Abstract
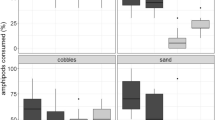
Predation of fish assemblages in seagrass meadows was examined in the field and in tank experiments. Lure trolling indicated that (1) total abundance of fish was higher on bare sediment where small fish (<5 cm), including juveniles, predominated; (2) abundance was lowest in seagrass where large fish (>15 cm) predominated; (3) large ambush predators, primarily the grass goby and European eel, were almost completely restricted to seagrass; (4) the predation mode in seagrass was almost entirely ambushing or stalk-attacking, while the predation mode on bare sediment was almost entirely chase-attacking; (5) ambush predation was far more successful than chase-attack predation; and (6) overall predation risk was approximately three times higher in seagrass. Tank experiments showed that piscivory success of the grass goby was higher than that of the most common chase-attacker, the black goby, and the presence or absence of artificial seagrass, regardless of density, had no significant effect on predation success of either species. Guts of the grass goby contained food items of a wider size range that averaged twice the size of those of the black goby. Our results confirm our prediction that the risk of predation, especially of small/juvenile fish, is higher in seagrass meadows than at adjacent bare substrate, and this risk differential is explained by the presence of larger, more efficient ambush predators restricted to seagrass, and the scarcity of large chase-attack predators in the Novigrad Sea.








Similar content being viewed by others
References
Abrams, P. A., 2007. Habitat choice in predator–prey systems: spatial instability due to interacting adaptive movements. The American Naturalist 169: 581–594.
Allen, M. R., 2007. Measuring and modeling dispersal of adult zooplankton. Oecologia 153: 135–143.
Anderson, M. J., 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences 58: 626–639.
Aronson, R. B., J. Heck, L. Kenneth & J. F. Valentine, 2001. Measuring predation with tethering experiments. Marine Ecology Progress Series 214: 311–312.
Beck, M. W., K. L. Heck, K. W. Able, D. L. Childers & D. B. Eggleston, 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51: 633–641.
Bonaca, M. O. & L. Lipej, 2005. Factors affecting habitat occupancy of fish assemblage in the Gulf of Trieste (Northern Adriatic Sea). Marine Ecology: An Evolutionary Perspective 26: 42–53.
Endler, J. A., 1980. Natural-selection on color patterns in Poecilia reticulata. Evolution 34: 76–91.
Endler, J. A., 1991. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Research 31: 587–608.
Flynn, A. & D. Ritz, 1999. Effect of habitat complexity and predatory style on the capture success of fish feeding on aggregated prey. Journal of the Marine Biology Association of the United Kingdom 79: 487–494.
Franco, A., S. Malavasi, M. Zucchetta, P. Franzoi & P. Torricelli, 2006. Environmental influences on fish assemblage in the Venice lagoon, Italy. Chemistry and Ecology 22: 105–118.
Gabriel, K., 1971. The biplot graphical display of matrices with application to principal component analysis. Biometrica 58: 453–467.
Gloeckner, D. R. & J. J. Luczkovich, 2008. Experimental assessment of trophic impacts from a network model of a seagrass ecosystem: direct and indirect effects of gulf flounder, spot and pinfish on benthic polychaetes. Journal of Experimental Marine Biology and Ecology 357: 109–120.
Guidetti, P., 2000. Differences among fish assemblages associated with nearshore Posidonia oceanica seagrass beds, rocky-algal reefs and unvegetated sand habitats in the Adriatic Sea. Estuarine Coastal and Shelf Science 50: 515–529.
Guidetti, P., F. Boero & J. Dulcic, 2002. Mass mortality of gilt sardine, Sardinella aurita (Clupeidae), in the Adriatic and Ionian seas. Cybium 26: 317–319.
Heck, K. L. & L. M. Orth, 1980a. Seagrass habitats: the role of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In Kennedy, V. (ed.), Estuarine Perspectives. Academic Press, New York: 449–464.
Heck, K. L. & R. J. Orth, 1980b. Structural components of eelgrass (Zostera marina) meadows in the lower Chesapeake Bay, Decapod Crustacea. Estuaries 3: 289–295.
Heck, K. L. & R. J. Orth, 1983. Predator–prey relationships in seagrass ecosystems – a reexamination of hypotheses. Estuaries 6: 256–257.
Heck, K. L. & T. A. Thoman, 1981. Experiments on predator–prey interactions in vegetated aquatic habitats. Journal of Experimental Marine Biology and Ecology 53: 125–134.
Heck, K. L., G. Hays & R. J. Orth, 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136.
Heck, K. L., J. F. Valentine, J. R. Pennock, G. Chaplin & P. M. Spitzer, 2006. Effects of nutrient enrichment and grazing on shoalgrass halodule wrightii and its epiphytes: results of a field experiment. Marine Ecology Progress Series 326: 145–156.
Holsman, K. K., P. S. Mcdonald & D. A. Armstrong, 2006. Intertidal migration and habitat use by subadult dungeness crab Cancer magister in a Ne Pacific estuary. Marine Ecology Progress Series 308: 183–195.
Horinouchi, M., 2007. Review of the effects of within-patch scale structural complexity on seagrass fishes. Journal of Experimental Marine Biology and Ecology 350: 111–129.
Jackson, E. L., A. A. Rowden, M. J. Attrill, S. J. Bossey & M. B. Jones, 2001. The importance of seagrass beds as a habitat for fishery species. Oceanography and Marine Biology 39: 269–303.
James, P. L. & K. L. Heck, 1994. The effects of habitat complexity and light-intensity on ambush predation within a simulated seagrass habitat. Journal of Experimental Marine Biology and Ecology 176: 187–200.
Job, S. D. & J. Shand, 2001. Spectral sensitivity of larval and juvenile coral reef fishes: implications for feeding in a variable light environment. Marine Ecology Progress Series 214: 267–277.
Koutrakis, A. & E. T. Tsikliras, 2003. Length–weight relationships of fishes from three northern Aegean estuarine systems (Greece). Journal of Applied Ichthyology 19: 258–260.
Labropoulou, M., G. Tserpes & N. Tsimenides, 1998. Age, growth and feeding habits of the brown comber Serranus hepatus (Linnaeus, 1758) on the Cretan shelf. Estuarine Coastal and Shelf Science 46: 723–732.
Laurel, J., A. W. Stoner, C. H. Ryer, T. P. Hurst & A. A. Abookire, 2007. Comparative habitat associations in juvenile pacific cod and other gadids using seines, baited cameras and laboratory techniques. Journal of Experimental Marine Biology and Ecology 351: 42–55.
Lima, S. L., 1992. Strong preferences for apparently dangerous habitats – a consequence of differential escape from predators. Oikos 64: 597–600.
Lindstrom, M. J. & D. M. Bates, 1990. Nonlinear mixed effects models for repeated measures data. Biometrics 46: 673–687.
Lindstrom, M. J. & D. M. Bates, 1994. Newton–Raphson and Em algorithms for linear mixed effects models for repeated-measures data. Journal of the American Statistical Association 89: 1572–1573.
Main, K. L., 1987. Predator avoidance in seagrass meadows – prey behavior, microhabitat selection, and cryptic coloration. Ecology 68: 170–180.
Matić-Skoko, S., M. Peharda, A. Pallaoro & M. Franičević, 2005. Species composition, seasonal fluctuations, and residency of inshore fish assemblages in the Pantan estuary of the eastern middle Adriatic. Acta Adriatica 46: 201–212.
Matić-Skoko, S., M. Peharda, A. Pallaoro, M. Cukrov & B. Baždarić, 2007. Infralittoral fish assemblages in the Zrmanja estuary, Adriatic sea. Acta Adriatica 48: 45–55.
Moreira, F., C. Assis, P. Almeida, J. Costa & M. Costa, 1992. Trophic relationships in the community of the upper Tagus estuary (Portugal): a preliminary approach. Estuarine Coastal and Shelf Science 34: 617–623.
Nagelkerken, I., M. Dorenbosch, W. C. E. P. Verberk, E. C. de la Moriniere & G. van der Velde, 2000. Day-night shifts of fishes between shallow-water biotopes of a Caribbean bay, with emphasis on the nocturnal feeding of haemulidae and lutjanidae. Marine Ecology Progress Series 194: 55–64.
Okamoto, M., G. Kawamura & Y. Tanaka, 2001. Selectivity of color of lure by Japanese sea bass Lateolabrax japonicus under different background colors. Nippon Suisan Gakkaishi 67: 449–454.
Ota, D., J. E. G. Downing & J. E. Cook, 1999. Neuronal and glial cell types revealed by Nadph-diaphorase histochemistry in the retina of a teleost fish, the grass goby (Zosterisessor ophocephalus, Perciformes Gobiidae). Anatomy and Embryology 200: 487–494.
Pearsons, T. N. & A. L. Fritts, 1999. Maximum size of Chinook salmon consumed by juvenile coho salmon. North American Journal of Fisheries Management 19: 165–170.
Peterson, C. H. & R. Black, 1994. An experimentalists challenge – when artifacts of intervention interact with treatments. Marine Ecology Progress Series 111: 289–297.
Pile, A. J., R. N. Lipcius, J. Vanmontfrans & R. J. Orth, 1996. Density dependent settler-recruit-juvenile relationships in blue crabs. Ecological Monographs 66: 277–300.
Schofield, P. J., 2005. Predation vulnerability of two gobies (Microgobius gulosus; Gobiosoma robustum) is not related to presence of seagrass. Florida Scientist 68: 25–34.
Schultz, S. T., C. Kruschel & T. Bakran-Petricioli, 2009. The influence of seagrass meadows on predator–prey habitat segregation in an Adriatic lagoon. Marine Ecology Progress Series 374: 85–99.
Sih, A., 1984. Optimal behavior and density-dependent predation. The American Naturalist 123: 314–326.
Stergiou, K. I. & V. S. Karpouzi, 2001. Feeding habits and trophic levels of Mediterranean fish. Reviews in Fish Biology and Fisheries 11: 217–254.
Sundstrom, L. F., M. Lohmus, R. H. Devlin, J. I. Johnsson, C. A. Biagi & T. Bohlin, 2004. Feeding on profitable and unprofitable prey: comparing behaviour of growth-enhanced transgenic and normal coho salmon (Oncorhynchus kisutch). Ethology 110: 381–396.
Taylor, R., 1984. Predation. Chapman and Hall, New York.
Valle, C., J. T. Bayle & A. A. Ramos, 2003. Weight–length relationships for selected fish species of the western Mediterranean sea. Journal of Applied Ichthyology 19: 261–262.
Wisenden, B. D. & T. A. Thiel, 2002. Field verification of predator attraction to minnow alarm substance. Journal of Chemical Ecology 28: 433–438.
Acknowledgements
We would like to thank S. Matić-Skoko, L. Lipej, T. Bakran-Petricioli, and 3 anonymous reviewers for insightful discussion of the research and comments on the manuscript. This work was supported by the University of Zadar, Department of Maritime Science, and by a research grant from the Croatian Ministry of Science to S.S., for the project Monitoring and ecology of benthic communities of the Croatian Adriatic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Holmer & N. Marbà / Dynamics and functions of seagrass ecosystems
Rights and permissions
About this article
Cite this article
Schultz, S.T., Kruschel, C. Frequency and success of ambush and chase predation in fish assemblages associated with seagrass and bare sediment in an Adriatic lagoon. Hydrobiologia 649, 25–37 (2010). https://doi.org/10.1007/s10750-010-0256-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0256-1