Abstract
The occurrence of various chrysophyte cyst morphotypes is unknown in Finland, with the exception of a few isolated lake studies. We set out to chart which cyst types are found in Finland and what their ecological preferences are, focusing on cyst-air temperature relationships that could be further utilized in reconstructing past winter/spring air temperatures and ice-free periods from sedimentary cyst assemblages. Surface sediment samples from lakes across Finland were analysed for their chrysophyte stomatocyst assemblages. Multivariate ecological techniques (e.g. canonical correspondence analysis, principal component analysis) were used to identify the environmental variables that most strongly affected the distribution of the cysts. This survey expanded the known geographical range for several cyst types. Lake water pH and ice-free periods (surrogate for air temperature) explained the statistically significant distribution and composition of the cyst assemblages studied. The results broaden our knowledge of cyst biogeography and strengthen the findings of previous studies of the environmental factors contributing to the occurrence of cysts. Highly variable and rich chrysophyte cyst assemblages in Finland are clearly associated with temperature, pH, electrical conductivity and total phosphorus, with good potential in contemporary and retrospective environmental assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chrysophytes, also known as golden algae or golden-brown algae (classes Chrysophyceae and Synurophyceae), usually form an essential part of algal communities in cool, high-latitude, oligotrophic lakes, such as the majority of lakes in Finland (Eloranta, 1995; Sandgren et al., 1995). All chrysophytes produce siliceous resting stages, often previously referred to as statospores, but now more properly called stomatocysts or simply cysts, to ensure the survival of the population under unfavourable environmental conditions in the form of a seed bank. Many phytoplankton groups produce resting stages with long-term persistence, in some cases up to at least a century (Ellegaard & Ribeiro, 2018). Germination from chrysophyte cysts at least 60 years old has been reported in lake sediments from Sweden (Cronberg, 1982), but the exact longevity of chrysophyte cysts remains yet unknown (Ellegaard & Ribeiro, 2018).
Chrysophyte cysts are produced endogenously, either asexually or sexually, resulting in an identical-looking cyst whose morphology is considered to be species-specific (Sandgren, 1983, 1991). Chrysophyte cysts possess a hard siliceous cyst wall resistant to dissolution, a characteristic that, together with the sheer numbers of cysts produced, makes them ideal subjects in palaeolimnological investigations. To date, only a small percentage of chrysophyte stomatocysts have successfully been linked with their vegetative stages. This shortcoming led to the development of an artificial naming system created by the International Statospore Working Group (Cronberg & Sandgren, 1986). These guidelines have been extensively modified and revised in subsequent taxonomic treatments (Duff et al., 1995; Wilkinson et al., 2001).
During recent decades, several cyst floras from different geographical regions have been published, e.g. Central Europe (Facher & Schmidt, 1996), the Azores (Hansen, 2001), the Pyrenees (Pla, 2001), sub-Antarctica (van de Vijver & Beyens, 1997, 2000) and China (Pang & Wang, 2014, 2016; Bai et al., 2018). The most expansive floras are the Atlas of Chrysophycean Cysts (Duff et al., 1995) and the Atlas of Chrysophycean Cysts Volume II (Wilkinson et al., 2001), focusing largely on North American temperate and Arctic locations. In addition to cyst identification floras, several palaeolimnological studies throughout the world have successfully linked cyst morphotypes with various environmental variables in the form of cyst-based transfer functions, e.g. for pH (Rybak et al., 1991; Facher & Schmidt, 1996; Pla & Anderson, 2005), temperature (Kamenik & Schmidt, 2005; Pla-Rabes & Catalan, 2005; de Jong et al., 2016; Hernández-Almeida et al. 2015a), salinity (Zeeb & Smol, 1995) and water chemistry (Duff & Smol, 1995; Pla et al., 2003). However, chrysophyte cysts are not as widely used as diatoms or pollen in palaeolimnological research, mostly due to the challenges in linking chrysophyte species with the stomatocysts they produce. The studies that have utilized cysts have revealed their great potential for reconstructing past environmental conditions, particularly winter- and spring-related climate parameters, which is a great advantage, since most other palaeobioindicators are related to summer conditions only (e.g. Kamenik & Schmidt, 2005; Pla-Rabes & Catalan, 2005).
Even though chrysophyte stomatocysts have even been associated with carnivorous plants (Wolowski et al., 2011) and have also been found in a thermal spring in Egypt (Piątek et al., 2009), there still remain geographic regions where no comprehensive knowledge of the cyst flora is available. One of these areas is Northern Continental Europe. This is the first large-scale study aiming to describe the distribution and ecological preference of various chrysophyte cyst types in Finland, ‘the land of a thousand lakes’.
Materials and methods
Study sites
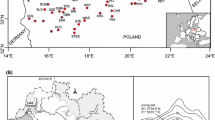
The study sites comprised 59 lakes located throughout Finland, ranging from the clear-water ultraoligotrophic cold lakes in the Hemiarctic Zone to the warmer, more nutrient-rich humic lakes in the Hemiboreal Zone (Fig. 1). The ice-free period varies typically from approximately 8 months in the southern parts to only 4 months in northernmost Finnish Lapland (Table 1). Spring mixing usually occurs during May in the south, autumnal mixing in October. In northern Finnish Lapland, spring mixing has been observed in July and a longer autumnal mixing in September–October (Forsström et al., 2005). The study lakes were chosen according to their available water chemistry data (Oiva, 2015), with the main object of keeping the water pH close to neutral (pH 7), total phosphorus (tot-P) concentration below 20 µg/l and lakes with as little human influence in the drainage area as possible. Electrical conductivity values varied between 5 and 164 µS/cm.
The surface sediment chrysophyte cyst assemblages from 59 lakes were sampled in 2007 and 2008 with a Limnos-type sediment corer. The top 1 cm of sediment was retrieved from the deepest part of the lake and stored in the dark in Minigrip bags at + 4°C prior to laboratory analysis. Nineteen of the lakes are located in southern or central Finland (SF), seven in southern and eastern Lapland (SYKE) and 33, mostly in western Finnish Lapland (WFL), both above and below the tree line (Fig. 1).
Sample preparation
The sediment samples were cleaned according to the standard treatment for diatoms (Weckström et al., 1997; Battarbee et al., 2001), i.e. by digesting the sediment in hydrogen peroxide (H2O2) and adding a few drops of hydrochloric acid (HCl, 37%) before repeated washing with distilled water. The cleaned chrysophyte cyst aliquot suspension was dried on scanning electron microscope (SEM) stubs and left uncoated. A minimum of 200 cysts per sample were counted and identified from SEM (JEOL JSM-840A, Hitachi S-4800) images. Unornamented cysts in which the pore morphology could not be observed were classified, using probabilistic counting, i.e. they were classified as a specific morphotype based on the percentage distribution of different morphotypes in the same sample. The cyst identification was based on Duff et al. (1995), Facher & Schmidt (1996), Pla (2001), Wilkinson et al. (2001) and Baumann et al. (2010).
Water chemistry data
The water chemistry data were retrieved from the Finnish Environment Institute’s Oiva database (Oiva, 2015) for nationwide lake water chemistry information. The used data are average values of 1–51 in situ measurement conducted between years 1997 and 2008.
Climate data
The meteorological data are part of the Finnish Meteorological Institute´s ClimGrid dataset described in Aalto et al. (2016), derived from the spatial data for research and teaching database (PaiTuli). The daily and monthly temperature data are based on a spatial resolution of 10 × 10 km. Due to the variable sediment-accumulation rates in lakes located in different vegetation/climatic zones, and thus a different temporal extent of the topmost 1-cm sediment sample, daily and monthly temperature data for 10 years were used for sites located in the Middle, Southern and Hemiboreal zones, 20 years for Northern Boreal Zone lakes and 30 years for lakes in the Hemiarctic (Weckström et al., 2014). The temperature data for different seasons were based on the monthly mean temperatures of 3 months per season (i.e. March, April and May for spring; June, July and August for summer; September, October and November for autumn; and December, January and February for winter). The ice-free period for each lake was calculated, using the melting degree-day value of + 130 and a freezing degree-day value of − 30, based on Thompson et al. (2005) and Weckström et al. (2014).
Numerical methods
In total, 17 environmental variables (altitude, alkalinity, conductivity, total nitrogen, total-phosphorus (tot-P), pH, surface area, sampling depth, spring mean temperature, summer mean temperature, autumn mean temperature, winter mean temperature, July mean temperature, January mean temperature, ice-free period, latitude, longitude) were used to study the relationship between the chrysophyte cysts and the environmental variables. All the environmental data except pH were log10- transformed prior to the statistical analyses.
Principal component analysis (PCA) was used to summarize the major patterns of variation within the 10 environmental variables screened (Fig. 2). The vegetation zones (Hemiarctic, Northern Boreal, Middle Boreal, Southern Boreal and Hemiboreal) were added as supplementary, i.e. passive, variables. Species richness at constant sample counts was estimated by rarefaction, which is well suited for stratigraphical species data with different count sizes between individual samples (Birks & Line, 1992). The Shannon H´ diversity index was used to evaluate the species diversity in the samples (Shannon & Weaver, 1949). Rarefaction and Shannon H´ analysis were conducted, using Palaeontological Statistics (PAST) statistics software 3.1. (Hammer et al., 2001). A species abundance diagram (Fig. 3) was created, using the program C2 (version 1.7.2., Juggins, 2007). Due to the high species turnover rate (DCA axis one length of 2.3 SD) between the surface sediment assemblages, canonical correspondence analysis (CCA) was used to analyse the species distribution across the environmental gradients measured. Monte Carlo permutation tests with 999 permutations were used to assess the statistical significance of the primary ordination axis and overall models. To assess the sensitivity of the chrysophyte cysts to the climate variables (mean July temperature and ice-free period), pH, and tot-P, the optima and tolerances for independent chrysophyte cysts were calculated, using the weighted-averaging (WA) regression technique (ter Braak & van Dam, 1989) in the program C2. All cysts were transformed to percentage abundances and square-root-transformed prior to the statistical analyses, which were performed using CANOCO 5.01 (ter Braak & Šmilauer, 2007–2012). All taxa were included in the PCA and CCA; however, only the most common taxa (the same as in Fig. 3) were plotted in Fig. 4.
Principal component analysis of the study lakes and their environmental variables in Finland. The vegetation zones indicated with star symbols were added as passive variables. Lake symbols refer to the vegetational zones shown in Fig. 1, square = Hemiboreal, triangle = Southern Boreal, swirl = Middle Boreal, circle = Northern Boreal, diamond = Hemiarctic (Tot-N = total nitrogen, Tot-P = total phosphorus)
Results
The study lakes are divided into three groups according to their location. The WFL lakes are mostly located in the Hemiarctic Vegetation Zone at a higher elevation, with the drainage area consisting of mountain birch (Betula pubescens ssp. czerepanovii (Orl.) Hämet-Ahti) forest or barren land if located above the treeline (Fig. 1). The PCA reveals that the WFL lakes are associated with low temperatures, low phosphorus concentrations, low conductivity and shorter ice-free periods (Fig. 2). The SYKE and SF lakes (Fig. 2) with coniferous forests or mixed vegetation in the drainage area belong to the Northern, Middle, Hemi- and Southern Boreal zones. These lakes are associated with higher temperatures for all seasons, higher tot-P concentrations, higher conductivity and longer ice-free periods. The SYKE lakes form a continuum between the WFL and SF lakes.
In all, 253 cyst morphotypes or collective categories were identified from the Finnish sediment material, 32 of which occurred at > 7% proportions in at least one lake (Fig. 3). SEM images of some of the most common cyst types found in Finland are shown in Online Resource 1. The number of morphotypes or collective categories per lake varied between 30 and 66; in general, the number of different morphotypes decreased from the southern lakes towards the northern lakes with barren drainage areas. A similar trend can be observed in the Shannon H´ index. Unornamented cyst surfaces form the majority of all cyst ornamentation types in lake sediments from Finland, their relative occurrence ranging between 31% and 71% per sample, with an average of 54% of cysts counted per sample. No clear association between cyst ornamentation type and the various vegetation zones was observed.
The majority of the cysts found are featured in the two cyst atlases; codes from PEARL 1 to PEARL 243 refer to Duff et al. (1995) and the rest of the PEARL codes refer to Wilkinson et al. (2001). The most common cyst types in Finland are PEARL 1, PEARL 9 and PEARL 50&52 (Duff et al., 1995). These are all collective categories: fairly small, unornamented and most likely produced by several different species of chrysophytes (Duff et al., 1995). Cysts that occur throughout Finland also include cyst types PEARL 15, 10, 116, 41 (Dinobryon cylindricum Imhof; Donaldson & Stein, 1984), 94, 234, 89 and 120. Of the most common cyst types only two, PEARL 115 and PEARL 75, are clearly linked with the Southern Boreal Vegetation Zone, with no particular cysts associated only with the narrow Hemiboreal Vegetation Zone. Cysts associated with vegetation zones spanning from the Hemiboreal to the Northern Boreal Zone include PEARL 204, 161 and 178. Cyst types preferring the Hemiarctic Vegetation Zone include S016 (Pla, 2001), PEARL 31, small PEARL 152 and PEARL 57.
The initial 17 environmental variables captured 36.9% of the total variance of the cyst data. Based on the known ecological preferences of the cysts, a subset of four environmental variables (pH, conductivity, ice-free period and tot-P) were chosen to further determine the relationship between the enumerated cysts and the environment. These four variables captured 14.4 of the total variance of the cyst data and 39% of the total variance explained by the initial 17 environmental variables, with sole explanatory power of 5.4% (pH), 5% (conductivity), 4.8% (ice-free period) and 4.1% (tot-P). All of these variables were statistically significant (P < 0.001). The CCA showed that the majority of the most common cysts were located close to the origin, i.e. they showed no clear preference for any of the environmental variables (Fig. 4).
The calculated optima show that the cysts PEARL 115, 204, 161, 178, 116, 75 and 50&52 prefer longer ice-free periods and higher tot-P concentrations (Fig. 5a, b). In contrast, Chrysococcus furcatus (Dolgoff) K.H. Nicholls vegetative cell, S016 (Pla, 2001), PEARL 31, 16, and small 152 favour short ice-free times and low tot-P concentrations. The cysts PEARL 86, 16, 11, 223 and 57 seem to prefer pH of 6.5 or less, while PEARL 115, 161, 204, 178 and 116 prefer higher conductivity (> 50 µS/cm).
Discussion
The most frequently occurring cysts found in this study were mainly cosmopolitan types found commonly in many previous studies (e.g. Brown et al., 1997; Pla & Anderson, 2005). These cyst types were mostly collective categories, in which similar-looking cysts were most likely produced by different species, possibly with different environmental preferences. Many of these were unornamented, leaving only size and pore morphology as identification criteria. Cyst types in Finland concorded with previous observations that unornamented cysts seem to predominate in most lakes and are common in all habitats (Duff et al., 1992; Wilkinson et al., 1997; Stewart et al., 2000; Betts-Piper et al., 2004; Pla & Anderson, 2005).
Cyst types in different vegetation zones
Of the most frequently occurring cysts, one type, PEARL 115, occurred only in the Southern Boreal Vegetation Zone. It has previously been associated with productive lakes and has been most abundant in slightly acidic to circumneutral lakes (Zeeb et al., 1990; Duff et al., 1995). Brown et al. (1997) have also found this cyst type in lakes with relatively high temperature in northwestern Canada. Our results show that this cyst type favours lakes with higher tot-P values and conductivity, long ice-free periods, but unlike in previous studies, it has been found in Finland in a slightly alkaline lake. This may also have been an artificial result, since PEARL 115 was only found in the sediment of one lake in Finland, causing the environmental range for this cyst to be somewhat limited.
PEARL 75 also occurred mainly in lakes located in the Southern Boreal Vegetation Zone. This cyst type is most likely produced by Dinobryon bavaricum Imhof, which has been found in Finland throughout the ice-free season and prefers clear oligotrophic lakes, although it has often also been found in mesotrophic lakes (Eloranta, 1989; Lepistö, 1999). Other cyst types preferring lakes located in the southern and central parts of Finland include PEARL 204 and 161 produced by Uroglena volvox Ehrenberg (Kristiansen 1980) and by Dinobryon divergens Imhof, respectively. In Finland, Dinobryon divergens is a common species in all but the most eutrophic lake types (Lepistö & Rosenström, 1998).
Cyst types occurring from the Hemiboreal to the Northern Boreal zones include PEARL 11, Facher & Schmidt # 23 (Facher & Schmidt, 1996), resembling PEARL 11 without the characteristic ridge extending from the collar, and PEARL 223. PEARL 11 has previously been observed at least in Svalbard (Betts-Piper et al., 2004) and in Arctic peat in Siberia, Russia (Gilbert et al., 1997). In a seasonality study of one particular lake in Finland (Korkonen et al., 2017), PEARL 11 occurred in small numbers mainly in spring cyst assemblages. Cyst Facher & Schmidt # 23 was also found in high numbers in Central European lakes with no clear ecological preference (Facher & Schmidt, 1996).
Cysts associated with northern boreal and hemiarctic lakes included PEARL 189 and PEARL 308. PEARL 189 has also been found in, e.g. Greenland and Ellesmere Island in the Canadian Arctic Archipelago and British Columbia, Canada, which may indicate that it is produced by cold-tolerant species (Duff et al., 1992; Brown et al., 1994; Duff & Smol, 1994). Brown et al. (1997) found and described PEARL 308 in lakes with relatively high temperature in northwestern Canada at relatively low latitudes.
PEARL 31 has also previously been considered to be produced by a cold-tolerant species. In our study, it was among the few that showed the optimum for an ice-free period of 140 days or less, which supports this hypothesis. S016 (Pla, 2001) has been described from lakes in the Pyrenees, and in the Finnish material it has mainly been found in hemiarctic lakes, i.e. lakes at high altitude in Western Finnish Lapland. S016 differs from PEARL 16 in collar-pore morphology and size. Both forms were among the most common cyst types in the Finnish material but they showed differences in their temperature and pH preferences. PEARL 16 prefers longer ice-free times and lower pH. Although also occurring in small numbers in other vegetation zones, PEARL 57 is found at greatest abundance in the Hemiarctic Zone. It has previously been found in oligotrophic locations (Duff & Smol, 1991; Rybak et al., 1991), which is also supported by this study (Fig. 5b). In Finland, it has previously been linked with the summer season in a mesotrophic lake (Korkonen et al., 2017).
Cysts and environmental variables
Temperature, pH and conductivity were among the most important variables affecting chrysophyte communities and their resting stages in several studies (e.g. Siver & Hamer, 1992; Duff & Smol, 1995; Stewart et al., 2000). According to Hernández-Almeida et al. (2015b), the general trend with factors controlling cyst assemblages at different locations indicates that temperature is an important controller of cyst assemblages in alpine and subalpine lakes, while water chemistry, including conductivity, pH and nutrients plays a bigger role in low-lying lake assemblages (Kamenik & Schmidt, 2005; Pla & Anderson, 2005; Hernández-Almeida et al., 2015b; de Jong et al., 2016). Ice break-up controlled by spring air temperatures affects stomatocyst assemblages (Kamenik & Schmidt, 2005; de Jong & Kamenik, 2011). Siver (1995) suggested that the ability of chrysophytes to survive under ice cover gives them a head start once the ice melts, while the low grazing pressure may add to the maximal biomass (Sandgren & Walton, 1995). The importance of chrysophyte cyst relationships to winter and spring conditions is highlighted in the scarcity of the palaeobioindicators available for reconstruction of past winter and spring temperatures.
In this study, pH, ice-free period (a surrogate for temperature), conductivity and tot-P were shown to statistically significantly explain the distribution of morphotypes in Finland (Fig. 4). Many of the morphotypes in Fig. 4 not showing clear preferences for the environmental variables, i.e. PEARL 1, 9, 15, 120, 150, 189, 234, PEARL 50&52, RidgeS and SpineS are collective categories produced by several different species (Duff et al., 1995). PEARL 115 stands out in the most commonly occurring cyst assemblages by displaying the highest optima (Fig. 5) for ice-free periods, pH, tot-P and conductivity. This may have been an artefact caused by the fact that this cyst type was only found in one lake, and the data range was therefore limited.
Chrysophytes are among the first algae to bloom in cold spring waters, some of the taxa even living under the ice (Cronberg, 1980; Siver & Hamer, 1992). The calculated ice-free period optima for cysts in Finland support previous studies in which a clear link has been found between spring temperatures/ice-cover times and cyst assemblages (Kamenik & Schmidt, 2005; Hernández-Almeida et al., 2015a). In the Pyrenees (Pla, 2001), S016 occurred only in low-elevation lakes, while in the Finnish lake material it displayed the shortest ice-free optimum and was mainly found in one lake in Western Finnish Lapland. A small version of PEARL 152 was found only in lakes in western Finnish Lapland thus displaying short ice-free period optimum. PEARL 152 was previously reported at least in Minnesota USA (Zeeb & Smol, 1993), where it was interpreted as an indicator of earlier springs, British Columbia in Canada (Duff & Smol, 1994) and Poland (in Rybak, 1987 as Cyst 2). PEARL 31 and 16 have also previously been interpreted as being produced by cold-tolerant species, as was the species Chrysococcus furcatus, whose vegetative cell in this study was associated with low tot-P levels, low conductivity and short ice-free time (Duff et al., 1995).
Cysts that were more commonly reported in lakes with longer ice-free periods include PEARL 204, produced by Uroglena volvox, and PEARL 161, produced by Dinobryon divergens, a species commonly found in phytoplankton assemblages in lakes in Finland. Dinobryon divergens has also been known to bloom under the ice cover, with the highest stomatocyst formation just before the onset of ice cover under oligotrophic, low-light, high-bacterial abundance conditions (Watson et al., 2001). In Finland, the highest accumulations of the cyst PEARL 161 were reported during summer (Korkonen et al., 2017). Our study indicates that in Finland, PEARL 116 prefers long ice-free periods, unlike in Elk Lake, Minnesota (Zeeb & Smol, 1993), where this cyst was suggested to be more common in lakes with later-than-average ice-out and deep snow.
Unlike many other phytoplankton groups in which a higher number of species can be observed in eutrophic habitats, chrysophytes usually thrive under oligotrophic conditions (Eloranta, 1986; Willén, 1987; Siver, 1995; Lepistö & Rosenström, 1998). Lakes with high phosphorus and nitrogen levels have been observed sustaining a decreased abundance of cysts (Smol, 1985; Wilkinson & Smol, 1998). The data range in our study for nutrients was not wide enough for this trend to be observed, since the lakes in this study are either oligotrophic or mesotrophic. PEARL 115 and 204 produced by Uroglena volvox and PEARL 116, 178 and 161 produced by Dinobryon divergens have also previously been associated with productive lakes (Duff et al., 1995; Lepistö, 1999). PEARL 116 has been linked with meso/eutrophy in palaeolimnological studies (Zeeb et al., 1990; Zeeb & Smol, 1993). PEARL 75, the cyst of Dinobryon bavaricum, however, has previously been linked with oligotrophic lakes (Duff et al., 1995; Eloranta, 1995). Lepistö (1999) showed that this chrysophyte species is also often observed in mesotrophic lakes. Our study on cysts associates PEARL 75 with slight mesotrophic conditions (Fig. 5b). Cysts associated with low phosphorus levels in this study include S016, originally described from material from Pyrenean lakes with low phosphorus content (Pla, 2001). PEARL 16 was also an indicator of low phosphorus in this study, the environmental preferences being similar to S016 regarding conductivity, but the pH and ice-free period optima differed considerably. Duff et al. (1995) described PEARL 31 as having a wide range for trophic status. In Finland, PEARL 31 can be interpreted as an indicator for low phosphorus levels, the cyst being mainly found in oligotrophic and ultraoligotrophic cold, clear-water northern lakes.
Our research supports previous studies in which pH was one of the most important variables that controlled the distribution of chrysophytes and their cysts (Duff & Smol, 1991; Carney et al., 1992; Siver, 1995; Duff et al., 1997; Wilkinson & Smol, 1998; Hansen, 2001; Pang & Wang, 2014). The most diverse cyst assemblages have been found in neutral and slightly acidic lakes and ponds, as in most of the lakes in this study (Adam & Mahood, 1981; Hansen, 2001; Pang & Wang, 2014). PEARL 86, 11, 16 and 57, found in Finland, seem to be produced by chrysophyte species tolerant of lower-pH environments, which is also backed up by previous studies elsewhere (Rybak et al., 1991; Facher & Schmidt, 1996; Duff et al., 1997; Betts-Piper et al., 2004). A cyst similar to PEARL 86, S086 (Pla, 2001), has a higher pH optimum, but since its size is recorded as being considerably bigger than that reported by Duff et al. (1995), it could be produced by a different species. Dinobryon divergens has been found in many types of lakes in Finland, including very acidic, species-poor lakes (Lepistö & Rosenström, 1998). Our study, however, associates the cyst PEARL 161, produced by this species, at the higher end of our pH range.
Conductivity is one of the key factors controlling cyst assemblages (Duff et al., 1997; Pla & Anderson, 2005; Hernández-Almeida et al., 2015b; de Jong et al., 2016). Cumming & Smol (1993) and Duff et al. (1997) stated that conductivity (i.e. salinity) probably affects algal species through the cells’ adaptability to external osmotic pressure changes in their surroundings. Most cysts in lakes in Finland prefer conductivity levels between 20 and 50 µS/cm. Our results show that the cysts PEARL 115, 161, 204, 178 and 116 could be considered as indicators of slightly higher conductivity values. PEARL 204 and 116 have also previously been associated with high conductivity values (Duff et al., 1995; Zeeb & Smol, 1995).
Conclusions
Highly diverse chrysophyte cyst assemblages are preserved in Finnish lake sediments, adding Northern Continental Europe to the areas where chrysophyte cysts have been surveyed on a larger scale. While many cyst types are common and found in various locations, our study expands the known geographical range for several of these cyst types to previously uncharted territory. Chrysophyte cyst assemblages in Finland are clearly associated with pH, ice-free periods (as a surrogate for temperature), electrical conductivity and tot-P, providing an additional tool with high potential for use in palaeolimnological environmental assessments, particularly in reconstructing past winter/spring temperatures, a challenging area due to the current scarcity of suitable palaeoproxies.
References
Aalto, J., P. Pirinen & K. Jylhä, 2016. New gridded daily climatology of Finland: permutation-based uncertainty estimates and temporal trends in climate. Journal of Geophysical Research: Atmospheres 121: 3807–3823.
Adam, D. P. & A. D. Mahood, 1981. Chrysophyte cysts as potential environmental indicators. Geological Society of America Bulletin Part 1(92): 839–844.
Bai, X., Z. J. Bu & X. Chen, 2018. Morphology of Chrysophycean stomatocysts in three peatlands in central China. Mires and Peat 21(19): 1–16.
Battarbee, R. W., V. J. Jones, R. J. Flower, N. G. Cameron, H. Bennion, L. Carvalho & S. Juggins, 2001. Diatom analysis. In Smol, J. P., H. J. B. Birks & W. M. Last (eds), Tracking Environmental Change Using Lake Sediments, Vol. 3., Terrestrial, Algal, and Siliceous Indicators Kluwer Academic Publishers, Dordrecht: 155–202.
Baumann, E., R. de Jong & C. Kamenik, 2010. A description of sedimentary chrysophyte stomatocysts from high-Alpine Lake Silvaplana (Switzerland). Nova Hedwigia Beiheft 136: 71–86.
Betts-Piper, A. M., B. A. Zeeb & J. P. Smol, 2004. Distribution and autecology of chrysophyte cysts from high Arctic Svalbard lakes: preliminary evidence of recent environmental change. Journal of Paleolimnology 31: 467–481.
Birks, H. J. B. & J. M. Line, 1992. The use of rarefaction analysis for estimating palynological richness from Quaternary pollen-analytical data. Holocene 2: 1–10.
Brown, K. M., M. S. V. Douglas & J. P. Smol, 1994. Siliceous microfossils in a Holocene, High Arctic peat deposit (Nordvestø, northwestern Greenland). Canadian Journal of Botany 72: 208–216.
Brown, K. M., B. A. Zeeb, J. P. Smol & R. Pienitz, 1997. Taxonomic and ecological characterization of chrysophyte stomatocysts from northwestern Canada. Canadian Journal of Botany 75: 842–863.
Carney, H. J., M. C. Whiting, K. E. Duff & D. R. Whitehead, 1992. Chrysophycean cysts in Sierra Nevada (California) lake sediments: paleoecological potential. Journal of Paleolimnology 7: 73–94.
Cronberg, G., 1980. Cyst development in different species of Mallomonas (Chrysophyceae) studied by scanning electron microscopy. Archiv für Hydrobiologie 56: 421–434.
Cronberg, G., 1982. Changes in the phytoplankton of Lake Trummen induced by restoration. Hydrobiologia 86: 185–193.
Cronberg, G. & C. D. Sandgren, 1986. A proposal for the development of standardized nomenclature and terminology for chrysophycean statospores. In Kristiansen, J. & A. Andersen (eds), Chrysophytes: aspects and problems. Cambridge University Press, Cambridge: 317–328.
Cumming, B. F. & J. P. Smol, 1993. Development of diatom-based salinity models for paleoclimatic research from lakes in British Columbia (Canada). Hydrobiologia 269(270): 179–196.
de Jong, R. & C. Kamenik, 2011. Validation of a chrysophyte stomatocyst-based cold-season climate reconstruction from high-Alpine Lake Silvaplana, Switzerland. Journal of Quaternary Science 26: 268–275.
de Jong, R., T. Schneider, I. Hernández-Almeida & M. Grosjean, 2016. Recent temperature trends in the South Central Andes reconstructed from sedimentary chrysophyte stomatocysts in Laguna Escodida (1742 m a.s.l., 38° 28 S, Chile). Global and Planetary Change 137: 24–34.
Donaldson, D. A. & J. R. Stein, 1984. Identification of planktonic Mallomonadaceae and other Chrysophyceae from selected lakes in the lower Fraser Valley, British Columbia (Canada). Canadian Journal of Botany 62: 525–539.
Duff, K. E. & J. P. Smol, 1991. Morphological descriptions and stratigraphic distributions of the Chrysophycean stomatocysts from a recently acidified lake (Adirondack Park, N.Y.). Journal of Paleolimnology 5: 73–113.
Duff, K. E. & J. P. Smol, 1994. Chrysophycean cyst flora from British Columbia (Canada) lakes. Nova Hedwigia 58: 353–389.
Duff, K. E. & J. P. Smol, 1995. Chrysophycean cyst assemblages and their relationship to water chemistry in 71 Adirondack Park (New York, USA) lakes. Archiv für Hydrobiologie 134: 307–336.
Duff, K. E., M. S. V. Douglas & J. P. Smol, 1992. Chrysophyte cysts in 36 Canadian high arctic ponds. Nordic Journal of Botany 12: 471–499.
Duff, K. E., B. A. Zeeb & J. P. Smol, 1995. Atlas of Chrysophycean Cysts. Developments in hydrobiology 99. Kluwer Academic Publishers, Dordrecht.
Duff, K. E., B. A. Zeeb & J. P. Smol, 1997. Chrysophycean cyst biogeographical and ecological distributions: a synthesis. Journal of Biogeography 24: 791–812.
Ellegaard, M. & S. Ribeiro, 2018. The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks’. Biological Reviews 93: 166–183.
Eloranta, P., 1986. Phytoplankton structure in different lake types in central Finland. Holarctic Ecology 9: 214–224.
Eloranta, P., 1989. Scaled chrysophytes (Chrysophyceae and Synurophyceae) from national park lakes in southern and central Finland. Nordic Journal of Botany 8: 671–681.
Eloranta, P., 1995. Biogeography of chrysophytes in Finnish lakes. In Sandgren, C. D., J. P. Smol & J. Kristiansen (eds), Chrysophyte Algae. Ecology, Phylogeny and Development. Cambridge University Press, Cambridge: 214–223.
Facher, E. & R. Schmidt, 1996. A siliceous chrysophycean cyst-based pH transfer function for Central European lakes. Journal of Paleolimnology 16: 275–321.
Forsström, L., S. Sorvari, A. Korhola & M. Rautio, 2005. Seasonality of phytoplankton in subarctic Lake Saanajärvi in NW Finnish Lapland. Polar Biology 28: 846–861.
Gilbert, S., B. A. Zeeb & J. P. Smol, 1997. Chrysophyte stomatocyst flora from a forest peat core in the Lena River Region, northeastern Siberia. Nova Hedwigia 64: 311–352.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Paleontologia Electronica 4: 1–9.
Hansen, P., 2001. Chrysophyte stomatocysts in the Azores—biogeographical implications and 110 new morphotypes. Opera Botanica 138: 1–96.
Hernández-Almeida, I., M. Grosjean, R. Przybylak & W. Tylmann, 2015a. A chrysophyte-based quantitative reconstruction of winter severity from varved lake sediments in NE Poland during the past millennium and its relationship to natural climate variability. Quaternary Science Reviews 122: 74–88.
Hernández-Almeida, I., M. Grosjean, W. Tylmann & A. Bonk, 2015b. Chrysophyte cyst-inferred variability of warm season lake water chemistry and climate in northern Poland: training set and downcore reconstruction. Journal of Paleolimnology 53: 123–138.
Juggins, S., 2007. C2 Version 1.5: Software for Ecological and Palaeoecological Data Analysis and Visualization. University of Newcastle, Newcastle upon Tyne.
Kamenik, C. & R. Schmidt, 2005. Chrysophyte resting stages a tool for reconstructing winter/spring climate from alpine lake sediments. Boreas 34: 477–489.
Korkonen, S., A. Ojala, E. Kosonen & J. Weckström, 2017. Seasonality of chrysophyte cyst and diatom assemblages in varved Lake Nautajärvi—implications for palaeolimnological studies. Journal of Limnology 76: 366–379.
Kristiansen, J., 1980. Chrysophyceae from some Greek lakes. Nova Hedwigia 33: 167–194.
Lepistö, L., 1999. Phytoplankton Assemblages Reflecting the Ecological Status of Lakes in Finland. Monographs of the Boreal Environment Research. Monograph No. 16. Finnish Environment Institute, Helsinki.
Lepistö, L. & U. Rosenström, 1998. The most typical phytoplankton taxa in four types of boreal lakes. Hydrobiologia 369(370): 89–97.
OIVA, 2015. OIVA—the environmental and geographical information service, Finland’s environmental administration. Accessed Oct 2015. http://wwwp2.ymparisto.fi/scripts/oiva.asp.
Pang, W. & Q. Wang, 2014. Chrysophycean stomatocysts from the Aershan Geological Park (Inner Mongolia), China. Phytotaxa 187: 1–92.
Pang, W. & Q. Wang, 2016. Chrysophycean stomatocysts from Xinjian Province, China. Phytotaxa 288: 41–50.
Piątek, J., M. Piątek, B. A. Zeeb & A. El Shahed, 2009. Chrysophyte stomatocysts in Africa: the first description of an assemblage in the recent sediments of a thermos-mineral spring in Egypt. Phycologia 48: 13–23.
Pla, S., 2001. Chrysophycean Cysts from the Pyrenees. Bibliotheca Phycologica, Vol. 109. Cramer in der Gebrüder Borntraeger, Berlin.
Pla, S. & N. J. Anderson, 2005. Environmental factors correlated with chrysophyte cyst assemblages in low arctic lakes of southwest Greenland. Journal of Phycology 41: 957–974.
Pla, S., L. Camarero & J. Catalan, 2003. Chrysophyte cyst relationships to water chemistry in Pyrenean lakes (NE Spain) and their potential for environmental reconstruction. Journal of Paleolimnology 30: 21–34.
Pla-Rabes, S. & J. Catalan, 2005. Chrysophyte cysts from lake sediments reveal the submillennial winter/spring climate variability in the northwestern Mediterranean region throughout the Holocene. Climate Dynamics 24: 263–278.
Rybak, M., 1987. Fossil chrysophycean cyst flora of Racze Lake, Wolin island (Poland) in relation to paleoenvironmental conditions. Hydrobiologia 150: 257–272.
Rybak, M., I. Rybak & K. Nicholls, 1991. Sedimentary chrysophycean cyst assemblages as paleoindicators in acid sensitive lakes. Journal of Paleolimnology 5: 19–72.
Sandgren, C. D., 1983. Morphological variability in populations of chrysophycean resting cysts. I. Genetic (interclonal) and encystment temperature effects on morphology. Journal of Phycology 19: 64–70.
Sandgren, C. D., 1991. Chrysophyte reproduction and resting cysts: a paleolimnologist’s primer. Journal of Paleolimnology 5: 1–9.
Sandgren, C. D., J. P. Smol & J. Kristiansen (eds), 1995. Chrysophyte Algae: Ecology, phylogeny and development. Cambridge University Press, Cambridge UK.
Sandgren, C. D. & W. E. Walton, 1995. The influence of zooplankton herbivory on the biogeography of chrysophyte algae. In Sandgren, C. D., J. P. Smol & J. Kristiansen (eds), Chrysophyte Algae. Ecology, Phylogeny and Development. Cambridge University Press, Cambridge: 269–302.
Shannon, C. E. & W. Weaver, 1949. The Mathematical Theory of Communication. The University of Illinois Press, Urbana, IL.
Siver, P. A., 1995. The distribution of chrysophytes along environmental gradients: their use as biological indicators. In Sandgren, C. D., J. P. Smol & J. Kristiansen (eds), Chrysophyte Algae: Ecology, phylogeny and development. Cambridge University Press, Cambridge: 232–268.
Siver, P. A. & J. S. Hamer, 1992. Seasonal periodicity of Chrysophyceae and Synurophyceae in a small New-England lake—implications for paleolimnological research. Journal of Phycology 28: 186–198.
Smol, J. P., 1985. The ratio of diatom frustules to chrysophycean statospores: a useful paleolimnological index. Hydrobiologia 123: 199–208.
Stewart, K., I. Gregory-Eaves, B. A. Zeeb & J. P. Smol, 2000. Covariation among Alaskan chrysophyte stomatocysts assemblages and environmental gradients: a comparison with diatom assemblages. Nordic Journal of Botany 20: 357–368.
ter Braak, C. J. F. & H. van Dam, 1989. Inferring pH from diatoms: a comparison of old and new calibration methods. Hydrobiologia 178: 209–223.
ter Braak, C. J. F. & P. Šmilauer, 2007–2012. Canoco Reference Manual and User’s Guide: Software for 623 Ordination (version 5.0). Itaca: Microcomputer power.
Thompson, R., D. Price, N. Cameron, V. Jones, C. Bigler, P. Rosén, R. I. Hall, J. Catalan, J. García, J. Weckström & A. Korhola, 2005. Quantitative calibration of remote mountain-lake sediments as climatic recorders of air temperature and ice-cover duration. Arctic, Antarctic, and Alpine Research 37: 626–635.
van de Vijver, B. & L. Beyens, 1997. The Subfossil chrysophyte cyst flora of some peat samples from Kerguelen islands. Archiv für Protistenkunde 148: 491–503.
van de Vijver, B. & L. Beyens, 2000. Chrysophycean stomatocysts from freshwater habitats of the Strømness Bay area, South Georgia, Antarctica. Canadian Journal of Botany 78: 97–99.
Watson, S. B., T. Satchwill, E. Dixons & E. McCauley, 2001. Under-ice blooms and source-water odour in a nutrient-poor reservoir: biological, ecological and applied perspectives. Freshwater Biology 46: 1553–1567.
Weckström, J., A. Korhola & T. Blom, 1997. Temperature patterns over the past eight centuries in northern Fennoscandia inferred from sedimentary diatoms. Quaternary Research 66: 78–86.
Weckström, J., S. Hanhijärvi, L. Forsström, E. Kuusisto & A. Korhola, 2014. Reconstructing lake ice cover in subarctic lakes using a diatom-based inference model. Geophysical Research Letters 41: 2026–2032.
Wilkinson, A. N. & J. P. Smol, 1998. Chrysophycean stomatocyst flora from south-central Ontario lakes. Canadian Journal of Botany 76: 836–862.
Wilkinson, A. N., B. A. Zeeb, J. P. Smol & M. S. V. Douglas, 1997. Chrysophyte stomatocyst assemblages associated with periphytic high arctic pond environments. Nordic Journal of Botany 17: 95–112.
Wilkinson, A. N., B. A. Zeeb & J. P. Smol, 2001. Atlas of chrysophycean cysts, Vol. II. Kluwer Academic Publishers, The Netherlands.
Willén, E., 1987. Phytoplankton and reversed eutrophication in Lake Mälaren, Central Sweden, 1965–1983. British Phycological Journal 22: 193–208.
Wolowski, K., J. Piątek & B. J. Plachno, 2011. Algae and stomatocysts associated with carnivorous plants. First report of chrysophyte stomatocysts from Virginia, USA. Phycologia 50: 511–519.
Zeeb, B. A. & J. P. Smol, 1993. Postglacial chrysophycean cyst record from Elk Lake, Minnesota. In Bradbury, J. P. & W. E. Dean (eds), Elk Lake, Minnesota: Evidence for Rapid Climate Change in the North-Central United States. Geological Society of America Special Paper 276. Boulder, Colorado.
Zeeb, B. A. & J. P. Smol, 1995. A weighted-averaging regression and calibration model for interfering lakewater salinity using chrysophycean stomatocysts from lakes in western Canada. International Journal of Salt Lake Research 4: 1–23.
Zeeb, B. A., K. E. Duff & J. P. Smol, 1990. Morphological descriptions and stratigraphic profiles of chrysophycean stomatocysts from the recent sediments of Little Round Lake, Ontario. Nova Hedwigia 51: 361–380.
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. This work was made possible by personal grants to S. Korkonen from the Tellervo and Juuso Walden Foundation and the Societas pro Fauna et Flora Fennica. Steve Juggins, Emma Pearson and Juha Niemistö are acknowledged for helping out in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Korkonen, S., Weckström, J. & Korhola, A. Biogeography and ecology of freshwater chrysophyte cysts in Finland. Hydrobiologia 847, 487–499 (2020). https://doi.org/10.1007/s10750-019-04112-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04112-0