Abstract
Two pennate diatoms, Amphora coffeaeformis and Nitzschia ovalis, were used to evaluate potential responses to the future CO2 and temperature increases with respect to cell-specific growth rate, elemental composition, size, population growth rate, and carrying capacity. Diatoms were subjected to four different treatments over a 2 week period (approximately 4 generations): a control (28°C and present-day CO2, ~400 ppm), high CO2 (28°C with high CO2, ~750 ppm), high temperature (31°C and present-day CO2, ~400 ppm), and greenhouse-effect treatment (31°C with high CO2, ~750 ppm). The results indicated that both the cell-specific growth rates and the carrying capacity of A. coffeaeformis decreased at the higher temperature treatment, whereas N. ovalis did not differ among all treatments. No significant difference was found in either species’ elemental cell composition, but higher C:N and C:P ratios were observed for A. coffeaeformis and N. ovalis, respectively, in high CO2 and greenhouse-effect treatments. Smaller cell sizes were observed for both species under the greenhouse-effect treatment, a phenomenon that could alter benthic food webs in the future.
Similar content being viewed by others
Introduction
Atmospheric CO2 is predicted to increase from a present concentration of 390–750 ppm by the end of the century (Houghton, 2001). In fact, ambient concentrations in the atmosphere recently exceeded 400 ppm for the first time in recorded history. Increase in atmospheric CO2 will elevate the dissolved CO2 in the ocean (Stumm & Morgan, 1996) and lead to a decrease in pH, or acidification, of the ocean water (Houghton, 2001). Field studies and laboratory experiments have revealed that increases in CO2 concentration can affect phytoplankton in many ways, including growth (Riebesell et al., 2007; Sun et al., 2011; McCarthy et al., 2012), inorganic carbon uptake (Rost et al., 2003; Tortell et al., 2008; Trimborn et al., 2009), nitrogen fixation in cyanobacteria (Hutchins et al., 2007), elemental ratio (Burkhardt et al. 1999a; Tortell et al., 2000; Engel et al., 2005; Reinfelder 2012, b), cell size (Hoogstraten et al., 2012), and change of species composition within an ecosystem (Tortell et al., 2002, 2008). Increases in temperature may also change the phytoplankton community structure (Hare et al., 2007; Schabhuttl et al., 2013), growth (Lurling et al., 2013), cell size (Morabito et al., 2007; Peter & Sommer, 2012; Polovina & Woodworth, 2012), elemental ratio (Xiu & Chai, 2012), and biogeochemical components (Wohlers-Zollner et al., 2012), among others.
Diatoms are responsible for about 40% of marine primary productivity (Falkowski et al., 2004) and usually dominate the phytoplankton assemblage in nutrient-rich ocean regions (Irigoien et al., 2002). As such, diatoms are the primary algal group that supports higher trophic levels (Gao et al., 2012). Some studies have revealed that high CO2 concentration will increase the growth rate of diatoms (Wu et al., 2010; McCarthy et al., 2012; Li & Campbell, 2013), whereas other showed that rising CO2 will reduce diatom growth (Torstensson et al., 2012) and productivity (Gao et al., 2012). Thus, it appears that the effect of elevated CO2 on diatoms is species specific or dependent on other environmental conditions.
Carbon dioxide is a well-known greenhouse gas that traps reflected infrared radiation and results in rapid warming of both terrestrial and ocean ecosystems (Gao et al., 2012). Temperature, like CO2, has varied effects on diatoms. An inverse relationship between diatom cell size and temperature has been documented in certain diatom species, increasing in cell size with decreasing temperature (Mohan et al., 2011; Li et al., 2012). In contrast, others found no change in diatom cell size with temperature (Vanden Byllaardt & Cyr, 2011). Scholz & Liebezeit (2012) showed that some diatom species can adapt to wide variations in temperature with no significant effect on growth. Yet, larger diatoms may respond differently under high temperature conditions relative to smaller diatoms, and a small surface area to volume ratio may be advantageous for diatoms under high temperature (Yun et al., 2010). Changes in temperature may result in an altered distribution of the different diatom communities, which will alter the basis of the aquatic food web (Silina & Zhukova, 2007).
In tropical areas, much effort has been invested in determining the effects of global warming on corals and coral reef ecosystems (e.g., Ferreira et al., 2013), with little emphasis regarding the effects on microscopic algae. In this study, we simulated a tropical condition by setting the baseline, or control temperature, at 28°C with a CO2 concentration near that of present day (~400 ppm); we investigated the main and interactive effects of elevated temperature (31°C) and CO2 concentrations (750 ppm) that are projected to occur by 2100 (IPCC, 2007). Using these simulated conditions, we determined the potential effects of global climate change on the cell-specific growth rate, cell elemental composition, and cell size of two cosmopolitan pennate diatoms, Amphora coffeaeformis (~20 μm), a fouling diatom (Rasmussen & Ostgaard, 2001), and Nitzschia ovalis (~10 μm). Both diatoms are benthic species, which have received less attention in terms of determining their responses to global climate change.
Materials and methods
Cultures and growth conditions
Marine diatoms Amphora coffeaeformis CCMP128 and Nitzschia ovalis CCMP1118 stock cultures were obtained from the Provasoli–Guillard National Center for Marine Algae and Microbiota (NCMA). Although these benthic diatom species are widely distributed, the strains used in this study were isolated from tropical areas. Given the similar environments that these species have experienced, we expected them to respond similarly to the various treatments. Stock cultures were incubated at 28°C with a light:dark cycle of 12:12 and an incident photon flux density of ~100 μmol photons m−2 s−1. All cultures were grown in filtered (GF/F) and autoclaved natural seawater (collected from the nearby coast) with f/2 medium (purchased from NCMA) prepared according to the f/2 recipe (Guillard, 1975). The algae were acclimated to 28°C for at least 10 generations (when constant growth rate was reached) before the experiments began. The population growth of each culture was monitored on a daily basis by chl-a measurements. A semi-continuous culture approach was used during the experiment, i.e., the same amount of f/2 medium was added whenever a sample was taken from a flask during the experiment.
Experimental design
Cultures were incubated in triplicate 1-l glass bottles containing 0.2 μm filtered and autoclaved seawater using stock culture growth conditions. A total of 10 ml of stock culture were put into 1 l of water, with a final algal density of 103 cell ml−1. Diatoms were subjected to four different treatments: a control (28°C and present-day CO2, 400 ppm), high CO2 (28°C with high CO2, 750 ppm), high temperature (31°C and present-day CO2, ~400 ppm), and a greenhouse-effect treatment (31°C with high CO2, 750 ppm). The experiments were conducted in a CO2 growth chamber (F-130 CO, Highpoint, Taiwan) for 14 days. Present-day CO2 concentration (~400 ppm) was obtained by gentle bubbling with filtered ambient air using an aquarium pump, whereas the projected year 2100 CO2 concentration (750 ppm, IPCC, 2007) was obtained by bubbling with a commercially prepared air/CO2 mixture (Jingshang Co. Kaohsiung, Taiwan). The air temperature and CO2 concentration were monitored daily throughout the experiment. The CO2 concentrations were 418 ± 44 ppm (±SD) and 752 ± 40 ppm in the growth chamber for low and high CO2 experiments, respectively. The pH, total alkalinity, HCO3 −, CO −23 , and CO2 in natural water were 8.255 ± 0.029, 2225 ± 30, 1657 ± 47, 230 ± 10, and 14.7 ± 1.1 μmol/kgSW, respectively.
Chl-a measurement
Cells for chl-a analysis were filtered through a 0.45-μm GF/F filter paper (Whatman, UK) every 2 days throughout the experiment and stored frozen at −20°C. Once thawed, the filter paper was placed in a 15-ml centrifuge tube with 5 ml 90% acetone, extracted in complete darkness for 20 h (Strickland & Parsons, 1972), and measured with a fluorometer (Turner Model 450). Specific growth rate (day−1) of each species was calculated according to Levasseur et al. (1993). The growth curves of both species under different treatments during the experiment were analyzed quantitatively using the logistic growth equations (Barnes & Hughes, 1999) and the least-squares curve-fitting algorithm (Marquardt, 1963) using SigmaPlot 10.0 (Jandel Scientific, California). The equation for logistic growth is given by
where N is the chl-a concentration, r the intrinsic growth rate, and K the carrying capacity (Barnes & Hughes, 1999).
Carbon, nitrogen, and phosphorous elemental analysis
Elemental cell analysis was performed at the end of the experiment. Cell samples for determinations of particulate C and N were collected under low vacuum (<17 kPa) onto precombusted 13-mm Gelman TCLP glass fiber filters (nominal pore size 0.7 μm; Pall Corporation, East Hills, NY, USA) and stored frozen at −20°C. Particulate C and N samples were dried at 50°C for 8 h and analyzed using an elemental combustion system (Elementar VarioEL III, Scientech, USA), calibrated with EDTA (C:N = 4.29), and phenylalanine (C:N = 7.72) as reference materials.
For particulate organic P (POP) determinations, 10-ml cultures were filtered onto precombusted 25-mm GF/F glass fiber filters and placed in an Erlenmeyer flask with 50 ml of deionized water. After persulfate digestion, the dissolved phosphate was measured colorimetrically using a spectrophotometer (APHA, 2005).
Cell size
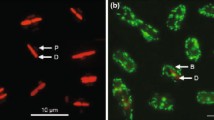
Samples from day 1 and day 14 were fixed in unbuffered 4% paraformaldehyde and collected on a filter paper. Drying was done by heat at 50°C overnight in an oven. After drying, the filters were affixed to a scanning electron microscope (SEM) stub and coated with platinum using an EMS 76 sputter coater. Specimens were observed in a SEM (Hitachi S 3500N, Japan), and the apical axis of 20 individuals was measured randomly to represent each population (Snoeijs et al., 2002).
Statistics
All values presented represent the mean (±SE) of triplicate bottles for each treatment. Differences in specific growth rate, elemental composition, and size of each species among treatments were determined using one-way ANOVA. Significant differences were further analyzed with a post-hoc Tukey’s test (α = 0.05) to determine which categories differed. Data were loge-transformed when necessary to meet the assumption of normality and homogeneity of variance. Statistical analyses were computed with the software package SigmaStat 2.03 (SPSS, 1997).
Results
Algal growth
The growth curves of both diatom species showed that they responded consistently to the high CO2 at normal temperature during early phase exponential growth, leading to the highest growth by the end of the experiment (Fig. 1). High temperature and greenhouse treatments resulted in slower growth (Fig. 1) and lower carrying capacity of A. coffeaeformis (Table 1), but the effects on N. ovalis were less profound (Fig. 1; Table 1).
Specific growth rates, i.e., the average daily growth rate, of the two diatom species tested were not different from the control (P > 0.05; Fig. 2). Among the treatment groups, growth rates of Amphora coffeaeformis were approximately two times higher in the high CO2 treatment than the high temperature and greenhouse groups (P < 0.05), whereas growth rate of Nitzschia ovalis did not differ under these four growth conditions (P > 0.05; Fig. 2).
Cellular carbon, nitrogen, phosphorus, and elemental ratios
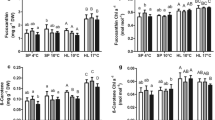
The increased in temperature and/or CO2 did not significantly change the elemental contents of A. coffeaeformis and N. ovalis. (Table 2), although there was a twofold increase in mean cellular composition of carbon (Q C) in high CO2 and greenhouse treatments for A. coffeaeformis (Table 2). There was also nearly 40 and 70% increases in Q C and cellular nitrogen (Q N), respectively, in the high CO2 and greenhouse treatments for N. ovalis. Compared with the effect of CO2, the effects of temperature on the cellular composition for A. coffeaeformis grown at high temperature (31°C) were 77, 175, and 20% higher for Q C, Q N, and cellular phosphorus (Q P), respectively (Table 2). Cellular composition remained largely unaffected in the high temperature treatment for N. ovalis (Table 2).
In A. coffeaeformis, increases in the carbon composition in cultures raised in high concentrations of CO2 generally resulted in higher C:N and C:P ratios, and higher N:P ratio when the temperature increased (Table 2). Whereas in N. ovalis, percent increases in Q N from high concentrations of CO2 was higher than the carbon and phosphorus composition, which resulted in a significant change in the N:P ratio for this species (Table 2).
Cell size
Differences in cell size among the treatments generally followed similar trends observed for cell-specific growth rate. High temperature reduced the cell size of both benthic diatom species (Fig. 3). Relative to the control, the cell size of A. coffeaeformis was reduced by 20 and 36% in the high temperature and greenhouse treatments (P < 0.05, Fig. 3). The cell size for N. ovalis cell size was reduced 23% relative to the control (P < 0.05), with no other differences among treatments (P > 0.05).
Discussion
In general, the cell-specific growth rates and the carrying capacity of A. coffeaeformis were lower in the higher temperature treatments but were nearly identical for N. ovalis under these four growth conditions. However, the growth during early exponential phase for both species was enhanced by increasing CO2 (Figs. 1, 2). Previous studies on the effects of increased temperature and elevated CO2 concentrations on phytoplankton have led to different conclusions. For example, Fu et al. (2007) found that pico-cyanobacteria Synechococcus increased in growth rate under high temperature and high CO2 concentration, whereas Prochlorococcus remained unaffected under the same conditions (Fu et al., 2007). Elevated CO2 has been reported to stimulate the growth of green algae Chlamydomonas reinhardtii, Chlorella pyrenoidosa, Scenedesmus obliquus, and Dunaliella tertiolecta (Beardall & Raven, 2004); diatoms Skeletonema costatum (Kim et al., 2006), Chaetoceros (Beardall & Raven, 2004), Thalassiosira pseudonana (McCarthy et al., 2012), and coccolithophore Emiliania huxleyi (McCarthy et al., 2012). In contrast, the elevated CO2 has also been reported to have neutral or decrease algal growth, as seen in the present study, diatom Navicula directa (Torstensson et al., 2012) and coccolithophore E. huxleyi (Iglesias-Rodriguez et al., 2008). The traditional view point has suggested that the response of phytoplankton to CO2 elevation is negligible (Raven, 1997); however, a recent model has suggested that as CO2 level raises to 700 ppm, the growth of marine phytoplankton may increase by as much as 40% (Schippers et al., 2004). The varying results from different studies could be attributed to different algal species, but varying experimental conditions such as light intensity (Li & Campbell, 2013), salinity (Ganguly et al., 2013; Tanimoto et al., 2013), day length (Burkhardt et al., 1999a, b), and temperature (Taucher et al., 2012) may also play an important role. The current study demonstrates the importance of temperature in the physiology of some diatom species. It suggests that a temperature increase of 3°C could significantly reduce algal growth and carrying capacity, as shown in the case of A. coffeaeformis (Figs. 1, 2), but the effect could be modest to other species (e.g., N. ovalis). Nonetheless, increase of both temperature and CO2 concentration, as we expect to occur in the future, will likely affect these algae in some other areas (reduced cell sizes in this study).
The elemental composition of marine phytoplankton naturally varies among species (Ho et al., 2003; Quigg et al., 2003) and within species (Reinfelder, 2012). It has been hypothesized that elemental ratios may be sensitive to the availability of CO2 given that algae actively take up CO2 (Burkhardt & Riebesell, 1997; Burkhardt et al. 1999a, b). Increase CO2 availability can enhance carbon uptake and therefore change the elemental composition of the algae. Previous studies on diatoms have found no general pattern for CO2-related changes in elemental compositions, and the C:N and C:P could either increase or decrease with increasing CO2, depending on the species tested (Burkhardt et al., 1999a, b). A recent study by Reinfelder (2012) showed that increases in CO2 resulted in decreased Q N of centric diatoms but varied little in pennate diatom, whereas Q P decreased as CO2 increase (Reinfelder, 2012). The results of our study indicated that the Q C and Q N of the two pennate diatoms tended to increase, although not significantly, under high CO2, whereas Q P did not change. C:N and C:P tended to increase with increasing CO2 due to higher Q C, and the effect was more profound on the larger species. The effect of temperature was reported to be negligible on cell elemental composition, despite the fact that changes in ambient temperature will directly influence metabolism (Raven & Geider, 1988). However, the N:P ratio of A. coffeaeformis in the high temperature and greenhouse treatment groups in our study was apparently higher, although not significant (Table 1). A recent study has shown that eukaryotic phytoplankton seem to require a lower density of phosphate-rich ribosomes to produce the required amounts of cellular protein in warmer oceans, which will lead to the production of higher organismal N:P ratio, in turn increasing demand for N (Toseland et al., 2013). The lack of a statistically significant effect of temperature on cell elemental composition in our study was probably a result of the lowest temperature tested being 28°C, which is within their tolerance range, albeit at the upper end. Low replication and high variation may have also contributed to the apparent absence of a biologically significant effect.
The cells for the elemental analysis were collected at the end of the experiment. With the exception of the high temperature group in A. coffeaeformis and the control group in N. ovalis, most of the treatments had reached their carrying capacities (Fig. 1; Table 1). The Q N of A. coffeaeformis in high temperature treatment was almost threefold higher than in the control (Table 2), leading to question that nitrogen could also be a limiting factor that constrained the population growth in the group. Measuring the elemental cell composition at the same growth phase (e.g., early exponential phase) would have reduced the potential bias that this may have resulted in and should be considered in future studies.
According to Reinfelder (2012), when nutrients are not limiting, increased CO2 concentration would lower the nitrogen demand and could therefore support higher diatom biomass per unit of nitrogen (Reinfelder, 2012). Cell size of Rhizosoleniales diatom (centric) Proboscia alata was found to increase 10 times under high CO2 (Hoogstraten et al., 2012) concentrations. A twofold increase in centric diatom size was observed as CO2 was increased from 150 to 380 ppm CO2, yet cell size only increased by 15% from 380 to 770 ppm (Reinfelder, 2012). Thus, if the CO2 increases from 380 to 750 ppm by the end of the century as projected (IPCC, 2007), only a small increase in centric diatom size would be expected. Pennate diatoms on the other hand, showed little to no response of nitrogen composition to CO2 (Sun et al., 2011; Reinfelder, 2012; this study), and the size of these diatoms would not be expected to vary with increased CO2. In contrary, the greenhouse effect might reduce the cell size, as shown in the two pennate diatoms in this study (Fig. 3).
In conclusion, the two pennate diatoms tested responded differently under the treatments contrary to our expectations. The specific growth rates of A. coffeaeformis decreased in higher temperature and greenhouse conditions, whereas N. ovalis stayed identical. No significant difference was found in both species’ elemental cell contents, but higher C:N and C:P ratios were observed for A. coffeaeformis and N. ovalis, respectively. Smaller cell sizes were observed for both species under the greenhouse-effect treatment, a phenomenon that might alter benthic food webs in the future.
References
APHA, 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington, DC.
Barnes, R. S. K. & R. N. Hughes, 1999. An Introduction to Marine Ecology. Blackwell Publishing, Malden, MA.
Beardall, J. & J. A. Raven, 2004. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 43: 26–40.
Burkhardt, S. & U. Riebesell, 1997. CO2 availability affects elemental composition (C:N:P) of the marine diatom Skeletonema costatum. Marine Ecology Progress Series 155: 67–76.
Burkhardt, S., I. Zondervan & U. Riebesell, 1999a. Effect of CO2 concentration on C:N:P ratio in marine phytoplankton: a species comparison. Limnology and Oceanography 44: 683–690.
Burkhardt, S., U. Riebesell & I. Zondervan, 1999b. Effects of growth rate, CO2 concentration, and cell size on the stable carbon isotope fractionation in marine phytoplankton. Geochimica Et Cosmochimica Acta 63: 3729–3741.
Engel, A., I. Zondervan, K. Aerts, L. Beaufort, A. Benthien, L. Chou, B. Delille, J. P. Gattuso, J. Harlay, C. Heemann, L. Hoffmann, S. Jacquet, J. Nejstgaard, M. D. Pizay, E. Rochelle-Newall, U. Schneider, A. Terbrueggen & U. Riebesell, 2005. Testing the direct effect of CO2 concentration on a bloom of the coccolithophorid Emiliania huxleyi in mesocosm experiments. Limnology and Oceanography 50: 493–507.
Falkowski, P. G., M. E. Katz, A. H. Knoll, A. Quigg, J. A. Raven, O. Schofield & F. J. R. Taylor, 2004. The evolution of modern eukaryotic phytoplankton. Science 305: 354–360.
Ferreira, B. P., M. B. S. F. Costa, M. S. Coxey, A. L. B. Gaspar, D. Veleda & M. Araujo, 2013. The effects of sea surface temperature anomalies on oceanic coral reef systems in the southwestern tropical Atlantic. Coral Reefs 32: 441–454.
Fu, F. X., M. E. Warner, Y. H. Zhang, Y. Y. Feng & D. A. Hutchins, 2007. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). Journal of Phycology 43: 485–496.
Ganguly, D. R. S., K. V. Robin, P. R. Vardhan, K. R. Muduli, S. Patra Abhilash & B. R. Subramanian, 2013. Variable response of two tropical phytoplankton species at different salinity and nutrient condition. Journal of Experimental Marine Biology and Ecology 440: 244–249.
Gao, K. S., J. T. Xu, G. Gao, Y. H. Li, D. A. Hutchins, B. Q. Huang, L. Wang, Y. Zheng, P. Jin, X. N. Cai, D. P. Hader, W. Li, K. Xu, N. N. Liu & U. Riebesell, 2012. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nature Climate Change 2: 519–523.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In Smith, W. L. & M. H. Chanley (eds), Culture of Marine Invertebrate Animals. Plenum Press, New York: 26–60.
Hare, C. E., K. Leblanc, G. R. DiTullio, R. M. Kudela, Y. Zhang, P. A. Lee, S. Riseman & D. A. Hutchins, 2007. Consequences of increased temperature and CO2 for phytoplankton community structure in the Bering Sea. Marine Ecology Progress Series 352: 9–16.
Ho, T. Y., A. Quigg, Z. V. Finkel, A. J. Milligan, K. Wyman, P. G. Falkowski & F. M. M. Morel, 2003. The elemental composition of some marine phytoplankton. Journal of Phycology 39: 1145–1159.
Hoogstraten, A., K. R. Timmermans & H. J. W. de Baar, 2012. Morphological and physiological effects in Proboscia alata (Bacillariophyceae) grown under different light and CO2 conditions of the modern southern ocean. Journal of Phycology 48: 559–568.
Houghton, R. A., 2001. Counting terrestrial sources and sinks of carbon. Climatic Change 48: 525–534.
Hutchins, D. A., F. X. Fu, Y. Zhang, M. E. Warner, Y. Feng, K. Portune, P. W. Bernhardt & m R Mulholland, 2007. CO2 control of Trichodesmium N-2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnology and Oceanography 52: 1293–1304.
Iglesias-Rodriguez, M. D., P. R. Halloran, R. E. M. Rickaby, I. R. Hall, E. Colmenero-Hidalgo, J. R. Gittins, D. R. H. Green, T. Tyrrell, S. J. Gibbs, P. von Dassow, E. Rehm, E. V. Armbrust & K. P. Boessenkool, 2008. Phytoplankton calcification in a high-CO2 world. Science 320: 336–340.
IPCC (Intergovernmental Panel on Climate Change), 2007. Climate Change 2007: synthesis report. Contributions of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva.
Irigoien, X., R. P. Harris, H. M. Verheye, P. Joly, J. Runge, M. Starr, D. Pond, R. Campbell, R. Shreeve, P. Ward, A. N. Smith, H. G. Dam, W. Peterson, V. Tirelli, M. Koski, T. Smith, D. Harbour & R. Davidson, 2002. Copepod hatching success in marine ecosystems with high diatom concentrations. Nature 419: 387–389.
Kim, J. M., K. Lee, K. Shin, J. H. Kang, H. W. Lee, M. Kim, P. G. Jang & M. C. Jang, 2006. The effect of seawater CO2 concentration on growth of a natural phytoplankton assemblage in a controlled mesocosm experiment. Limnology and Oceanography 51: 1629–1636.
Levasseur, M., P. A. Thompson & P. J. Harrison, 1993. Physiological acclimation of marine phytoplankton to different nitrogen sources. Journal of Phycology 29: 587–595.
Li, G. & D. A. Campbell, 2013. Rising CO2 interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana. Plos ONE 8: 13.
Li, Y. L., Z. J. Gong & J. Shen, 2012. Effects of eutrophication and temperature on Cyclotella rhomboideo-elliptica Skuja, endemic diatom to China. Phycological Research 60: 288–296.
Lurling, M., F. Eshetu, E. J. Faassen, S. Kosten & V. L. M. Huszar, 2013. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshwater Biology 58: 552–559.
Marquardt, D. W., 1963. An algorithm for least squares estimation of non-linear parameters. Journal of the Society for Industrial and Applied Mathematics 11: 431–441.
McCarthy, A., S. P. Rogers, S. J. Duffy & D. A. Campbell, 2012. Elevated carbon dioxide differentially alters the photophysiology of Thalassiosira pseudonana (Bacillariophyceae) and Emiliania huxleyi (Haptophyta). Journal of Phycology 48: 635–646.
Mohan, R., A. A. Quarshi, T. Meloth & M. Sudhakar, 2011. Diatoms from the surface waters of the Southern Ocean during the austral summer of 2004. Current Science 100: 1323–1327.
Morabito, G., A. Oggioni, E. Caravati & P. Panzani, 2007. Seasonal morphological plasticity of phytoplankton in Lago Maggiore (N. Italy). Hydrobiologia 578: 47–57.
Peter, K. H. & U. Sommer, 2012. Phytoplankton Cell Size: intra- and interspecific effects of warming and grazing. Plos ONE 7: 9.
Polovina, J. J. & P. A. Woodworth, 2012. Declines in phytoplankton cell size in the subtropical oceans estimated from satellite remotely-sensed temperature and chlorophyll, 1998–2007. Deep Sea Research Part II 77–80: 82–88.
Quigg, A., Z. V. Finkel, A. J. Irwin, Y. Rosenthal, T. Y. Ho, J. R. Reinfelder, O. Schofield, F. M. M. Morel & P. G. Falkowski, 2003. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425: 291–294.
Rasmussen, K. & K. Ostgaard, 2001. Adhesion of the marine fouling diatom Amphora coffeaeformis to non-solid gel surfaces. Biofouling 17: 103–115.
Raven, J. A., 1997. Putting the C in phycology. European Journal of Phycology 32: 319–333.
Raven, J. A. & R. J. Geider, 1988. Temperature and algal growth. New Phytologist 110: 441–461.
Reinfelder, J. R., 2012. Carbon dioxide regulation of nitrogen and phosphorus in four species of marine phytoplankton. Marine Ecology Progress Series 466: 57–67.
Riebesell, U., K. G. Schulz, R. G. J. Bellerby, M. Botros, P. Fritsche, M. Meyerhoefer, C. Neill, G. Nondal, A. Oschlies, J. Wohlers & E. Zoellner, 2007. Enhanced biological carbon consumption in a high CO2 ocean. Nature 450: 545–U10.
Rost, B., U. Riebesell, S. Burkhardt & D. Sultemeyer, 2003. Carbon acquisition of bloom-forming marine phytoplankton. Limnology and Oceanography 48: 55–67.
Schabhuttl, S., P. Hingsamer, G. Weigelhofer, T. Hein, A. Weigert & M. Striebel, 2013. Temperature and species richness effects in phytoplankton communities. Oecologia 171: 527–536.
Schippers, P., M. Lurling & M. Scheffer, 2004. Increase of atmospheric CO2 promotes phytoplankton productivity. Ecology Letters 7: 446–451.
Scholz, B. & G. Liebezeit, 2012. Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthorn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Research 27: 65–73.
Silina, A. V. & N. V. Zhukova, 2007. Growth variability and feeding of scallop Patinopecten yessoensis on different bottom sediments: evidence from fatty acid analysis. Journal of Experimental Marine Biology and Ecology 348: 46–59.
Snoeijs, P., S. Busse & M. Potapova, 2002. The importance of diatom cell size in community analysis. Journal of Phycology 38: 265–272.
SPSS, 1997. SigmaStat Statistical Software. SPSS Marketing Department, Chicago, IL.
Strickland, J. D. H. & T. R. Parsons, 1972. A Practical Handbook of Seawater Analysis. Fisheries Research Board of Canada, Ottawa, Canada: 261–279.
Stumm, W. & J. J. Morgan, 1996. Aquatic Chemistry. Chemical Equilibria and Rates in Natural Waters. Wiley Inc, New York.
Sun, J., D. A. Hutchins, Y. Y. Feng, E. L. Seubert, D. A. Caron & F. X. Fu, 2011. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnology and Oceanography 56: 829–840.
Tanimoto, Y., H. Yamaguchi, T. Yoshimatsu, S. Sato & M. Adachi, 2013. Effects of temperature, salinity and their interaction on growth of toxic Ostreopsis sp. 1 and Ostreopsis sp. 6 (Dinophyceae) isolated from Japanese coastal waters. Fisheries Science 79: 285–291.
Taucher, J., K. G. Schulz, T. Dittmar, U. Sommer, A. Oschlies & U. Riebesell, 2012. Enhanced carbon overconsumption in response to increasing temperatures during a mesocosm experiment. Biogeosciences 9: 3531–3545.
Torstensson, A., M. Chierici & A. Wulff, 2012. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa. Polar Biology 35: 205–214.
Tortell, P. D., G. R. DiTullio, D. M. Sigman & F. M. M. Morel, 2002. CO2 effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Marine Ecology Progress Series 236: 37–43.
Tortell, P. D., G. H. Rau & F. M. M. Morel, 2000. Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnology and Oceanography 45: 1485–1500.
Tortell, P. D., C. Payne, C. Gueguen, R. F. Strzepek, P. W. Boyd & B. Rost, 2008. Inorganic carbon uptake by Southern Ocean phytoplankton. Limnology and Oceanography 53: 1266–1278.
Toseland, A., S. J. Daines, J. R. Clark, A. Kirkham, J. Strauss, C. Uhlig, T. M. Lenton, K. Valentin, G. A. Pearson, V. Moulton & T. Mock, 2013. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nature Climate Change 3: 979–984.
Trimborn, S., D. Wolf-Gladrow, K. U. Richter & B. Rost, 2009. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. Journal of Experimental Marine Biology and Ecology 376: 26–36.
Vanden Byllaardt, J. & H. Cyr, 2011. Does a warmer lake mean smaller benthic algae? Evidence against the importance of temperature-size relationships in natural systems. Oikos 120: 162–169.
Wohlers-Zollner, J., A. Biermann, A. Engel, P. Dorge, A. M. Lewandowska, M. von Scheibner & U. Riebesell, 2012. Effects of rising temperature on pelagic biogeochemistry in mesocosm systems: a comparative analysis of the AQUASHIFT Kiel experiments. Marine Biology 159: 2503–2518.
Wu, Y., K. Gao & U. Riebesell, 2010. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7: 2915–2923.
Xiu, P. & F. Chai, 2012. Spatial and temporal variability in phytoplankton carbon, chlorophyll, and nitrogen in the North Pacific. Journal of Geophysical Research-Oceans 117: 17.
Yun, M. S., S. H. Lee & I. K. Chung, 2010. Photosynthetic activity of benthic diatoms in response to different temperatures. Journal of Applied Phycology 22: 559–562.
Acknowledgments
This research was supported by intramural funding from the National Museum of Marine Biology & Aquarium to KS Tew (99100304 and 99200343). We thank two anonymous reviewers for very professional suggestions and comments on the earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Jiang-Shiou Hwang & Koen Martens / Challenges in Aquatic Sciences
Rights and permissions
About this article
Cite this article
Tew, K.S., Kao, YC., Ko, FC. et al. Effects of elevated CO2 and temperature on the growth, elemental composition, and cell size of two marine diatoms: potential implications of global climate change. Hydrobiologia 741, 79–87 (2014). https://doi.org/10.1007/s10750-014-1856-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1856-y