluviatilis. To our knowledge, this study provides the first record of a parasitic trematode of the genus Plagioporus, most likely P. skrjabini, in the Northern-European form of T. fluviatilis. P. skrjabini has previously been described in the Ponto-Caspian region from T. fluviatilis being the first intermediate host, and from other hosts, including amphipods and predatory fish like gobies (Chernogorenko et al., 1978). Presumably, the trematode could have been introduced to the River Rhine system through the invasion of its second intermediate or final hosts, i.e. amphipods as Dikerogammarus villosus (Sowinsky, 1894) or Ponto-Caspian gobies, via the southern corridor. D. villosus has been discovered in the lower reaches of the River Rhine in 1994 (Bij de Vaate & Klink, 1995) and is nowadays the predominant amphipod species in the river (Alt et al., 2019). The bighead goby, Neogobius kessleri (Günther, 1861), and the monkey goby, Neogobius fluviatilis (Pallas, 1814), occur in the River Rhine since 2007 and 2009, respectively (van Kessel et al., 2009). The results of the present study resemble the findings of Hohenadler et al. (2018), who found that the Ponto-Caspian acanthocephalan parasite Pomphorhynchus laevis (Zoega in Müller, 1776) was recently co-introduced to European waterbodies together with different host species such as D. villosus and Neogobius melanostomus (Pallas, 1814). The introduction of P. cf. skrjabini into the River Rhine and its tributaries could be another example of a host-parasite system which originates from the Ponto-Caspian region.
The mechanism of the introduction of new parasite species to local hosts through an invasive species, and the resulting increase of prevalence in native host communities is called parasite spillover (e.g. Hohenadler et al., 2019). It has already been observed for example by an examination of the marine parasitic copepod Mytilicola orientalis Mori, 1935, in Northern Europe, which does not only infect its principal host, the invasive Pacific oyster Crassostrea gigas (Thunberg, 1793), but also native blue mussels Mytilus edulis Linné, 1758 and common cockles Cerastoderma edule (Linné, 1758) (Goedknegt et al., 2017). Hence, it is probable that a parasite spillover of P. cf. skrjabini from its Ponto-Caspian invasive hosts, e.g. amphipods and/or gobies, to the Northern-European T. fluviatilis occurred in the River Rhine system. Whilst the Danubian form potentially experienced some kind of co-evolution due to the already occurred infestation with P. skrjabini in its native range (Chernogorenko et al., 1978), the Northern European form may represent a new host for the parasitic trematode in its introduced region of the River Rhine and its tributaries. Histopathological findings in the snail’s organs can be associated with trematode infection as well as environmental stressors at the sampling sites. Trematode-associated effects on reproductive organs of infected snails are well studied, as parasites use these resource-rich tissues to grow and develop within hosts (reviewed by Sorensen & Minchella, 2001). Increased depositions of lipo-pigments and dilatations in epithelial cells and tubules of the mollusc’s organs, e.g. the digestive system can be associated with unspecific immune reactions (Watermann et al., 2008), either towards parasite infections or environmental toxicants. However, which factors are responsible for the high prevalence and which consequences may occur on the population level of infected native Northern-European T. fluviatilis are unknown so far.
As parasites have often been demonstrated as useful indicators of environmental pollution (reviewed by Sures et al., 2017), the high prevalence of the trematode Plagioporus cf. skrjabini in Northern-European T. fluviatilis might be fostered by environmental stressors. The rivers Jagst, Kocher and Tauber are characterised by moderate pollution (water quality class II; LAWA, 2002) and high species-richness regarding macroinvertebrates (unpublished data). If the difference in the prevalence between the two forms of T. fluviatilis is caused by environmental stressors, different susceptibility to parasite pressure or a combination of both factors needs to be addressed in future studies.
In conclusion, this study shows for the first time that native populations of the Northern-European form of T. fluviatilis have been infected by the Ponto-Caspian parasitic trematode P. cf. skrjabini. Presumably, the trematode has been co-introduced to the Upper River Rhine system through the invasion of its second intermediate and final hosts (Ponto-Caspian amphipods and gobies). The presence of both the non-indigenous species of parasites and hosts outside their natural ranges can have an essential impact on native biota. As the effects of interactions are difficult to estimate, emphasis should be placed on monitoring the condition of native populations such as the Northern-European form of T. fluviatilis.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alt, K. G., S. Emde, J. Kochmann, D. D. Doerge & S. Klimpel, 2019. The Main River and Main-Danube canal: a hub for Ponto-Caspian parasite invasion. In: Mehlhorn, H. & S. Klimpel (eds) Parasite and Disease Spread by Major Rivers on Earth. Parasitology Research Monographs 12. Springer, Cham.
Behrens-Chapuis, S., T. Malewski, E. Suchecka, M. F. Geiger, F. Matthias, F. Herder & W. Bogdanowicz, 2018. Discriminating European cyprinid specimens by barcode high–resolution melting analysis (Bar-HRM): a cost efficient and faster way for specimen assignment? Fisheries Research 204: 61–73.
Bij de Vaate, A. & A. G. Klink, 1995. Dikerogammarus villosus Sowinski (Crustacea: Gammaridae) a new immigrant in the Dutch part of the Lower Rhine. Lauterbornia 20: 51–54.
Bij de Vaate, A., K. Jazdzewski, H. A. M. Ketelaars, S. Gollasch & G. Van der Velde, 2002. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 59: 1159–1174.
Bunje, P. M. E., 2005. Pan-European phylogeography of the aquatic snail Theodoxus fluviatilis (Gastropoda: Neritidae). Molecular Ecology 14: 4323–4340.
Bush, A. O., K. D. Lafferty, J. M. Lotz, A. W. Shostak, et al., 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83: 575–583.
Chernogorenko, M. I., T. I. Komarova & D. P. Kurandina, 1978. The life cycle of the trematode Plagioporus skrjabini Kowal, 1951 (Allocreadiata, Opecoelidae). Parazitologiia 12: 479–486.
Cichy, A., A. Faltýnková & E. Zbikowska, 2011. Cercariae (Trematoda, Digenea) in European freshwater snails: a checklist of records from over one hundred years. Folia Malacologica 19: 165–189.
Cribb, T. H., 2002. Family Opecoelidae Ozaki, 1925. In: Keys to the Trematoda. Vol. 2: 768 pp.
Emde, S., J. Kochmann, T. Kuhn, M. Plath & S. Klimpel, 2014. Getting what is served? Feeding ecology influencing parasite-host interactions in invasive round goby Neogobius melanostomus. PLoS ONE 9: e109971.
Esch, G. W., M. A. Barger & K. J. Fellis, 2002. The transmission of digenetic trematodes: style, elegance, complexity. Integrative and Comparative Biology 42: 304–312.
Gergs, R., M. Koester, K. Grabow, F. Schöll, A. Thielsch & A. Martens, 2015. Theodoxus fluviatilis’ re-establishment in the River Rhine: a native relict or a cryptic invader? Conservation Genetics 16: 247–251.
Goedknegt, M. A., A.-K. Schuster, C. Buschbaum, R. Gergs, A. S. Jung, P. C. Luttikhuizen, J. van der Meer, K. Troost, K. M. Wegner & D. W. Thieltges, 2017. Spillover but no spillback of two invasive parasitic copepods from invasive Pacific oysters (Crassostrea gigas) to native bivalve hosts. Biological Invasions 19: 365–379.
Herbison, R., C. Lagrue & R. Poulin, 2018. The missing link in parasite manipulation of host behaviour. Parasites & Vectors 11: 222.
Hohenadler, M. A. A., M. Nachev, M. Freese, J. D. Pohlmann, R. Hanel & B. Sures, 2019. How Ponto-Caspian invaders affect local parasite communities of native fish. Parasitology Research 118: 2543–2555.
Hohenadler, M. A. A., M. Nachev, F. Thielen, H. Taraschewski, D. Grabner & B. Sures, 2018. Pomphorhynchus laevis: an invasive species in the river Rhine? Biological Invasions 20: 207–217.
Holdich, D. M., J. D. Reynolds, C. Souty-Grosset & P. J. Sibley, 2009. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowledge and Management of Aquatic Ecosystems 394–395: 11.
Huang, X. & A. Madan, 1999. CAP3: a DNA sequence assembly program. Genome Research 9: 868–877.
IKSR/CIPR/ICBR, 2012. Das Makrozoobenthos des Rheins 2012. IKSR/CIPR/ICBR-report 227, 56 pp.
van Kessel, N., M. Dorenbosch & F. Spikmans, 2009. First record of Pontian monkey goby, Neogobius fluviatilis (Pallas, 1814), in the Dutch Rhine. Aquatic Invasions 4: 421–424.
Koester, M. & R. Gergs, 2014. No evidence for intraguild predation of Dikerogammarus villosus (Sowinsky 1894) at an invasion front in the Untere Lorze, Switzerland. Aquatic Invasions 9: 489–497.
Koester, M. & R. Gergs, 2017. Laboratory protocol for genetic gut content analyses of aquatic macroinvertebrates using group-specific rDNA primers. Journal of Visualized Experiments 128: e56132.
LAWA, 2002. Gewässergüteatlas der Bundesrepublik Deutschland. Biologische Gewässergütekarte 2000. Länderarbeitsgemeinschaft Wasser (LAWA). LAWA-report, 70 pp.
Mulisch, M. & M. Welsch, 2010. Romeis-Mikroskopische Technik. Springer Spektrum, Berlin.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2020. Vegan: Community ecology package. Version 2.5-7, https://CRAN.R-project.org/package=vegan.
Posada, D., 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256.
Prenter, J., C. MacNeil, J. T. A. Dick & A. M. Dunn, 2004. Roles of parasites in animal invasions. Trends in Ecology and Evolution 19: 385–390.
R Core Team, 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Version 3.6.3, https://www.R-project.org/.
Ran, R., Q. Zhao, A. M. I. Abuzeid, Y. Huang, Y. Liu, Y. Sun, L. He, X. Li, J. Liu & G. Li, 2020. Mitochondrial genome sequence of Echinostoma revolutum from Red-Crowned Crane (Grus japonensis). Korean Journal of Parasitology 58: 73–79.
Richling, I. & K. Groh, 2018. Ergebnisse der Herbstexkursion der Arbeitsgemeinschaft Mollusken BW in Tauberfranken (Baden-Württemberg) im Oktober 2016. Mitteilungen der Deutschen Malakozoologischen Gesellschaft 98: 45–60.
Rothmeier, L. & A. Martens, 2019. Die Neubesiedlung des Oberrheins durch Theodoxus fluviatilis: die Ausbreitung im Rheinhafen Karlsruhe. Lauterbornia 86: 39–45.
Rothmeier, L. M., A. Martens, B. Watermann, M. Feibicke, J. Kullwatz & R. Gergs, 2020. Effects of copper ions on non-target species: a case study using the grazer Theodoxus fluviatilis (Gastropoda: Neritidae). Bulletin of Environmental Contamination and Toxicology 105: 62–66.
Schliep, K. P., 2011. Phangorn: phylogenetic analysis in R. Bioinformatics 27: 592–593.
Sorensen, R. E. & D. J. Minchella, 2001. Snail-trematode life-history interactions: past trends and future directions. Parasitology 123: S3–S18.
Sures, B., M. Nachev, C. Selbach & D. J. Marcogliese, 2017. Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology’. Parasites & Vectors 10: 65.
Söderhäll, K. & L. Cerenius, 1999. The crayfish plague fungus: history and recent advances. Freshwater Crayfish 12: 11–35.
van Steenkiste, N., S. A. Locke, M. Castelin, D. J. Marcogliese & C. L. Abbott, 2015. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Molecular Ecology Resources 15: 945–952.
Westermann, F., F. Schöll & A. Stock, 2007. Wiederfund von Theodoxus fluviatilis im nördlichen Oberrhein. Lauterbornia 59: 67–72.
Watermann, B., A. Thomsen, H. Kolodzey, B. Daehne, M. Meemken, U. Pijanowska & G. Liebezeit, 2008. Histopathological lesions of molluscs in the harbour of Norderney, Lower Saxony, North Sea (Germany). Helgoland Marine Research 62: 167–175.
Zettler, M. L., 2008. Zur Taxonomie und Verbreitung der Gattung Theodoxus Montfort, 1810 in Deutschland. Darstellung historischer und rezenter Daten einschließlich einer Bibliografie. Mollusca 26: 13–72.
Acknowledgements
Special thanks go to the Deutsche Bundesstiftung Umwelt (DBU) for a Ph.D. fellowship to Louisa Marie Rothmeier and to the German Environment Agency for financial support. We also thank Prof. Dr. Tadeusz Malewski from the Museum and Institute of Zoology of the Polish Academy of Sciences, Warsaw, Poland, for his supporting expertise regarding trematode genetics, and Anja Thomsen from LimnoMar Laboratory for Freshwater and Marine Research, Hamburg, Germany, for histological preparation of snails.
Funding
Open Access funding enabled and organized by Projekt DEAL. Louisa Marie Rothmeier was supported with a PhD fellowship by Deutsche Bundesstiftung Umwelt. Financial support was given by the German Environment Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest and no competing interests.
Additional information
Handling editor: Diego Fontaneto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rothmeier, L.M., Sahm, R., Watermann, B. et al. The Ponto-Caspian parasite Plagioporus cf. skrjabini reaches the River Rhine system in Central Europe: higher infestation in the native than in the introduced Danubian form of the gastropod Theodoxus fluviatilis. Hydrobiologia 848, 2569–2578 (2021). https://doi.org/10.1007/s10750-021-04578-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04578-x