Abstract
We investigated the feeding behaviour of the dominant microzooplankton of saline lakes in the East African Rift Valley. A set of grazing experiments revealed high ingestion rates of the two euryhaline rotifers Brachionus dimidiatus and Brachionus plicatilis and of the large-sized omnivorous ciliates Frontonia sp. and Condylostoma magnum reflecting the unique nature of tropical saline systems. The size spectrum of ingested particles was broad and even included filamentous cyanobacteria such as the commonly dominating Arthrospira fusiformis. Feeding selectivity on cyanobacteria, however, was rather low showing higher values for cryptomonads and small ciliates. Bacterial biomass was favoured by the presence of grazers, as small bacterivorous predators were reduced at an average of 13.9%, showing the cascading effect of large zooplankton on the food web structure. Overall, based on this first-time study of the microzooplankton feeding behaviour in East African soda lakes, a strong structuring effect of rotifers and large ciliates on microbial plankton communities is assumed, especially in times of high consumer biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grazing of zooplankton is a major factor structuring plankton communities (Gliwicz, 1975) and influencing biomass, competitiveness, growth rates and morphology of prey organisms (Arndt, 1993; Jurgens & Matz, 2002; Verschoor et al., 2007). Zooplankton in the size class 250–100 μm (hereafter referred to as microzooplankton) consists mainly of rotifers, naupliae and large ciliates and is able to ingest a wide range of food particles. Most studies have focused on the grazing impact on phytoplankton (Rothhaupt, 1990; Hansen et al., 1997). Nevertheless, microzooplankton is also important in channelling energy from the microbial loop to higher trophic levels because it ingests protozoans (Azam et al., 1983; Arndt, 1993) and even bacteria, although with lower efficiencies (Vadstein et al., 1993; Oomswilms, 1997). Feeding behaviour and grazing rates are influenced by several characteristics of food particles. Factors such as food quantity and quality, temperature, taste, digestibility, catchability and toxicity can play important roles (Demott, 1986; Montagnes et al., 2001; Mitra & Flynn, 2007). For rotifers, prey size is often considered most important (Rothhaupt, 1990; Hansen et al., 1997).
In shallow tropical lakes and especially in alkaline-saline (soda) lakes, phytoplankton communities are frequently dominated by large forms (Lewis, 1978; Fernando, 1994; Oduor & Schagerl, 2007a). This leads to an overlap in size between herbivorous grazers and phytoplankton (Vareschi & Jacobs, 1985; Work et al., 2005). Blooms of large-sized algae may be supported by a higher grazing resistance (Boon et al., 1994; Gragnani et al., 1999) and the lack of regular disturbance events (Talling, 2001).
Schagerl & Oduor (2008), however, demonstrated a relatively low impact of environmental factors on the phytoplankton community structure of soda lakes, which implies an increased importance of biological features. This indicates that, besides allelopathy and resource-competition, grazing by microzooplankton—the most important planktonic consumers in soda lakes (Vareschi & Jacobs, 1985)—could also play a fundamental role, despite the dominance of large-sized photoautotrophs.
Another feature of shallow soda lakes is their high abundance of microbial organisms. Bacterial densities often reach 108 ind ml−1 (Kilham, 1981), and the biomass of pelagic ciliates ranges among the highest values recorded worldwide (Finlay et al., 1987; Yasindi et al., 2002). Protozoan biomass is commonly dominated by large herbivorous and carnivorous ciliates, whereas bacterivorous protozoa only occasionally reach high abundances. Zinabu & Taylor (1997) argue that carnivorous microzooplankton could be a major reason for the characteristic microbial community structure in tropical saline lakes. They discovered a stronger correlation between chlorophyll a values and bacterial numbers in soda lakes than in freshwater systems and hypothesised that bacterial biomass in soda lakes is bottom up controlled. Top down predation pressure could be reduced because of the heavy rotifer and ciliate grazing on heterotrophic nanoflagellates (HNF).
So far, grazing impacts of microzooplankton in tropical soda lakes have not been studied empirically; they were calculated based on either assumptions (Vareschi & Jacobs, 1984) or the subject of speculations (Zinabu & Taylor, 1997). Trying to fill this gap, we conducted grazing experiments under controlled laboratory conditions and focused on two central aspects of mircozooplankton community interactions: First, we tested the hypothesis that dominating filamentous cyanobacteria serve as a food source for microzooplankton in African soda lakes. We investigated ingestion rates on filamentous cyanobacteria and determined the selectivity of microzooplankton taxa when a variety of natural food particles are offered simultaneously. Second, we explored the shaping force of four commonly dominating microzooplankton taxa of tropical African soda lakes on protozoan and bacterial populations. By measuring changes of near-natural communities under the presence and absence of microzooplankton grazers, we monitored the interactions between different members of the microbial loop.
Materials and methods
General
Two sets of grazing experiments were conducted with animals from the two saline-alkaline Kenyan Rift Valley Lakes Bogoria and Nakuru. Both lakes represent typical examples of hypersaline tropical soda lakes and have been used as model systems before (Vareschi & Jacobs, 1985; Harper et al., 2003). For a detailed description of their physical and chemical properties refer to Oduor & Schagerl (2007a, b). All animals used for grazing experiments were isolated from lake water, starved for 6 h and acclimated stepwise to conductivity levels in case experimental conditions differed from natural conditions (change rate of 5 mS cm−1 h−1). Experiments were conducted in complete darkness with a constant conductivity of 45 mS cm−1 at 22°C, which is close to the average lake temperature (Vareschi, 1982; Harper et al., 2003) and the recommended temperature of 25°C for grazing experiments of B. plicatilis (Montagnes et al., 2001).
Bacterial samples were fixed (5% formalin), stained using the SYBR Gold technique (Tuma et al., 1998) and counted under a compound microscope (Motic BA 400, Nikon, Tokyo) equipped with an epifluorescence device. Phytoplankton samples were fixed (5% formalin) and counted using an inverted microscope (Nikon Diaphot, Nikon, Tokyo) according to Utermöhl (1958). Two magnifications, one for pico and nanoplankton (1000×) and one for larger forms (200×), were used. Biovolumes of the various taxa were estimated using geometric formulae of the shapes of the respective phytoplankton cells (Sun & Liu, 2003). Protozoan samples were fixed with Bouin’s solution (5%) and stained using the Quantitative Protargol Staining Technique (QPS) by Montagnes & Lynn (1993) to facilitate counting.
We used direct counts to evaluate grazing impacts even though it is time-consuming and often associated with relatively high-standard deviations (Rott, 1981) because this method still yields the most detailed species-specific results when dealing with diverse plankton communities. Furthermore, other methods such as isotopic or radioactive labelling and coulter counter techniques bear the risk of delays because of incubation times, altering microbial community structure or leading to a misinterpretation of egested particle fragments and cell breakage occurring during ingestion (Harbison & Mcalister, 1980; Peters, 1984). We also avoided culturing because in vitro conditions can affect important factors such as food quality or colony size (Rothhaupt, 1995; Verschoor et al., 2007). We worked with organisms freshly isolated from the lakes to guarantee near-natural conditions.
Grazing on the natural phytoplankton community of L. Nakuru
Water samples were taken from an offshore station in L. Nakuru. Immediately after sampling, lake water was filtered through 40-μm sieves to remove large herbivorous zooplankton. As the phytoplankton community consisted mostly of unicellular algae and small fragments of filamentous cyanobacteria, the phytoplankton community structure was not significantly changed by the filtration process. All experiments started in triplicates within 24 h after water samples were taken. Thirty individuals of one of the four zooplankton taxa Brachionus dimidiatus Bryce, Brachionus plicatilis Mueller, Frontonia sp. (all isolated freshly from L. Nakuru) or Condylostoma magnum Spiegel (isolated from L. Bogoria) were placed in 50 ml of prefiltered lake water. Control experiments without added microzooplankton were conducted (n = 3). At the start and end of each experiment (after 24 h), samples were taken for bacteria, protozoa and phytoplankton counts.
Grazing on the filamentous cyanobacterium Arthrospira fusiformis
Dilution experiments were used to test the ingestion of filamentous cyanobacteria. Water samples from Lake Bogoria were taken because A. fusiformis (Vorochinin) Komárek, the phytoplankter commonly predominating in soda lakes, accounted for more than 90% of total plankton biomass in this lake. B. dimidiatus, B. plicatilis and Frontonia sp. were isolated from surface water samples with a micropipette; C. magnum was abstracted from subsurface water because this species showed much higher abundances at greater depths. A monospecific cyanobacteria concentrate of A. fusiformis (>99.9% biomass) was attained through multiple sieving through 40- and 100-μm sieves and sedimentation chambers making use of phytoplankton buoyancy. This concentrate was diluted in 50-ml containers with GF/F filtered lake water to concentrations of 1.3, 7.5, 14.0, 21.5 and 43.0 g C m−3, reflecting the natural spectrum of A. fusiformis densities in tropical soda lakes. To set up the grazing experiments, triplicates for each zooplankton taxon and each dilution approach were prepared by adding 25 individuals to every container. One set of triplicates without introduced grazers served as control. Experiments started within 24 h after lake water sampling, and zooplankton were allowed to graze for 24 h before samples were fixed for phytoplankton counts.
Grazing rates and selectivity
Grazing rates (G) were calculated according to the equation given by Frost (1972): G = (V/n) × ((ln C t − ln C tf)/t) × ((C tf − C 0)/(ln C tf − ln C 0 )), where V is the volume of the experimental containers, t is the duration of the experiment and n is the number of grazing individuals added. C 0, C t and C tf correspond to the initial particle concentration, the final concentration in the control and the final concentration in vessels with grazers, respectively.
We calculated the normalised forage ratio (NFR) after Paloheimo (1979) to evaluate the relative selectivity of different food types: NFR i = (r i /p i )/∑ (r i /p i ), where r i represents the biomass percentage of the food type i in the consumers diet and p i is the percentage of that food type in the total offered food spectrum. We included only food particles that were predominantly grazed by the introduced zooplankton. Our calculations were based on carbon content converted from biovolume with taxon-specific conversion factors (Table 1).
We tested the statistical significance of grazing impact with ANOVAs, followed by post hoc tests with FDR adjusted p values (Benjamini & Yekutieli, 2001). Data were transformed to achieve homogeneity of variance, if necessary. If data could not be transformed accordingly, Kruskal–Wallis one-way analysis of variance was used instead of ANOVA. All tests were performed with R, version 2.11 (R Development Core Team, 2010).
Results
Grazing on the natural phytoplankton community of L. Nakuru
At the beginning of the experiment, heterotrophic bacteria biomass was 6.9 g C m−3, contributing 22.2% to the total biomass of the plankton community <40 μm (Fig. 1). Protozoans accounted for 13.7% and phytoplankton for 64.1% or 20.1 g C m−3. Among protozoans, the omnivorous ciliate Euplotes and the bacterivorous ciliate Cyclidium dominated the biomass, although HNF also played a considerable role with a biomass of 0.9 g C m−3, equivalent to 12.9% of the biomass of heterotrophic bacteria, their major food source. Phytoplankton was dominated by the filamentous cyanobacterium Anabaenopsis abijatae Kebede et Willénoften, accounting for 9.6 g C m−3 or 46.6% of phytoplankton biomass (Table 2). Two Cryptomonas species, picophytoplankton (mainly 1–2.5 μm) and the cyanobacterium A. fusiformis were the other important taxa, contributing 28.2, 18.2 and 7.0% of phytoplankton biomass, respectively.
The microbial community in the control experiments changed significantly within 24 h (Fig. 2). The bacterial abundances decreased by 35% in the control setup (FDR post hoc test, P < 0.05), and the biomass of ciliates significantly increased by more than 60% (FDR post hoc test, P < 0.01). The introduction of grazers to experiments reduced the biomass of small ciliates in comparison to final values of controls (see Table 2 for statistics), although a trend to increased ciliate biomass relative to start values was still noticeable (Fig. 2B). In contrast, bacteria were favoured by the presence of introduced grazers compared with controls. Nevertheless, also in experiments with large microzooplankton, final bacterial biomass was always lower than the initial values (Fig. 2A). HNF (all <7 μm) showed the highest biomass at the beginning of the experiment. Controls and experiments with microzooplankton did not differ significantly.
Phytoplankton biomass showed no substantial change in the control treatments. All grazing experiments led to significantly decreased concentrations of A. abijatae, with the grazing impact of large ciliates being significantly higher than that of rotifers (FDR post hoc test, P < 0.05, Fig. 2C). There was also a clear trend of A. fusiformis biomass reduction because of the presence of grazers (Table 2). Although biomass reduction was not significant, the average filament length of the cyanobacterium significantly decreased in the presence of the two large ciliate taxa (FDR post hoc test, n = 300, P < 0.05). Cryptomonads were grazed on to various extents, with the smaller species showing significantly reduced concentrations (Table 2). Like HNF, picophytoplankton biomass had the highest values at the start of the experiment and a reduced biomass in all setups, including controls.
The grazing rates on phytoplankton were much higher for large ciliates than for rotifers. Among rotifers, B. plicatilis ingested 35% more food per individual than B. dimidiatus. The ciliate grazing rates were even higher, nearly double those of rotifers. When ingestion per unit grazer biomass was calculated, however, the small-bodied B. dimidiatus and Frontonia showed clearly higher grazing rates; values reached by B. plicatilis and C. magnum were one order of magnitude lower (Table 3).
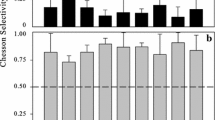
Selectivity indices for the different microzooplankton groups showed some accordance within microzooplankton taxa. The two Brachionus species both showed preferences for small bacterivorous ciliates, whereas filamentous cyanobacteria had lower selectivity values. The two ciliates, especially C. magnum, exhibited a more uniform ingestion, with a wide range of particles ingested at similar rates. Notably, when selectivity between filamentous cyanobacteria was compared, values for A. fusiformis were always lower than for A. abijatae (Fig. 3).
Grazing on Arthrospira fusiformis
The highest grazing rates on A. fusiformis were recorded for the large heterotrich ciliate C. magnum, which had a significant grazing impact (FDR post hoc test, P < 0.05) on the highest four A. fusiformis concentrations (Fig. 4). B. plicatilis also displayed high consumption rates, especially at A. fusiformis densities of 14.0 and 43.0 g C m−3. The other rotifer, B. dimidiatus, had little to no grazing impact on A. fusiformis, with only one significant reduction of A. fusiformis at intermediate phytoplankton densities.
Grazing rates on A. fusiformis concentrations of 1.3 g C m−3 were lowest for all microzooplankton organisms. All grazers, except C. magnum, showed no significant differences in grazing rates between the highest four A. fusiformis concentrations. This implies that the incipient limiting level of Frontonia and the two rotifers ranged between 1.3 and 7.5 g C m−3. Grazing rates of C. magnum significantly increased (FDR post hoc test, P < 0.05) until the second highest cyanobacterial biomass. Even highest A. fusiformis densities did not result in inhibitory effects on microzooplankton: None of the grazers showed significantly lower ingestion rates at highest cyanobacteria densities. Grazing rates measured in the two different sets of experiments were not significantly different for all microzooplankton taxa (FDR post hoc test, P > 0.05).
Discussion
Size spectrum of food particles and selectivity
High abundance of filamentous cyanobacteria is well documented for tropical soda lakes (Ballot et al., 2004; Schagerl & Oduor, 2008). In our experiments based on the natural plankton community of L. Nakuru, filaments of two cyanobacteria also accounted for 54.6% of phytoplankton biomass. The overlap in size between phytoplankton and dominant primary consumers, a typical phenomenon in shallow tropical lakes (Work et al., 2005), was also observed in our study. We showed that filamentous cyanobacteria can be ingested by microzooplankton despite their large dimension and coiled colony forms.
The two large ciliates Frontonia sp. and C. magnum showed high physical adaptability in food ingestion. Frontonia has a flexible cell wall (Dias & D’agosto, 2006) and we observed this species ingesting filaments substantially longer than its own body. C. magnum shows few size restrictions feeding on phytoplankton because of its own large body size. Sometimes C. magnum digested several large A. fusiformis filaments at once, and this genus is even known to occasionally ingest small rotifers (Arndt, 1993). It is commonly assumed that small food dominate the feeding spectrum of the suspension-feeding rotifer genus Brachionus (Rothhaupt, 1990). Studies on B. plicatilis from temperate regions defined an optimum food size between 5 and 10 μm (Hansen et al., 1997), and food particles in this range are also commonly used in aquaculture. In contrast, Pagano et al. (1998) showed that food selectivity of a tropical population of B. plicatilis reached almost maximum values when 20-μm particles were ingested (feeding on larger particles was not studied). Furthermore, tropical rotifer populations of Brachionus calyciflorus Pallas are able to ingest the filamentous cyanobacterium Cylindrospermopsis raciborskii Woloszynska (Soares et al., 2010), and Bouvy et al. (2001) measured high B. calyiflorus biomass when phytoplankton consisted nearly 100% of C. raciborskii. This indicates that brachionid rotifers populations in eutrophic tropical systems might have adapted to the dominance of large phytoplankton forms.
Selectivity indices, however, illustrated a lower selectivity of rotifers and Frontonia sp. for filamentous cyanobacteria. Especially B. plicatilis and B. dimidiatus preferred small ciliates over cyanobacteria. Variations in selectivity patterns could be caused by a number of factors ranging from specific nutrient requirements and food quality to prey catchability and the cell surface of prey items (Mohr & Adrian, 2000; Plath & Boersma, 2001). Nonetheless, size did not seem to be a factor limiting the ingestion of cyanobacteria in our study, as bacterivorous ciliates grazed by microzooplankton were considerably bigger.
Brachionus is often considered to be a size-selective grazer, unable to distinguish between particles of different quality (Demott, 1986; Rothhaupt, 1990, 1995; Hansen et al., 1997). In contrast, Snell (1998) pointed out the presence of chemoreceptors in the buccal field of this genus, indicating that other factors might also play a role for food selectivity. Some studies of Asian B. plicatilis populations have shown that selectivity cannot be explained by size alone (Hirayama et al., 1979; Zhou et al., 2009), and according to Kirk & Gilbert (1992), Brachionus can avoid toxic cyanobacteria via sensitive taste receptors. This seemed to be confirmed by another study (Lurling & Verschoor, 2003), which showed varying selectivity of Brachionus on Microcystis, depending on ratios between Microcystis and other available food particles. Gilbert & Starkweather (1977) have described three mechanisms of food rejection in B. calyciflorus: one for adjusting the maximum size of ingested particles and two others for selective rejection of single particles, probably connected to high energy costs for the grazing organism. Nonetheless, food selectivity in zooplankton has been shown to increase with particle size (Demott, 1986). Probably the amortization of energy-intensive rejection mechanisms may rise with increased particle size, leading to increased importance of other factors influencing selectivity patterns.
Interactions between microzooplankton and microbial food webs
Microzooplankton strongly influenced the abundance and biomass of other microorganisms in experiments with natural plankton communities (Fig. 2). Both rotifers and large ciliates caused cascading structural effects on the food chain by reducing numbers of bacterivorous grazers and thus stabilising bacterial biomass. Additionally, the positive reaction of bacterial abundances might also be attributed to other factors such as increased nutrient recycling because of metazooplankton presence (Guildford & Taylor, 2011). The biomass of an intermediate size group consisting of HNF and picophytoplankton decreased in both the control and experimental setups. This is because large microzooplankton and small ciliates feed on this particle size (Rothhaupt, 1990; Cheng et al., 2004). The increasing abundances of small omnivorous ciliates probably compensated the absence of large microzooplankton grazers in control vessels.
The positive effect of large microzooplankton grazers on heterotrophic bacteria, by reducing the biomass of bacterivorous protozoans, supports the hypothesis of Zinabu & Taylor (1997) that bacterial biomass in soda lakes is generally bottom up controlled. This is confirmed by the fact that large microzooplankton forms commonly occur in soda lakes at much higher densities than in our grazing experiments (Iltis & Riou-Duwat, 1971; Vareschi & Vareschi, 1984). A long-term investigation of microbial communities would provide a more detailed insight into this topic.
Grazing rates and structuring forces of zooplankton
Grazing rates in both sets of experiments were generally high. To our knowledge, only qualitative studies have been conducted on Frontonia sp. (Dias & D’agosto, 2006); grazing rates, however, have been reported for rotifers of the genus Brachionus and especially for B. plicatils. Most studies have been conducted with nanoalgae cultures and yielded rates in the range of 2–5 × 105 μm3 ind−1 h−1 (Hansen et al., 1997; Navarro, 1999; Montagnes et al., 2001). Vareschi & Jacobs (1984) did not directly measure grazing but calculated consumption based on the production and an energy transfer efficiency of 0.15%, with consumption rates more than 6 × 105 μm3 ind−1 h−1. Interestingly, the grazing rates of 8.2 ±1.8 × 105 μm3 ind−1 h−1 obtained in this study were substantially higher, which could be because of several factors. First, 24-h incubation times could have increased microzooplankton densities. Rotifers were randomly picked from natural populations without selecting against egg-carrying individuals. As egg rates (eggs females−1) were high, with 0.8 for B. plicatilis and 0.6 for B. dimidatus, and embryonic development time is low (Vareschi & Jacobs, 1984), freshly hatched individuals might have increased grazing rates. Second, sloppy feeding increases with food particle size (Moller, 2005). Accordingly, a larger proportion of ciliates and filamentous cyanobacteria in ingested food particles probably increased the importance of this factor, which is usually neglected in grazing experiments. Third, also food quality, quantity and gut transition time (GTT) have to be considered: maximum feeding rates can be increased by a factor of four through variation of these variables (Mitra & Flynn, 2007). If the quantity is high, GTT will depend on food quality because low-quality food remains shorter in the gut. This physiological acclimation ensures a high gradient of essential nutrients between the digestive system and the absorbing tissue, even if low-quality food is ingested (Willows, 1992; Jumars, 2000a, b). Conversely, if quality is high, then grazing rates are reduced and GTT is increased to maximise energy efficiency (Mitra & Flynn, 2007). Phytoplankton communities in tropical saline-alkaline lakes are commonly dominated by filamentous cyanobacteria, which are mostly considered as low-quality food (e.g. Arnold, 1971) leading to low zooplankton population growth rates (Brett et al., 2006). Thus, B. plicatilis populations in L. Nakuru and L. Bogoria might be adapted to this condition by keeping feeding rates high and GTT low.
When weight-specific grazing instead of individual rates is investigated, the two smaller species B. dimidiatus and Frontonia sp. display much higher ingestion rates. This might reflect higher metabolic requirements and higher production rates of the smaller microzooplankton taxa and is in line with Vareschi & Jacobs (1984), who found higher production rates for B. dimiatus than for B. plicatilis.
To estimate in situ community grazing rates, we multiplied microzooplankton of L. Bogoria and L. Nakuru at the sampling dates (Table 4) with grazing rates of rotifers and large ciliates in near-natural plankton communities. This yielded community grazing rates of 3.0 g C m2 day−1 for L. Nakuru, reflecting comparatively high microzooplankton densities (Vareschi & Vareschi, 1984), and 0.8 C day−1 for L. Bogoria, which had average to low densities at the sampling date. If community grazing is related to the average daily gross primary production rates of the two lakes (Oduor & Schagerl, 2007a), then rotifers and large ciliates consumed 45 and 17% of the daily autotrophic production of L. Nakuru and L. Bogoria, respectively.
Overall, we identified three different effects of the large microzooplankton on plankton communities: a negative one on large phytoplankton and small ciliates because of direct grazing, a neutral effect on HNF and picophytoplankton because microzooplankton consumption compensates reduced grazing pressure by small ciliates and finally a positive effect on heterotrophic bacteria (<1 μm).
Considering the high grazing rates and the sometimes outstandingly abundant rotifer populations (Iltis & Riou-Duwat, 1971; Vareschi & Vareschi, 1984), microzooplankton in tropical soda lakes certainly has the potential to shape phytoplankton communities. Especially during transitional phases—when phytoplankton biomass is low, rotifer abundances high and the phytoplankton community is switching between two alternative stable states (Vareschi & Jacobs, 1985)—grazing pressure might be a major controlling factor. Nevertheless, when A. fusiformis dominates, the influence of zooplankton should be very limited because of low consumer densities and a very high phytoplankton biomass (Vareschi, 1982). During such periods, even high community grazing rates would only minimally change the phytoplankton standing stock. Therefore, phytoplankton grazers can be excluded as the sole reason for A. fusiformis biomass breakdowns, which are observed stochastically in saline Rift Valley lakes. Nonetheless, enhanced nutrient recycling, lower selectivity of zooplankton for A. fusiformis and improved underwater light supply caused by grazing could play key roles in establishment of such blooms.
References
Ahlgren, G., 1983. Comparison of methods for estimation of phytoplankton carbon. Archiv für Hydrobiologie 98: 489–508.
Arndt, H., 1993. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)—a review. Hydrobiologia 255: 231–246.
Arnold, D. E., 1971. Ingestion, assimilation, survival, and reproduction by Daphnia-Pulex Fed 7 species of blue-green-algae. Limnology and Oceanography 16: 906–920.
Azam, F., T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyerreil & F. Thingstad, 1983. The ecological role of water-column microbes in the sea. Marine Ecology-Progress Series 10: 257–263.
Ballot, A., L. Krienitz, K. Kotut, C. Wiegand, J. S. Metcalf, G. A. Codd & S. Pflugmacher, 2004. Cyanobacteria and cyanobacterial toxins in three alkaline rift valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. Journal of Plankton Research 26: 925–935.
Bell, R. T., 1993. Estimating production of heterotrophic bacterioplankton via incorporation of tritiated thymidine. In Kemp, P. F., B. F. Sherr, E. B. Sherr & J. J. Cole (eds), Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton: 495–503.
Benjamini, Y. & D. Yekutieli, 2001. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics 29: 1165–1188.
Boon, P. I., S. E. Bunn, J. D. Green & R. J. Shiel, 1994. Consumption of cyanobacteria by fresh-water zooplankton—Implications for the success of top-down control of cyanobacterial blooms in Australia. Australian Journal of Marine and Freshwater Research 45: 875–887.
Bouvy, M., M. Pagano & M. Troussellier, 2001. Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (northeast Brazil). Aquatic Microbial Ecology 25: 215–227.
Brett, M. T., D. C. Muller-Navarra, A. P. Ballantyne, J. L. Ravet & C. R. Goldman, 2006. Daphnia fatty acid composition reflects that of their diet. Limnology and Oceanography 51: 2428–2437.
Cheng, S. H., S. Aoki, M. Maeda & A. Hino, 2004. Competition between the rotifer Brachionus rotundiformis and the ciliate Euplotes vannus fed on two different algae. Aquaculture 241: 331–343.
Demott, W. R., 1986. The role of taste in food selection by fresh-water zooplankton. Oecologia 69: 334–340.
Dias, R. J. P. & M. D’agosto, 2006. Feeding behavior of Frontonia leucas (Ehrenberg) (Protozoa, Cillophora, Hymenostomatida) under different environmental conditions in a lotic system. Revista Brasileira De Zoologia 23: 758–763.
Fernando, C. H., 1994. Zooplankton, fish and fisheries in tropical fresh-waters. Hydrobiologia 272: 105–123.
Finlay, B. J., C. R. Curds, S. S. Bamforth & J. M. Bafort, 1987. Ciliated protozoa and other microorganisms from 2 African Soda Lakes (Lake Nakuru and Lake Simbi, Kenya). Archiv Fur Protistenkunde 133: 81–91.
Frost, B. W., 1972. Effects of size and concentration of food particles on feeding behavior of marine planktonic copepod Calanus-Pacificus. Limnology and Oceanography 17: 805–815.
Gilbert, J. J. & P. L. Starkweather, 1977. Feeding in Rotifer Brachionus-Calyciflorus. 1. Regulatory Mechanisms. Oecologia 28: 125–131.
Gliwicz, Z. M., 1975. Effect of zooplankton grazing on photosynthetic activity and composition of phytoplankton. Verhandlungen des Internationalen Verein Limnologie 19: 1490–1497.
Gragnani, A., M. Scheffer & S. Rinaldi, 1999. Top-down control of cyanobacteria: a theoretical analysis. American Naturalist 153: 59–72.
Guildford, S. J. & W. D. Taylor, 2011. Evidence supporting the importance of nutrient regeneration by nano- and micrograzers for phytoplankton photosynthesis in Lake Malawi/Nyasa. Journal of Great Lakes Research 37: 54–60.
Hansen, B., T. Wernbergmoller & L. Wittrup, 1997. Particle grazing efficiency and specific growth efficiency of the rotifer Brachionus plicatilis (Muller). Journal of Experimental Marine Biology and Ecology 215: 217–233.
Harbison, G. R. & V. L. Mcalister, 1980. Fact and artifact in copepod feeding experiments. Limnology and Oceanography 25: 971–981.
Harper, D. M., R. B. Childress, M. M. Harper, R. R. Boar, P. Hickley, S. C. Mills, N. Otieno, T. Drane, E. Vareschi, O. Nasirwa, W. E. Mwatha, J. P. E. C. Darlington & X. Escuté-Gasulla, 2003. Aquatic biodiversity and saline lakes: Lake Bogoria National Reserve, Kenya. Hydrobiologia 500: 259–276.
Hirayama, K., K. Takagi & H. Kimura, 1979. Nutritional effect of eight species of marine phytoplankton on population growth of the rotifer, Brachionus plicatilis. Bulletin of the Japanese Society of Scientific Fisheries 45: 11–16.
Iltis, A. & S. Riou-Duwat, 1971. Variations saisonnières du peuplement en rotifères des eaux natronées du Kanem (Tchad). Cah O.R.S.T.O.M., ser. Hydriobiol 2: 101–112.
Jumars, P. A., 2000a. Animal guts as ideal chemical reactors: maximizing absorption rates. American Naturalist 155: 527–543.
Jumars, P. A., 2000b. Animal guts as nonideal chemical reactors: partial mixing and axial variation in absorption kinetics. American Naturalist 155: 544–555.
Jurgens, K. & C. Matz, 2002. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek (International Journal of General and Molecular Microbiology) 81: 413–434.
Kilham, P., 1981. Pelagic bacteria—extreme abundances in African Saline Lakes. Naturwissenschaften 68: 380–381.
Kirk, K. L. & J. J. Gilbert, 1992. Variation in herbivore response to chemical defenses—zooplankton foraging on toxic cyanobacteria. Ecology 73: 2208–2217.
Lewis, W. M., 1978. Dynamics and succession of the phytoplankton in a tropical lake—Lake Lanao, Philippines. Journal of Ecology 66: 849–880.
Lurling, M. & A. M. Verschoor, 2003. F-0-spectra of chlorophyll fluorescence for the determination of zooplankton grazing. Hydrobiologia 491: 145–157.
Menden-Deuer, S. & E. J. Lessard, 2000. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography 45: 569–579.
Mitra, A. & K. J. Flynn, 2007. Importance of interactions between food quality, quantity, and gut transit time on consumer feeding, growth, and trophic dynamics. American Naturalist 169: 632–646.
Mohr, S. & R. Adrian, 2000. Functional responses of the rotifers Brachionus calyciflorus and Brachionus rubens feeding on armored and unarmored ciliates. Limnology and Oceanography 45: 1175–1180.
Moller, E. F., 2005. Sloppy feeding in marine copepods: prey-size-dependent production of dissolved organic carbon. Journal of Plankton Research 27: 27–35.
Montagnes, D. J. S. & D. H. Lynn, 1993. A quantitative protargol stain (QPS) for ciliates and other protists. In Kemp, P. F., et al. (eds), Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton: 229–240.
Montagnes, D. J. S., S. A. Kimmance, G. Tsounis & J. C. Gumbs, 2001. Combined effect of temperature and food concentration on the grazing rate of the rotifer Brachionus plicatilis. Marine Biology 139: 975–979.
Navarro, N., 1999. Feeding behaviour of the rotifers Brachionus plicatilis and Brachionus rotundiformis with two types of food: live and freeze-dried microalgae. Journal of Experimental Marine Biology and Ecology 237: 75–87.
Oduor, S. O. & M. Schagerl, 2007a. Phytoplankton primary productivity characteristics in response to photosynthetically active radiation in three Kenyan Rift Valley saline-alkaline lakes. Journal of Plankton Research 29: 1041–1050.
Oduor, S. O. & M. Schagerl, 2007b. Temporal trends of ion contents and nutrients in three Kenyan Rift Valley saline-alkaline lakes and their influence on phytoplankton biomass. Hydrobiologia 584: 59–68.
Oomswilms, A. L., 1997. Are bacteria an important food source for rotifers in eutrophic lakes? Journal of Plankton Research 19: 1125–1141.
Pagano, M., L. Saint-Jean, R. Arfi, M. Bouvy & D. Guiral, 1998. Zooplankton food limitation and grazing impact in a eutrophic brackish-water tropical pond (Cote d’Ivoire, West Africa). Hydrobiologia 390: 83–98.
Paloheimo, J. E., 1979. Indexes of food type preference by a predator. Journal of the Fisheries Research Board of Canada 36: 470–473.
Peters, R. H., 1984. Methods for the study of feeding, grazing and assimilation by zooplankton. In Downing, J. A. & F. H. Rigler (eds), A Manual on Methods for the Assessment of Secondary Productivity in Freshwater. IBP Handbook 17. Blackwell Scientific Publications, Oxford: 336–412.
Plath, K. & M. Boersma, 2001. Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology 82: 1260–1269.
R Development Core Team, 2010. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org.
Rothhaupt, K. O., 1990. Differences in particle size-dependent feeding efficiencies of closely related rotifer species. Limnology and Oceanography 35: 16–23.
Rothhaupt, K. O., 1995. Algal nutrient limitation affects rotifer growth rate but not ingestion rate. Limnology and Oceanography 40: 1201–1208.
Rott, E., 1981. Some results from phytoplankton counting intercalibrations. Schweizerische Zeitschrift Fur Hydrologie (Swiss Journal of Hydrology) 43: 34–62.
Schagerl, M. & S. O. Oduor, 2008. Phytoplankton community relationship to environmental variables in three Kenyan Rift Valley saline-alkaline lakes. Marine & Freshwater Research 59: 125–136.
Snell, T. W., 1998. Chemical ecology of rotifers. Hydrobiologia 388: 267–276.
Soares, M. C. S., M. Lurling & V. L. M. Huszar, 2010. Responses of the rotifer Brachionus calyciflorus to two tropical toxic cyanobacteria (Cylindrospermopsis raciborskii and Microcystis aeruginosa) in pure and mixed diets with green algae. Journal of Plankton Research 32: 999–1008.
Strathmann, R., 1967. Estimating organic carbon content of phytoplankton from cell volume or plasma volume. Limnology and Oceanography 12: 411–418.
Sun, J. & D. Y. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346.
Talling, J. F., 2001. Environmental controls on the functioning of shallow tropical lakes. Hydrobiologia 458: 1–8.
Telesh, I. V., M. Rahkola & M. Viljanen, 1998. Carbon content of some freshwater rotifers. Hydrobiologia 387: 355–360.
Tuma, R., M. Beaudet, S. Yue, X. Jin, V. Paragas, T. Steinberg, L. Jones & V. L. Singer, 1998. SYBR (R) gold nucleic acid gel stain: a new dye optimized for use with 300 nm transilluminators. FASEB Journal 12: A1399.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitteilung Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Vadstein, O., G. Oie & Y. Olsen, 1993. Particle-size dependent feeding by the rotifer Brachionus-Plicatilis. Hydrobiologia 255: 261–267.
Vareschi, E., 1982. The ecology of Lake Nakuru (Kenya). 3. Abiotic factors and primary production. Oecologia 55: 81–101.
Vareschi, E. & J. Jacobs, 1984. The ecology of Lake Nakuru (Kenya). 5. Production and consumption of consumer organisms. Oecologia 61: 83–98.
Vareschi, E. & J. Jacobs, 1985. The ecology of Lake Nakuru. 6. Synopsis of production and energy-flow. Oecologia 65: 412–424.
Vareschi, E. & A. Vareschi, 1984. The ecology of Lake Nakuru (Kenya). 4. Biomass and distribution of consumer organisms. Oecologia 61: 70–82.
Verschoor, A. M., Y. S. Zadereev & W. M. Mooij, 2007. Infochemical-mediated trophic interactions between the rotifer Brachionus calyciflorus and its food algae. Limnology and Oceanography 52: 2109–2119.
Willows, R. I., 1992. Optimal digestive investment—a model for filter feeders experiencing variable diets. Limnology and Oceanography 37: 829–847.
Work, K., K. Havens, B. Sharfstein & T. East, 2005. How important is bacterial carbon to planktonic grazers in a turbid, subtropical lake? Journal of Plankton Research 27: 357–372.
Yasindi, A. W., D. H. Lynn & W. D. Taylor, 2002. Ciliated protozoa in Lake Nakuru, a shallow alkaline-saline lake in Kenya: seasonal variation, potential production and role in the food web. Archiv fur Hydrobiologie 154: 311–325.
Zhou, W. L., X. X. Tang, X. Y. Qiao, Y. Wang, R. J. Wang & L. Feng, 2009. Ingestion of Brachionus plicatilis under different microalgae conditions. Chinese Journal of Oceanology and Limnology 27: 473–479.
Zinabu, G. M. & W. D. Taylor, 1997. Bacteria–chlorophyll relationships in Ethiopian lakes of varying salinity: are soda lakes different? Journal of Plankton Research 19: 647–654.
Acknowledgments
This study was funded by the Austrian Science Fund Project No. P19911 “Factors controlling abundance of A. fusiformis.” Our sincere thanks go to all team members of the project for their support, especially to Dr Steve Odour for his help in organising this study and to Pauline Macharia and Ben Simiyu for their assistance during laboratory work at Egerton University, Kenya. We also acknowledge three anonymous reviewers for their valuable comments on the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Zhengwen Liu, Bo-Ping Han & Ramesh D. Gulati / Conservation, management and restoration of shallow lake ecosystems facing multiple stressors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Burian, A., Schagerl, M. & Yasindi, A. Microzooplankton feeding behaviour: grazing on the microbial and the classical food web of African soda lakes. Hydrobiologia 710, 61–72 (2013). https://doi.org/10.1007/s10750-012-1023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1023-2