Abstract
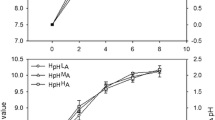
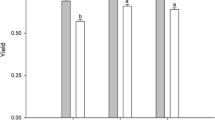
Humic substances (HSs) have been shown to influence growth, photophysiology, and redox homeostasis in phototrophs. However, many ecological studies deliver controversial results indicating inhibitory or stimulating effects depending on the kind of phototrophic organism or type of HSs applied. Here we analyzed the effect of Huminfeed® (HF), a preparation from leonardite associated with lignite deposits, on growth and photosynthetic performance of three different coccal green algae. Concentrations of HF in the range of 2–20 mg l−1 dissolved organic carbon (DOC) did neither affect the growth rate nor the light-adapted photosynthetic electron transport. Even photoinhibitory light intensities of 1,600 μmol photons m−2 s−1, representing the tenfold of growth light intensity, did not result in a decline of the maximal photosynthetic rate in HF-treated algae. In HF-grown algae, a very subtle decrease by about 10% could be observed in thermoluminescent light emission, a sensitive method to detect changes in photosystem II (PSII) chemistry. However, thermoluminescence (TL) represents only 3% of the light-induced charge separated states in PSII. Hence, rather small changes will not have significant effects on overall photosynthetic performance and growth. Therefore, the physiological effect of HSs on freshwater phototrophs has to be revisited.





Similar content being viewed by others
Notes
The use of HuminFeed® is not an advertisement for this product. For more information of this commercial product, the reader is referred to Humintech (2011).
Abbreviations
- Chl a :
-
Chlorophyll a
- DOC:
-
Dissolved organic carbon
- HF:
-
Huminfeed®
- HS:
-
Humic substance
- NOM:
-
Natural organic matter
- PSII:
-
Photosystem II
- rETR:
-
Relative electron transport rate
- TL:
-
Thermoluminescence
References
Allard, B. & J. Templier, 2000. Comparison of neutral lipid profile of various trilaminar outer cell wall (TLS)-containing microalgae with emphasis on algaenan occurrence. Phytochemistry 54: 369–380.
Atkinson, A. W., B. E. S. Gunning & P. C. L. John, 1972. Sporopollenin in the cell wall of Chlorella and other algae: ultrastructure, chemistry and incorporation of 14C-acetate, studied in synchronous cultures. Planta 107: 1–32.
Costa, A. R. & M. N. de Pinho, 2002. The role of membrane morphology on ultrafiltration for natural organic matter removal. Desalination 145: 299–304.
Devol, A. H., A. Dos Santos, B. R. Forsberg & T. M. Zaret, 1984. Nutrient addition experiments in Lago Jacaretinga, Central Amazon, Brazil: 2. The effect of humic and fulvic acids. Hydrobiologia 109: 97–103.
Ducruet, J.-M., 2003. Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. Journal of Experimental Botany 54: 2419–2430.
Ducruet, J.-M. & I. Vass, 2009. Thermoluminescence: experimental. Photosynthesis Research 101: 195–204.
Eilers, P. H. C. & J. C. H. Peeters, 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling 42: 199–215.
Farjalla, V. F., A. M. Amado, A. L. Suhett & F. Meirelles-Pereira, 2009. DOC removal paradigms in highly humic aquatic ecosystems. Environmental Science and Pollution Research 16: 531–538.
Gilbert, M., H. Wagner, I. Weingart, J. Skotnica, K. Nieber, G. Tauer, F. Bergmann, H. Fischer & C. Wilhelm, 2004. A new type of thermoluminometer: a highly sensitive tool in applied photosynthesis research and plant stress physiology. Journal of Plant Physiology 161: 641–651.
Gjessing, E. T., J. J. Alberts, A. Bruchet, P. K. Egeberg, E. Lydersen, L. B. McGown, J. J. Mobed, U. Münster, J. Pempkowika, M. Perdue, H. Ratnawerra, D. Rybacki, M. Takacs & G. Abbt-Braun, 1998. Multi-method characterisation of natural organic matter isolated from water: characterisation of reverse osmosis-isolates from water of two semi-identical dystrophic lakes basins in Norway. Water Research 32: 3108–3124.
Gleason, F. K. & J. L. Paulson, 1984. Site of action of the natural algicide, cyanobacterin, in the blue-green alga, Synechococcus sp. Archives of Microbiology 138: 273–277.
Hegewald, E., A. Aldave & E. Schnepf, 1978. Investigations on the lakes of Peru and their phytoplankton, 2. The algae of pond La Laguna, Huanuco, with special reference to Scenedesmus intermedius and S. armatus. Archiv für Hydrobiologie 82: 207–215.
Heil, C. A., 2005. Influence of humic, fulvic and hydrophilic acids on the growth, photosynthesis and respiration of the dinoflagellate Prorocentrum minimum (Pavillard) Schiller. Harmful Algae 4: 603–618.
Humintech, 2011. Product information HuminFeed®. http://www.humintech.com/001/animalfeeds/products/huminfeed.html. Accessed July 2011.
Imai, A., T. Fukushima & K. Matsushige, 1999. Effects of iron limitation and aquatic humic substances on the growth of Microcystis aeruginosa. Canadian Journal of Fisheries and Aquatic Sciences 56: 1929–1937.
Izawa, S., J. M. Gould, D. R. Ort, P. Felker & N. E. Good, 1973. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. III. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochimica et Biophysica Acta 305: 119–128.
Izawa, S., 1977. Inhibitors of electron transport. In Trebst, A. & M. Avron (eds), Encyclopedia of Plant Physiology, New Series, Vol. 5, Photosynthesis I. Springer, Berlin: 266–282.
Jackson, T. A. & R. E. Hecky, 1980. Depression of primary productivity by humic matter in lake and reservoir water of the boreal forest zone. Canadian Journal of Fisheries and Aquatic Sciences 37: 2300–2317.
Karasyova, T. A., E. O. Klose, R. Menzel & C. E. W. Steinberg, 2007. Natural organic matter differently modulates growth of two closely related coccal green algal species. Environmental Science and Pollution Research 14: 88–93.
Kulikova, N. A., I. V. Perminova, G. A. Badun, M. G. Chernysheva, O. V. Koroleva & E. A. Tsvetkova, 2010. Estimation of uptake of humic substances from different sources by Escherichia coli cells under optimum and salt stress conditions by use of tritium-labeled humic materials. Applied and Environmental Microbiology 76: 6223–6230.
Kullberg, A., K. H. Bishop, A. Hargeby, M. Jansson & R. C. Petersen Jr, 1993. The ecological significance of dissolved organic carbon in acidified waters. Ambio 22: 331–337.
Leenheer, J. A. & C. E. Rostad, 2004. Tannins and terpenoids as major precursors of Suwannee River fulvic acids. U.S. Geological Survey Scientific Investigations Report 2004-5276: 16 pp.
Lovley, D. R., J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips & J. C. Woodward, 1996. Humic substances as electron acceptors for microbial respiration. Nature 382: 445–448.
Maxwell, K. & G. N. Johnson, 2000. Chlorophyll fluorescence - a practical guide. Journal of Experimental Botany 51: 659–668.
Meinelt, T., T. M. Phan, E. Zwirnmann, A. Krüger, A. Paul, A. Wienke & C. E. W. Steinberg, 2007. Reduction in vegetative growth of the water mold Saprolegnia parasitica (Coker) by humic substances of different origin. Aquatic Toxicology 83: 93–103.
Menzel, R., S. Stürzenbaum, A. Bärenwaldt, J. Kulas & C. E. W. Steinberg, 2005. Humic material induces behavioral and global transcriptional responses in the nematode Caenorhabditis elegans. Environmental Science and Technology 39: 8324–8332.
Mittler, R., 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7: 405–410.
Münster, U., 1985. Investigation about structure, distribution and dynamics of different organic substrates in the DOM of lake Plusssee. Archiv für Hydrobiologie 70: 429–480.
Nicklisch, A., T. Shatwell & J. Köhler, 2008. Analysis and modelling of the interactive effects of temperature and light on phytoplankton growth and relevance for the spring bloom. Journal of Plankton Research 30: 75–91.
Oettmeier, W., R. Dostatni & H. J. Santel, 1987. Irreversibly binding photosynthetic electron transport inhibitors. II: halogen-substituted 1,4-naphthoquinones and halogenmethyl-1,4-quinones. Zeitschrift für Naturforschung 42c: 693–697.
Oettmeier, W., K. Masson & A. Donner, 1988. Anthraquinone inhibitors of photosystem II electron transport. FEBS Letters 231: 259–262.
Oettmeier, W., R. Dostatni, C. Majewski, G. Höfle, T. Fecker, B. Kunze & H. Reichenbach, 1990. The aurachins, naturally occurring inhibitors of photosynthetic electron flow through photosystem II and the cytochrome b6/f-complex. Zeitschrift für Naturforschung 45c: 322–328.
Pollio, A., G. Pinto, R. Ligrone & G. Aliotta, 1993. Effects of the potential allelochemical α-asarone on growth, physiology and ultrastructure of two unicellular green algae. Journal of Applied Phycology 5: 395–403.
Pörs, Y., A. Wüstenberg & R. Ehwald, 2010. A batch culture method for microalgae and cyanobacteria with CO2 supply through polyethylene membranes. Journal of Phycology 46: 825–830.
Prakash, A. & M. A. Rashid, 1968. Influence of humic substances on the growth of marine phytoplankton: Dinoflagellates. Limnology and Oceanography 13: 598–606.
Prakash, A., M. A. Rashid, A. Jensen & D. V. Subba Rao, 1973. Influence of humic substances on the growth of marine phytoplankton: Diatoms. Limnology and Oceanography 18: 516–524.
Prokhotskaya, V. Yu. & C. E. W. Steinberg, 2007. Differential sensitivity of a coccal green algal and a cyanobacterial species to dissolved natural organic matter (NOM). Environmental Science and Pollution Research 14: 11–18.
Richardson, K. & G. E. Fogg, 1982. The role of dissolved organic material in the nutrition and survival of marine dinoflagellates. Phycologia 21: 17–26.
Roháĉek, K., 2002. Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40: 13–29.
Sachse, A., D. Babenzien, G. Ginzel, J. Gelbrecht & C. E. W. Steinberg, 2001. Characterization of dissolved organic carbon (DOC) in a dystrophic lake and an adjacent fen. Biogeochemistry 54: 279–296.
Sarvala, J., V. Ilmavirta, L. Paasivirta & K. Salonen, 1981. The ecosystem of the oligotrophic Lake Pääjärvi 3. Secondary production and an ecological energy budget of the lake. Verhandlungen des Internationalen Verein Limnologie 21: 422–427.
Schreiber, U., W. Bilger & C. Neubauer, 1995. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Schulze, E. D. & M. M. Caldwell (eds), Ecophysiology of Photosynthesis. Springer, Berlin: 49–70.
Schreiber, U., 1998. Chlorophyll fluorescence: new instruments for special applications. In Garab, G. (ed.), Photosynthesis: Mechanisms and Effects, Vol. V. Kluwer Academic Publishers, Dordrecht: 4253–4258.
Shapiro, J., 1957. Chemical and biological studies on the yellow organic acids of lake water. Limnology and Oceanography 2: l61–l179.
Skulberg, O. M., 1967. Algal cultures as a means to assess the fertilizing influence of pollution. In Jaag, O. & H. Liebman (eds), Advances in Water Pollution Research. Water Pollution Control Federation, Washington: 113–138.
Smith, G. D. & N. T. Doan, 1999. Cyanobacterial metabolites with bioactivity against photosynthesis in cyanobacteria, algae and higher plants. Journal of Applied Phycology 11: 337–344.
Steinberg, C. E. W., 2003. Ecology of humic substances in freshwaters. Determinants from geochemistry to ecological niches. Springer, Berlin.
Steinberg, C. E. W., A. Paul, S. Pflugmacher, T. Meinelt, R. Klöcking & C. Wiegand, 2003. Pure humic substances have the potential to act as xenobiotic chemicals - a review. Fresenius Environmental Bulletin 12: 391–401.
Steinberg, C. E. W., S. Kamara, V. Yu. Prokhotskaya, L. Manusadžianas, T. Karasyova, M. A. Timofeyev, J. Zhang, A. Paul, T. Meinelt, V. F. Farjalla, A. Y. O. Matsuo, B. K. Burnison & R. Menzel, 2006. Dissolved humic substances - ecological driving forces from the individual to the ecosystem level? Freshwater Biolgy 51: 1189–1210.
Steinberg, C. E. W., N. Saul, K. Pietsch, T. Meinelt, S. Rienau & R. Menzel, 2007. Dissolved humic substances facilitate fish life in extreme aquatic environments and have the potential to extend lifespan of Caenorhabditis elegans. Annals of Environmental Science 1: 81–90.
Steinberg, C. E. W., N. Ouerghemmi, S. Herrmann, R. Bouchnak, M. A. Timofeyev & R. Menzel, 2010. Stress by poor food quality and exposure to humic substances: Daphnia magna responds with oxidative stress and life-span extension, but reduced offspring numbers. Hydrobiologia 652: 223–236.
Suhett, A. L., A. M. Amado, A. Enrich-Prast, F. D. A. Esteves & V. F. Farjalla, 2007. Seasonal changes of dissolved organic carbon photo-oxidation rates in a tropical humic lagoon: the role of rainfall as a major regulator. Canadian Journal of Fisheries and Aquatic Sciences 64: 1266–1272.
Sun, B., Y. Tanji & H. Unno, 2006. Extinction of cells of cyanobacterium Anabaena circinalis in the presence of humic acid under light. Applied Microbiology and Biotechnology 72: 823–828.
Thurman, E. M., R. L. Wershaw, R. L. Malcolm & D. J. Pinckney, 1982. Molecular size of aquatic humic substances. Organic Geochemistry 4: 27–35.
Toledo, A. P. P., J. G. Tundisi & V. A. D’Aquino, 1980. Humic acid influence on the growth and copper tolerance of Chlorella sp. Hydrobiologia 71: 261–263.
Trebst, A., 1972. Measurement of Hill reactions and photoreduction. In San Pietro, A. (ed.), Methods in Enzymology, Vol. 24: Photosynthesis and Nitrogen Fixation - Part B. Academic Press, New York: 146–165.
Trebst, A., 1980. Inhibitors in electron flow: tools for the functional and structural localization of carriers and energy conservation sites. In San Pietro, A. (ed.), Methods in Enzymology, Vol. 69: Photosynthesis and Nitrogen Fixation - Part C. Academic Press, New York: 675–715.
van Hoecke, K., K. A. C. De Schamphelaere, P. van der Meeren, S. Lucas & C. R. Janssen, 2008. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: importance of surface area. Environmental Toxicology and Chemistry 27: 1948–1957.
Vavilin, D. V. & J.-M. Ducruet, 1998. The origin of 115–130°C thermoluminescence bands in chlorophyll-containing material. Photochemistry and Photobiology 68: 191–198.
Vavilin, D. V., J.-M. Ducruet, D. N. Matorin, P. S. Venediktov & A. B. Rubin, 1998. Membrane lipid peroxidation, cell viability and photosystem II activity in the green alga Chlorella pyrenoidosa subjected to various stress conditions. Journal of Photochemistry and Photobiology B 42: 233–239.
Warburg, O. & W. Lüttgens, 1944. Weitere Experimente zur Kohlensäureassimilation. Die Naturwissenschaften 32: 301.
Wetzel, R. G., 2001. Limnology: Lake and river ecosystems, 3rd edn. Academic Press, San Diego.
Ziegler, R. & K. Egle, 1965. Zur quantitativen Analyse der Chloroplastenpigmente. I. Kritische Überprüfung der spektralphotometrischen Chlorophyllbestimmung. Beiträge zur Biologie der Pflanzen 41: 11–37.
Acknowledgments
The help given by some people of the stress ecology laboratory is gratefully acknowledged, particularly by Shumon Chakrabati for assisting the laboratory work, Andreas Nicklisch for general advices and Yvonne Pörs and Ulrich Schreiber for advices with the Phyto-PAM. We also thank the Deutsche Forschungsgemeinschaft (DFG) for supporting the scientific work (Grant Ste 673/17-1 and Wi 764/17-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Tobias Heinze and Hanno Bährs contributed equally to this article.
Rights and permissions
About this article
Cite this article
Heinze, T., Bährs, H., Gilbert, M. et al. Selected coccal green algae are not affected by the humic substance Huminfeed® in term of growth or photosynthetic performance. Hydrobiologia 684, 215–224 (2012). https://doi.org/10.1007/s10750-011-0985-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0985-9