Abstract
Predation is important in maintaining the community structure, functioning and ecological resilience of estuarine seascapes. Understanding how predator community structure, seascape context and habitat condition combine to influence predation is vital in managing estuarine ecosystems. We measured relationships between predator species richness, predator abundance and individual species abundances as well as seascape context and habitat condition, on relative predation probability in mangrove forests, seagrass meadows and unvegetated sediment across 11 estuaries in Queensland, Australia. Predation was quantified using videoed assays of tethered invertebrates (i.e. ghost nippers, Trypaea australiensis) and fish assemblages were surveyed using remote underwater video systems. Yellowfin bream (Acanthopagrus australis) dominated predation in all three habitats; however, predation was not correlated with yellowfin bream abundance. Instead, predation increased fourfold in mangroves and threefold in unvegetated sediment when predatory species richness was highest (> 3 species), and increased threefold in seagrass when predator abundance was highest (> 10 individuals). Predation in mangroves increased fourfold in forests with a lower pneumatophore density (< 50/m2). In seagrass, predation increased threefold at sites that had a greater extent (> 2000 m2) of seagrass, with longer shoot lengths (> 30 cm) and at sites that were closer to (< 2000 m) the estuary mouth. Predation on unvegetated sediment increased threefold when more extensive salt marshes (> 15000 m2) were nearby. These findings demonstrate the importance of predator richness and abundance in supplementing predation in estuaries, despite the dominance of a single species, and highlight how seascape context and habitat condition can have strong effects on predation in estuaries.
Similar content being viewed by others
Introduction
Estuaries are important sites for human settlements, commerce and transport and are, therefore, subjected to increased human use which results in declines in water quality (i.e. pollution and terrestrial runoff), habitat removal (i.e. habitat fragmentation and degradation) and intense fishing pressure (i.e. commercial, recreational and artisanal fisheries) (Kennish 2002; Blaber 2013; Cloern et al. 2016). This leads to changes to the distribution, diversity and abundance of most animal and plant groups within estuaries as these systems are impacted by stressors originating from both land and sea (Lobry et al. 2008; Evans et al. 2018; Whitfield et al. 2018). However, estuaries also experience many natural stresses such as rapid changes to physio-chemical water conditions (e.g. salinity and turbidity) (Elliott et al. 2007). These anthropogenic and natural influences commonly lead to estuaries having reduced fish species richness when compared with adjacent marine ecosystems (Martino and Able 2003; Whitfield and Harrison 2021). While species richness is typically low in estuarine ecosystems, estuaries can often favour generalist (e.g. omnivores) over specialist (e.g. piscivores) species (Elliott and Whitfield 2011; Stuart-Smith et al. 2015; Bishop et al. 2017). Generalist species have wide ecological niches and broad habitat requirements, and can adapt to the physical conditions that often characterise estuarine ecosystems (Clavel et al. 2011; Borland et al. 2022b). These generalists are particularly dominant in modified estuaries because these species have wider trophic niches and can cope with the loss, reduction or change of resources better than those with specialised diets (Richmond et al. 2005; Clavel et al. 2011; Henderson et al. 2020a). Due to having broad functional niches, these generalists may be important in shaping rates of important ecological functions such as opportunistic predation in estuaries (Johnson et al. 2014).
Predation rates manage the abundance and diversity of species at lower trophic levels, and control feeding behaviours and food web properties within estuarine seascapes (Silliman and Bertness 2002; Duffy et al. 2005; Atwood et al. 2015). As estuaries are considered a low-diversity system (Henderson et al. 2020b), predation is likely to be dominated by a single species across a range of estuarine habitats including unvegetated sediment (Duncan et al. 2019), salt marsh (Jones et al. 2020), seagrass (Smith et al. 2011) and oyster reefs (Duncan et al. 2019). Many of the species that dominate predation are generalists that feed opportunistically on a range of benthic invertebrates and fish across habitats, and can thrive throughout broad environmental conditions (e.g. broad salinity and temperature tolerances) (Clavel et al. 2011; Curley et al. 2013; Duncan et al. 2019). Rates of predation and other ecological functions such as carrion consumption (which are performed by similar species) often correlate with the abundance of a dominant species, resulting in limited functional redundancy (i.e. few species use the same resources and perform an identical function) (Loreau 2004; Henderson et al. 2020b; Jones et al. 2020). Alternatively, estuaries may exhibit moderate functional complementarity in which species that have overlapping niches perform predation under different contexts such as within different habitat types, under different environmental conditions, or during distinct stages of diel and tidal cycles (Cardinale et al. 2012; Olds et al. 2018). However, understanding if predation and predatory fish assemblages are characterised by functional redundancy and complementarity within estuaries has rarely been quantified (Gross et al. 2017; Henderson et al. 2020b). Furthermore, it is unclear if predation is influenced by species richness or the abundance of dominant species across a range of habitats such as mangrove forests, seagrass meadows and unvegetated sediment that form heterogenous estuarine seascapes.
Mangrove forests and seagrass meadows are highly productive and important habitats in many estuarine seascapes (Boström et al. 2006; Friess et al. 2019). These habitats serve as important foraging areas for predatory fish as they support a large biomass of prey items (Laegdsgaard and Johnson 2001; Sheaves et al. 2015; Whitfield 2017). Additionally, unvegetated sediment habitats are key transition zones between a range of estuarine habitats such as mangrove forests and seagrass meadows and serve as complementary feeding grounds for predatory fish as they support an abundance of food items (e.g. crustaceans, polychaetes) (Hosack et al. 2006; Pessanha et al. 2021). Generally, higher rates of predation and a greater abundance of predatory fish are found in habitat patches that are near other habitat types (e.g. seagrass patches located in close proximity to mangrove patches) (Hammerschlag et al. 2010; Skilleter et al. 2017; Jones et al. 2020). This occurs because well-connected habitats often support an elevated abundance and biomass of food items, and predatory fish can migrate between these distinct habitat types during different tidal cycles (Hyndes et al. 2014; Whitfield 2017). However, these patterns are not always consistent as greater rates of predation can also be recorded in habitats that are isolated from other habitat types (see Duncan et al. 2019). Predation is often greatest along the edges of mangrove and seagrass patches compared to habitat interiors (Bologna and Heck Jr 1999; Nanjo et al. 2011; Smith et al. 2011) and nearby unvegetated sediment (Peterson et al. 2001; Gorman et al. 2009; Hammerschlag et al. 2010). This occurs as many predators forage along the edges of seagrass and mangrove patches and consume prey items (e.g. juvenile fish and invertebrates) that are vulnerable to predation as they are less protected by the complex structures (i.e. mangrove roots and seagrass blades) within these habitats that prey use as predation refugia (Boström et al. 2011; Whitfield 2017).
The structural complexity and configuration of mangrove and seagrass patches (i.e. habitat condition) can shape their value for coastal fish by influencing the abundance and distribution of prey species (e.g. benthic invertebrates) and the foraging behaviours of predatory fish (Heck Jr and Orth 2006; Whitfield 2017). This habitat condition can be quantified over a variety of spatial scales from broad patch-scale complexity (e.g. species composition and cover) to a much finer scale (e.g. mangrove pneumatophore and root density, seagrass shoot length and density) (Boström et al. 2006; Goodridge Gaines et al. 2022). Patch-scale complexity can shape predation by influencing the abundance and diversity of predatory fishes, the foraging efficiency of predators and the survival of prey items (Orth et al. 1984; Nagelkerken et al. 2008). Within seagrass meadows, increased survival rates of prey items are often seen when a greater coverage of seagrass is present within the patch as this provides increased protection from predation (Irlandi et al. 1995; Laurel et al. 2003; Gorman et al. 2009). However, these patterns are not always consistent as fragmented seagrass meadows may provide increased protection for invertebrate prey items such as blue crabs (Callinectes sapidus) (see Hovel and Lipcius 2001; Hovel and Fonseca 2005). In mangroves, tree species composition, coverage, canopy height and canopy cover can influence prey availability and predation refugia (Kathiresan and Bingham 2001; Ellis and Bell 2004; Nagelkerken et al. 2008); however, the effects of this patch-scale complexity in shaping predation in mangrove forests are lacking empirical information and are, therefore, comparatively unclear. At a finer scale, the structurally complex features of mangroves and seagrass, such as roots, rhizomes, stems and leaves can also influence rates of predation (Heck and Thoman 1981; Boström et al. 2006; Nagelkerken et al. 2010). In mangroves, higher densities of mangrove pneumatophores and roots within the forests offers increased protection for prey items such as juvenile fish and invertebrates (e.g. shrimps and crabs) from predatory fishes (Wilson 1989; Primavera 1997; Macia et al. 2003; Nanjo et al. 2011, 2014). In seagrass, a higher shoot density often leads to lower rates of predation on invertebrates (e.g. blue crabs) (Hovel and Lipcius 2001; Heck Jr and Orth 2006). This occurs as a higher density of mangrove pneumatophores and roots and seagrass shoots offer increased protection for prey items and restrict the mobility of predators, resulting in lower predation efficiency and detection rates (Chacin and Stallings 2016; Glazner et al. 2020). In addition, a greater coverage of algae in mangrove forests and seagrass meadows can also offer increased protection for prey items from predatory fish (Adams et al. 2004; Jaxion-Harm and Speight 2012). However, a greater abundance of prey items (e.g. invertebrates) within mangroves and seagrass is expected to offset the difficulties encountered by predatory fish when foraging within these structurally complex habitats (Whitfield 2017).
Determining factors that shape predation on invertebrates is vital in establishing the functional value of estuarine seascapes as foraging areas for coastal fishes (Duncan et al. 2019; Jones et al. 2020). This is of particular importance as a range of coastal fishes that consume invertebrates within estuaries are often the target of commercial and recreational fishers (Curley et al. 2013). Predation on invertebrates by fish is performed by a wide variety of species within estuaries (Curley et al. 2013; Jones et al. 2020). However, the relationship between predation and predator species richness and abundance, and how functional redundancy and complementarity characterise predation has rarely been examined within estuaries. In addition, it remains relatively unknown if the environmental factors (e.g. seascape context and habitat condition) that shape predation within estuaries are consistent across a range of important estuarine habitats such as mangrove forests, seagrass meadows and unvegetated sediment. This study used estuarine seascapes in eastern Australia to test if (1) relative predation probability on tethered live invertebrates varies in three key estuarine habitats (mangroves, seagrass and unvegetated sediment); (2) relative predation probability in each habitat correlates with predator abundance, predator richness or the abundance of individual species that are performing the function; (3) variation in the broader coastal seascape (e.g. proximity to nearby habitats) and habitat condition (e.g. species composition and coverage of habitat patches) influences relative predation probability across mangroves, seagrass and unvegetated sediment; and (4) variation in the broader coastal seascape and habitat condition influences predator species richness and abundance across mangroves, seagrass and unvegetated sediment. We hypothesised that relative predation probability would be greatest in mangroves and seagrass and that a higher predator abundance and richness would lead to increased predation probability in mangroves, seagrass and unvegetated sediment. We also expected that relative predation probability and predator species richness and abundance would be greater in habitat patches that were near other habitat types and that were in better habitat condition.
Methods
Study Seascape
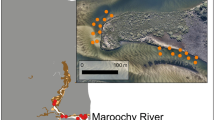
We surveyed relative fish predation on tethered invertebrates and fish assemblage composition at 130 sites across 11 estuaries in southeast Queensland, Australia. These sites spanned ~240 km of coastlines from the Noosa River (−26°22ʹS, 153°04ʹE) in the north to Tallebudgera Creek (−28°13ʹS, 153°19ʹE) in the south (Fig. 1). Estuaries and the sites within them were selected to maximise variation in spatial features within the region and range from relatively natural waterways with abundant mangroves and seagrass (e.g. Noosa River) to highly modified systems dominated by artificial structures (e.g. Nerang River) (Gilby et al. 2018; Olds et al. 2018). We sampled fish assemblages from three estuarine habitats: (1) mangrove forests: dominated in the region by grey (Avicennia marina), red (Rhizophora stylosa), orange (Bruguiera gymnorhiza) and river (Aegiceras corniculatum) mangroves; (2) seagrass meadows: dominated in the region by eelgrass (Zostera muelleri); and (3) unvegetated sediment. We intended to survey five sites in each habitat per estuary (following Henderson et al. 2019; Borland et al. 2022a). However, this was not always possible as some estuaries did not support seagrass or contained mangrove and seagrass patches that were too closely spaced or too small to ensure spatial independence between sites. The intent of this study was to determine seascape and habitat condition factors that shaped predation, thus to minimise the confounding effects of salinity, all surveys were conducted within the marine extent (i.e. from 30 to 35 Practical Salinity Units) of each estuary (based on 10 years of salinity data; following Gilby et al. 2017b). We attempted to space all five replicates for each habitat as evenly as possible from the mouth of the estuary to the point at which salinity reached 30 Practical Salinity Units (following Borland et al. 2022a); however, this was not always possible for seagrass, due to the sparsity of seagrass patches in the region. All surveys were conducted during the austral winter (June through August) of 2020 and within 2 h of high tide in water depths of 1–2.0 m, to account for the possible confounding effects of variable water depth, quality and clarity and to maximise the area of intertidal habitat available for fish (following, Gilby et al. 2017a).
Location of 11 study estuaries in eastern Australia. Insets illustrate the location of sampling sites along (top) a relatively natural estuary with abundant mangroves and seagrass (Noosa River); and (bottom) a highly urbanised estuary where shorelines are dominated by artificial structures (Nerang River)
Predation Assays
Predation assays are used to quantify factors that shape the rates and distribution of predation in estuarine seascapes (Baker and Sheaves 2007; Duncan et al. 2019). We measured relative predation probability (henceforth, predation probability) within the study and note that predation probability on tethered prey may not reflect natural predation on untethered prey as tethering may alter the behaviour and defensive strategies of prey items (Aronson and Heck 1995; Bessey and Heithaus 2013). There is also potential for the artefacts of tethering to interact with treatments, particularly for comparisons among habitats with differing structural complexity (Peterson and Black 1994). However, by deploying tethered prey on unvegetated sediment at the edge of focal habitats and filming all predation assays, we were able to identify and minimise experimental artefacts that may bias our results (Baker and Waltham 2020). We quantified predation probability using assays that were constructed of a GoPro camera recording in high definition, fixed to a 3 kg weighted frame that was attached to a 1-m-long PVC pipe. We tethered live ghost nippers (Trypaea australiensis), a species of burrowing shrimp that is an abundant prey species across the study region (Skilleter et al. 2005; Dunn et al. 2019). Tethered ghost nippers were all similar in length (45–60 mm) and were captured from the same estuary they were deployed in using a yabby pump at low tide. They were housed in an aerated 30-L holding tank with regular water exchange and were kept for no longer than a 24-h period. Ghost nippers were tethered through the tail with a sewing needle (0.65 mm diameter) via thin monofilament fishing line (6 lb breaking strain, 20 cm long) that was attached to a 20-cm-long bamboo stake. The length of this tether prevented ghost nippers from burrowing in the sediment and limited the range of movement to within the field of view of the camera. The bamboo stake was then fastened to the camera unit via the PVC pipe at a distance of 50 cm from the camera so that the ghost nipper was visible at all times. Predation assays were deployed for 1 h in mangrove forests, seagrass meadows and unvegetated sediment with up to 5 replicates occurring in each habitat type per estuary. The number of replicates was dependent on the extent of each habitat per estuary to ensure deployments were separated by a minimum distance of 200 m. In mangroves and seagrass meadows, assays were placed on unvegetated sediment at the edge (< 1 m) of the focal habitat and the field of view of the camera was faced parallel to the edge of the focal habitat itself to ensure the field of view was not obstructed by the habitat (following Borland et al. 2022a; Goodridge Gaines et al. 2022) and to prevent ghost nippers becoming entangled on mangrove roots and seagrass blades. Predation events were recorded if ghost nippers were absent from their tethers in the first 60 min of video footage. The species performing predation events were quantified by viewing video footage from each deployment. This filmed underwater video approach also allowed for any ghost nippers that were removed from the tether without a predation event occurring (i.e. escaped) to be recorded (Baker and Waltham 2020); however, this number was very low in this study (n = 1). Video footage from predation assays was also used to confirm that tethered ghost nippers did not burrow in the sediment for the duration of deployments (n = 0).
Fish Assemblage Surveys
Fish assemblages were surveyed using remote underwater video stations (RUVS) which are widely used to survey fish assemblages in a range of coastal ecosystems (Bradley et al. 2017; Zarco-Perello and Enríquez 2019). RUVS were chosen over baited cameras as they ensure fish are not attracted from other habitats (Gilby et al. 2018). RUVS were constructed of a GoPro camera recording in high definition, fixed to a 3 kg weight, which was buoyed at the surface for retrieval and to ensure the rope does not enter the video’s field of view. RUVS were deployed for 30 min and were placed at the same sites as the predation assays (n = 130). RUVS were always deployed on the same day as predation assays and were always deployed first, to eliminate potential sampling bias (i.e. potential for tethered ghost nippers attracting fish into a habitat). RUVS in mangroves and seagrass were positioned on unvegetated sediment at the edge (< 1 m) of the focal habitat and the field of view of the camera was faced parallel to the edge of the focal habitat itself to ensure the field of view was not obstructed by the habitat. Fish assemblage composition was quantified using the standard MaxN statistic; the maximum number of individuals of the same species that could be seen in one frame of the video footage, which is widely employed as the standard method for processing RUVS footage (Brook et al. 2018). We then used this to calculate predator species richness (number of predator species) and predator abundance (the sum of the MaxN of all predatory species). Species were classified as predators of invertebrates based on species diet literature (Elliott et al. 2007; Froese and Pauly 2021).
Quantifying Seascape Context, Habitat Condition and Water Quality
To investigate how seascape context influenced the probability of predation, we measured a range of spatial factors that are known to influence both fish species composition and predation within estuaries in the region (Gilby et al. 2017a; Duncan et al. 2019; Goodridge Gaines et al. 2022). These included (1) the proximity of each site to the nearest seagrass, mangrove, salt marsh and intertidal flat and the distance to urban land and the estuary mouth; (2) the area of seagrass, mangroves, salt marsh, intertidal flats and urban land within a 500 m buffer (to represent the likely maximum limit of single tidal movements of common estuarine fish species) of each site (following Gilby et al. 2017a); and (3) water depth (m), temperature (°C) and turbidity (water column light penetration in metres using a Secchi disc) at the time of deployment (Table S1). All seascape context and connectivity measures (i.e. proximity and area of relevant habitats and the level of urbanisation) were quantified in QuantumGIS (QGIS Development Team 2022) from vector spatial data that had been obtained from existing benthic habitats maps within the southeast Queensland region (Queensland Government 2019). These habitat maps were then ground-truthed in the field.
We undertook a range of infield habitat surveys to assess the habitat condition of seagrass meadows and mangrove forests surrounding each predation assay and RUV deployment (following Goodridge Gaines et al. 2022). In mangrove forests, we recorded the number and species of each tree, as well as tree diameter (at both the base and breast height), sediment type (% of mud and sand), and canopy cover and height within a 10 × 10 m plot (Table S2). We also measured the cover of benthic algae, the number of invertebrate burrows and the number of mangrove pneumatophores in four 50 × 50 cm quadrats placed haphazardly within the broader 10 × 10 m plot (Table S2). The condition of seagrass meadows was quantified by recording the seagrass species composition and cover within a 10 × 10 m plot surrounding each predation assay and RUV deployment. We also measured seagrass shoot density (number of individual shoots) and seagrass shoot lengths in four 50 × 50 cm quadrats placed haphazardly within the broader 10 × 10 m plot (Table S2).
Data Analysis
We examined how different components of the fish assemblage (e.g. predator species richness, predator abundance, abundance of individual species performing the function) and probability of predation (number of predation assays consumed and not consumed) correlated with seascape context and habitat condition in three estuarine habitats (mangroves, seagrass and unvegetated sediment). Environmental metrics were tested for co-linearity using Pearson’s correlation coefficient in the R statistical framework (R Core Team 2022); subsequently, distance to intertidal flats (correlated with distance to seagrass) was removed from the analysis (Pearson’s r = ≥ 0.7). All other metrics did not correlate greater than 0.7 or less than −0.7 r values, and were included in further analysis.
We used generalised linear models (GLMs) in the R statistical framework (R Core Team 2022) to identify (1) whether predation differed between our focal habitats by including the variables ‘habitat’ (three levels; mangroves, seagrass and unvegetated sediment),and ‘estuary’ (eleven levels; corresponding to each sampled estuary) in these analyses (Table S3). After this step, separate GLMs for each habitat (mangroves, seagrass and unvegetated sediment) were then used to assess; (2) if predation probability correlated with predator species richness, predator abundance or the abundance of individual species performing the function (Table S3); (3) if predation probability varied with changes in habitat condition and seascape context (Table S3); and (4) if predator species richness and abundance varied with changes in habitat condition and seascape context (Table S3). GLMs were fitted with binomial distributions (1 = consumed and 0 = not consumed). Model overfitting was reduced by running all possible combinations on four or fewer factors. Best-fit GLM models were identified using reverse stepwise simplification based on Aikaike Information Criterion (AIC); best-fit models were those with the lowest AIC values. To assess the efficiency of our sampling design, we then constructed species accumulation curves to ensure our samples represented the full predatory fish community in each habitat (Fig. S1).
Results
Predatory Fish Assemblages
We recorded a total of 16 species that were categorised as predators that are known to feed upon benthic invertebrates. In mangrove forests, we recorded a total of 11 predatory species, 7 predatory species in seagrass meadows and 9 species on unvegetated sediment (Table 1). The yellowfin bream (Acanthopagrus australis) was the most abundant predatory species in all three habitats accounting for 53% of the total abundance: 52% in mangroves, 60% in seagrass and 44% in unvegetated sediment (Table 1). Three other species were found across all three habitats: common silver biddy (Gerres subfasciatus), common toadfish (Tetractenos hamiltoni) and sand whiting (Silago ciliata) while two species were found in both seagrass and unvegetated sediment (Table 1). Two species were found exclusively in seagrass, four in unvegetated sediment and seven in mangroves (Table 1).
Predation Across Different Estuarine Habitats
Predation probability on tethered ghost nippers by fish differed between mangrove forests, seagrass meadows and unvegetated sediment (p = 0.03, X2 = 6.86). The probability of predation was highest in mangrove forests (predation events occurring on 52% of tethered ghost nippers, Fig. 2, Table S4), followed by seagrass meadows (predation events occurring on 43% of tethered ghost nippers, Fig. 2, Table S4), with unvegetated sediment having the lowest probability of predation (predation events occurring on 26% of tethered ghost nippers, Fig. 2, Table S4). Given the strong and consistent effects of habitat on predation, all subsequent analyses considered our focal habitats (mangroves, seagrass and unvegetated sediment) separately.
(Top) Pie charts represent the proportion of tethered ghost nippers consumed by each predator in the three focal habitats; percentages in the middle of the pie charts show the amount of tethered ghost nippers that were consumed in each habitat (mangroves 24/46, seagrass 14/33, unvegetated sediment (13/51). (Bottom) Outputs of best-fit generalised linear models (GLMs) for relationships between the probability of predation (0 = not consumed and 1 = consumed) and predator species richness or predator abundance in each habitat: shaded regions indicate 95% confidence intervals
Across all the sampled estuaries, six fish species consumed tethered ghost nippers in mangrove forests: yellowfin bream (Acanthopagrus australis), common toadfish (Tetractenos hamiltoni), crescent grunter (Terapon jabua), banded toadfish (Marilyna pleurostricta), sand whiting (Silago ciliata) and the weeping toadfish (Torquigener pleurogramma) (Fig. 2, Table S5). Three species consumed tethered ghost nippers in seagrass meadows: yellowfin bream, eastern striped trumpeter (Pelates sexlineatus) and common toadfish (Fig. 2, Table S5) and six species consumed tethered ghost nippers on unvegetated sediment: yellowfin bream, common silver biddy (Gerres subfasciatus), common toadfish, narrow-lined puffer (Arothron manilensis), trumpeter whiting (Silago maculata) and dusky flathead (Platycephalus fuscus) (Fig. 2, Table S5). Predation was predominately performed by one species, the yellowfin bream, which consumed greater than 50% of all deployments in which predation events were recorded in all three habitats: 54% in mangrove forests, 71% in seagrass meadows and 53% on unvegetated sediment (Fig. 2, Table S5). Predation probability was positively correlated with predator richness in mangroves, and unvegetated sediment and predator abundance in seagrass (Fig. 2, Table 2). While yellowfin bream largely performed predation across all habitats, the probability of predation was not correlated with the abundance of yellowfin bream in mangroves (p = 0.69, X2 = 0.01), seagrass (p = 0.79, X2 = 0.06) or unvegetated sediment (p = 0.36, X2 = 0.83).
Seascape Context and Habitat Condition Shape Estuarine Predation
In mangrove forests, predation probability was greatest when a lower mangrove pneumatophore density (< 50 n/m2) was present in the forest (Fig. 3, Tables 3 and S6). In seagrass meadows, the probability of predation was greatest when sites were closer (< 200 m) to the estuary mouth (Fig. 3, Tables 3 and S7), had a greater extent of seagrass (> 3000 m2) nearby (Fig. 3, Tables 3 and S7) and in meadows with longer (> 30 cm) seagrass shoot lengths (Fig. 3, Tables 3 and S7). Predation probability on unvegetated sediment was greatest at sites that had a greater extent (> 15,000 m2) of salt marsh nearby (Fig. 3, Tables 3 and S8). All other variables were removed during the reverse stepwise model simplification, and therefore did not influence predation in this study.
Seascape Context and Habitat Condition Shape Predator Species Richness and Abundance
In mangrove forests, predator abundance was greatest when a greater extent (> 300,000 m2) of mangroves was nearby (Fig. 4, Table 4), and both predator richness and abundance were greatest when a lower pneumatophore density (< 50 n/m2) was present in the forest (Fig. 4, Table 4). In seagrass meadows, predator abundance was greatest when sites were nearer (< 200 m) to the estuary mouth (Fig. 4, Table 4), had longer (> 30 cm) shoot lengths (Fig. 4, Table 4) and when a greater extent of urban land (> 40,000 m2) was nearby (Fig. 4, Table 4). Predator abundance on unvegetated sediment was greatest at sites that were closer (< 250 m) to mangroves (Fig. 4, Table 4) and had a greater extent (> 400,000 m2) of urban land nearby (Fig. 4, Table 4). GLMs assessing environmental factors influencing predator richness in seagrass and unvegetated sediment contained no factors in best-fit models.
Outputs of best-fit generalised linear models (GLMs) for relationships between predator species richness and abundance and seascape context and condition factors in each habitat: shaded regions indicate 95% confidence intervals. Models assessing seascape context and condition factors that influenced predator species richness in seagrass and unvegetated sediment contained no factors in best-fit models
Discussion
Quantifying factors that shape predation within estuarine seascapes is important in understanding their resilience to disturbance (Elliott and Whitfield 2011; Atwood et al. 2015). The structure of predator communities can influence predation within estuarine seascapes as different species compete to use the same resources and distinct species can perform the function in spatially distinct areas (Bruno and O'Connor 2005; Olds et al. 2018). In this study, predation was largely performed by one species, the yellowfin bream (Acanthopagrus australis). However, predation probability was not linked to the abundance of this species and instead was correlated with predator species richness in mangrove forests and unvegetated sediment, and predatory fish abundance in seagrass meadows. Our findings, therefore, suggest that the remaining abundance and diversity of predators are still important in shaping predation within estuaries. Additionally, we identified different groups of species consuming tethered invertebrates within mangroves, seagrass and unvegetated sediment. These findings indicate the presence of functional complementarity across habitats as many predatory species share similar functional niches within estuaries (Olds et al. 2018). Predation probability was higher in mangroves and seagrass than in unvegetated sediment (Peterson et al. 2001; Gorman et al. 2009; Garside and Bishop 2014); however, this may be influenced by our method of deploying predation assays on mangrove and seagrass edges and not habitat interiors (Wilson et al. 1990; Laegdsgaard and Johnson 2001; Canion and Heck 2009). Nevertheless, these results support the notion that vegetated habitats may hold higher value as foraging areas for a range of coastal fish while compared to unvegetated sediment (Alongi 2002; Boström et al. 2006; Espadero et al. 2020). We also highlight the importance of seascape context (e.g. seagrass area and distance to estuary mouth for seagrass habitats, salt marsh area for unvegetated sediment) and habitat condition (i.e. pneumatophore density and seagrass shoot length) in explaining predation probability and predator species richness and abundance and illustrates the significance of maintaining heterogeneous seascapes within estuaries.
As estuaries are considered a low-diversity seascape, the importance of predator species richness in shaping predation in estuarine ecosystems is unclear (Henderson et al. 2020b; Whitfield and Harrison 2021). The use of videoed predation assays in recent years has allowed for predatory fish species consuming assays to be identified (Bessey and Heithaus 2013; Baker and Waltham 2020). Studies utilising videoed predation assays show that predation is often dominated by a single species (Smith et al. 2011; Duncan et al. 2019). Rates of predation can also be positively correlated with the abundance of this dominant species, suggesting that estuaries have low functional redundancy and complementarity (Henderson et al. 2020b; Jones et al. 2020). In this study, predation probability was not linked to the abundance of yellowfin bream and instead was correlated with predator species richness in mangroves and unvegetated sediment. Surprisingly, seagrass had the lowest diversity of predators consuming tethered ghost nippers with the dominance of yellowfin bream being more pronounced in this habitat; therefore, the predation probability was correlated with predator abundance instead of predator species richness. We suggest that while predation on invertebrates within estuaries may appear to have relatively low redundancy due to the dominance of a single generalist species across all habitats, the remaining predator community is important in supplementing predation. Yellowfin bream are highly abundant within the region, have aggressive behaviour and feed opportunistically on a variety of prey items including live animals (i.e. invertebrates, fish) and carrion (Hadwen et al. 2007; Sheaves et al. 2014; Henderson et al. 2020b). Similarly, many of the other species that performed predation in this study (e.g. toadfishes and whiting) were less abundant and have smaller body sizes (Froese and Pauly 2021). This wide trophic niche, aggressive behaviour and high abundance are likely to allow yellowfin bream to out-compete many other predators in estuaries (Henderson et al. 2020b). Additionally, we show that various species within estuaries exhibit functional complementarity by performing predation in different habitat types (Loreau 2004; Olds et al. 2018). However, finding consistency in these patterns has been difficult to quantify as low functional complementarity has also been reported within estuaries (Henderson et al. 2020b). Future studies that focus on a single habitat are required to further quantify levels of redundancy and complementarity that are present in each habitat for predation within estuaries.
Greater rates of predation and a higher abundance and diversity of predatory fish are often recorded in larger habitats that are located near other habitat types (Sheaves 2009; Hammerschlag et al. 2010; Sridharan and Namboothri 2015; Skilleter et al. 2017; Jones et al. 2020). We found similar effects in this study, as greater predation probability was found in seagrass meadows that were more extensive and located closer to the estuary mouth, and within unvegetated sediments that had a greater extent of salt marsh nearby. Furthermore, a greater predator abundance was found in mangrove forests that were more extensive and a higher predator abundance was seen at unvegetated sediment sites that were near mangroves. Sites closer to the estuary mouth concentrate a diversity of predators and a high abundance of important predatory species due to the stability of salinity levels and improved water quality (i.e. lower turbidity) (Henderson et al. 2017; Jones et al. 2020). Urban structures, which are typically concentrated at the mouth of estuaries, can also provide novel habitat for estuarine fish, particularly for generalist predators (e.g. yellowfin bream) that commonly utilise this additional habitat for foraging (Clynick et al. 2008; Olds et al. 2018). In this study, we recorded a higher abundance of predatory fish in seagrass and unvegetated sites that had a greater extent of urban land nearby. Fish movement across seascapes is dependent on the composition (e.g. type of connected habitats) and configuration (e.g. connectivity between habitats) of habitat patches (Grober-Dunsmore et al. 2009; Boström et al. 2011). Larger habitat patches that are in close proximity to other habitat types often support a higher abundance and biomass of food items for predatory fish, as they utilise these different habitat types as supplementary or complementary foraging grounds (Hyndes et al. 2014; Whitfield 2017). Therefore, these results highlight the importance of maintaining heterogeneous seascapes and well-connected habitats for predation in estuarine seascapes.
The condition of mangroves, seagrasses and other adjacent vegetated habitats such as salt marshes combine to influence the abundance and diversity of species, and the rates of predation in estuarine seascapes (Heck Jr and Orth 2006; Nanjo et al. 2014; Whitfield 2017). Mangroves, seagrass and unvegetated sediment habitats contain a large diversity and biomass of prey items (e.g. polychaetes, crustaceans, juvenile fish) that are consumed by predatory fish (Boström et al. 2006; Whitfield 2017). In mangrove forests, pneumatophore density can affect the habitat value of mangroves for some fish species by modifying the availability of food and predation refugia (Macia et al. 2003; Sheridan and Hays 2003; Nagelkerken et al. 2010). In this study, we found greater predation probability and a higher predator species richness and abundance when a lower number of pneumatophores were present in mangrove forests. We suggest that this relationship reflects the level of protection that a higher number of mangrove pneumatophores affords a vast range of invertebrates that are preyed upon by fish along mangrove edges (Primavera 1997; Macia et al. 2003; Glazner et al. 2020). However, studies have also shown that mangrove pneumatophores can have little to no effect on predation within mangrove forests (Smith and Hindell 2005). Here, we deployed assays on the edge of mangrove forests to ensure we could identify the species consuming tethered ghost nippers (i.e. to ensure the field of view of the camera was not blocked by the habitat). We posit that a higher density of mangrove pneumatophores will likely restrict the mobility and foraging efficiency of predators in these shallow intertidal mangroves, and may deter predatory fish from foraging in these areas (Glazner et al. 2020). This may explain why we found a lower predatory species richness and abundance in forests with a higher density of pneumatophores in the forest. In addition, mangrove forests with higher pneumatophore density also allow for increased algal growth and thereby provide a greater level of protection for a range of prey species such as invertebrates (Jaxion-Harm and Speight 2012; Goodridge Gaines et al. 2020).
We found different effects of habitat condition and complexity in seagrass meadows as predation probability and predator abundance were greatest in meadows with longer shoot lengths. Seagrass meadows with a greater density and longer shoot lengths offer increased protection for prey items (Heck and Thoman 1981; Hovel and Lipcius 2001; Heck Jr and Orth 2006; Chacin and Stallings 2016; Reiss et al. 2019). However, assays within the study were placed on habitat edges which may explain why we found no effect of seagrass density and an increase in predation probability in meadows with longer seagrass shoot lengths. Rates of predation are often greatest along seagrass edges compared to habitat interiors as this offers less protection for prey items (Bologna and Heck Jr 1999; Gorman et al. 2009; Smith et al. 2011). Despite this, we posit that longer seagrass shoot lengths represent well-established meadows of better habitat condition that supported a higher abundance of predatory fish that forage along seagrass edges (Hori et al. 2009). Particularly, a high abundance of species such as yellowfin bream and eastern striped trumpeter that are common in seagrass meadows and utilise these habitats as foraging areas (Sanchez-Jerez et al. 2002; Taylor et al. 2018). The condition of habitats can, therefore, modify predation and should be considered in restoration and conservation initiatives that aim to improve ecological functions such as predation in estuarine seascapes (Goodridge Gaines et al. 2020).
Predation was dominated by many generalist species (i.e. yellowfin bream and toadfish species) rather than specialists in this study, indicating that these species may be important for maintaining predation in highly modified systems such as estuaries (Jones et al. 2020). Modified ecosystems can be dominated by these generalist species with broad functional niches, that can forage across many distinct habitat types, and can therefore confer functional redundancy and complementarity to the ecosystem (Clavel et al. 2011; Bishop et al. 2017). These findings highlight the relevance of identifying functionally important species (e.g. yellowfin bream) that should be considered targets for refined spatial management (i.e. harvesting restrictions, habitat conservation), as this protection might help to maintain predation in estuarine seascapes (Oliver et al. 2015; Winfree et al. 2015). While many generalist species such as yellowfin bream are common and abundant in the region, this does not guarantee that they are safe from precipitous decline, particularly when such species are also heavily harvested across their range in both commercial and recreational fisheries (Curley et al. 2013; Webley et al. 2015). Furthermore, protecting habitats frequented by generalist species such as yellowfin bream also protects more specialised species that share those habitats. Determining the environmental attributes of estuarine ecosystems such as mangroves, seagrass and unvegetated sediment and the surrounding seascapes that shape predation could be helpful in optimising the design of restoration and rehabilitation projects or the placement of conservation-focused initiatives (Whitfield 2017; Goodridge Gaines et al. 2020). Quantifying how functional redundancy and complementarity characterise predation in estuarine seascapes can help inform conservation and restoration initiatives that aim to maintain predation within estuaries (Duffy 2006; Barbier et al. 2011; Gilby et al. 2020). Managing ecosystems and fisheries to sustain the abundance and diversity of species that perform predation may help to maintain the functioning of estuarine ecosystems and improve the resilience of these systems to disturbance.
Data Availability
Data for this publication is available upon request from the corresponding author.
References
Adams, A.J., J.V. Locascio, and B.D. Robbins. 2004. Microhabitat use by a post-settlement stage estuarine fish: Evidence from relative abundance and predation among habitats. Journal of Experimental Marine Biology and Ecology 299: 17–33.
Alongi, D.M. 2002. Present state and future of the world’s mangrove forests. Environmental Conservation 29: 331–349.
Aronson, R.B., and K.L. Heck. 1995. Tethering experiments and hypothesis testing in ecology. Marine Ecology Progress Series 121: 307–310.
Atwood, T.B., R.M. Connolly, E.G. Ritchie, C.E. Lovelock, M.R. Heithaus, G.C. Hays, J.W. Fourqurean, and P.I. Macreadie. 2015. Predators help protect carbon stocks in blue carbon ecosystems. Nature Climate Change 5: 1038–1045.
Baker, R., and M. Sheaves. 2007. Shallow-water refuge paradigm: Conflicting evidence from tethering experiments in a tropical estuary. Marine Ecology Progress Series 349: 13–22.
Baker, R., and N. Waltham. 2020. Tethering mobile aquatic organisms to measure predation: A renewed call for caution. Journal of Experimental Marine Biology and Ecology 523: 151270.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.W. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81: 169–193.
Bessey, C., and M.R. Heithaus. 2013. Alarm call production and temporal variation in predator encounter rates for a facultative teleost grazer in a relatively pristine seagrass ecosystem. Journal of Experimental Marine Biology and Ecology 449: 135–141.
Bishop, M.J., M. Mayer-Pinto, L. Airoldi, L.B. Firth, R.L. Morris, L.H.L. Loke, S.J. Hawkins, L.A. Naylor, R.A. Coleman, S.Y. Chee, and K.A. Dafforn. 2017. Effects of ocean sprawl on ecological connectivity: Impacts and solutions. Journal of Experimental Marine Biology and Ecology 492: 7–30.
Blaber, S.J.M. 2013. Fishes and fisheries in tropical estuaries: The last 10 years. Estuarine, Coastal and Shelf Science 135: 57–65.
Bologna, P.A.X., and K.L. Heck Jr. 1999. Differential predation and growth rates of bay scallops within a seagrass habitat. Journal of Experimental Marine Biology and Ecology 239: 299–314.
Borland, H.P., B.L. Gilby, C.J. Henderson, R.M. Connolly, B. Gorissen, N.L. Ortodossi, A.J. Rummell, I. Nagelkerken, S.J. Pittman, M. Sheaves, and A.D. Olds. 2022a. Seafloor terrain shapes the three-dimensional nursery value of mangrove and seagrass habitats. Ecosystems. https://doi.org/10.1007/s10021-022-00767-4.
Borland, H.P., B.L. Gilby, C.J. Henderson, R.M. Connolly, B. Gorissen, N.L. Ortodossi, A.J. Rummell, S.J. Pittman, M. Sheaves, and A.D. Olds. 2022b. Dredging transforms the seafloor and enhances functional diversity in urban seascapes. The Science of the Total Environment 831: 154811.
Boström, C., E.L. Jackson, and C.A. Simenstad. 2006. Seagrass landscapes and their effects on associated fauna: A review. Estuarine, Coastal and Shelf Science 68: 383–403.
Boström, C., S.J. Pittman, C. Simenstad, and R.T. Kneib. 2011. Seascape ecology of coastal biogenic habitats: Advances, gaps, and challenges. Marine Ecology Progress Series 427: 191–217.
Bradley, M., R. Baker, and M. Sheaves. 2017. Hidden components in tropical seascapes: Deep-estuary habitats support unique fish assemblages. Estuaries and Coasts 40: 1195–1206.
Brook, T., B. Gilby, A. Olds, R. Connolly, C. Henderson, and T. Schlacher. 2018. The effects of shoreline armouring on estuarine fish are contingent upon the broader urbanisation context. Marine Ecology Progress Series 605: 195.
Bruno, J.F., and M.I. O’Connor. 2005. Cascading effects of predator diversity and omnivory in a marine food web. Ecology Letters 8: 1048–1056.
Canion, C.R., and K.L. Heck. 2009. Effect of habitat complexity on predation success: Re-evaluating the current paradigm in seagrass beds. Marine Ecology. Progress Series (halstenbek) 393: 37–46.
Cardinale, B.J., J.E. Duffy, A. Gonzalez, D.U. Hooper, C. Perrings, P. Venail, A. Narwani, G.M. Mace, D. Tilman, D.A. Wardle, A.P. Kinzig, G.C. Daily, M. Loreau, J.B. Grace, A. Larigauderie, D.S. Srivastava, and S. Naeem. 2012. Biodiversity loss and its impact on humanity. Nature 486: 59.
Chacin, D.H., and C.D. Stallings. 2016. Disentangling fine- and broad- scale effects of habitat on predator-prey interactions. Journal of Experimental Marine Biology and Ecology 483: 10–19.
Clavel, J., R. Julliard, and V. Devictor. 2011. Worldwide decline of specialist species: Toward a global functional homogenization? Frontiers in Ecology and the Environment 9: 222–228.
Cloern, J.E., P.C. Abreu, J. Carstensen, L. Chauvaud, R. Elmgren, J. Grall, H. Greening, J.O.R. Johansson, M. Kahru, E.T. Sherwood, J. Xu, and K. Yin. 2016. Human activities and climate variability drive fast-paced change across the world’s estuarine-coastal ecosystems. Global Change Biology 22: 513–529.
Clynick, B.G., M.G. Chapman, and A.J. Underwood. 2008. Fish assemblages associated with urban structures and natural reefs in Sydney, Australia. Austral Ecology 33: 140–150.
Curley, B.G., A.R. Jordan, W.F. Figueira, and V.C. Valenzuela. 2013. A review of the biology and ecology of key fishes targeted by coastal fisheries in south-east Australia: Identifying critical knowledge gaps required to improve spatial management. Reviews in Fish Biology and Fisheries 23: 435–458.
Duffy, J.E. 2006. Biodiversity and the functioning of seagrass ecosystems. Marine Ecology Progress Series 311: 233–250.
Duffy, J.E., J.P. Richardson, and K.E. France. 2005. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecology Letters 8: 301–309.
Duncan, C.K., B.L. Gilby, A.D. Olds, R.M. Connolly, N.L. Ortodossi, C.J. Henderson, and T.A. Schlacher. 2019. Landscape context modifies the rate and distribution of predation around habitat restoration sites. Biological Conservation 237: 97–104.
Dunn, R.J.K., D.T. Welsh, P.R. Teasdale, F. Gilbert, J.C. Poggiale, and N.J. Waltham. 2019. Effects of the bioturbating marine yabby Trypaea australiensis on sediment properties in sandy sediments receiving mangrove leaf litter. Journal of Marine Science and Engineering 7: 1–10.
Elliott, M., and A.K. Whitfield. 2011. Challenging paradigms in estuarine ecology and management. Estuarine, Coastal and Shelf Science 94: 306–314.
Elliott, M., K. Whitfield Alan, C. Potter Ian, J.M. Blaber Stephen, P. Cyrus Digby, G. Nordlie Frank, and D. Harrison Trevor. 2007. The guild approach to categorizing estuarine fish assemblages: A global review. Fish and Fisheries 8: 241–268.
Ellis, W.L., and S.S. Bell. 2004. Conditional use of mangrove habitats by fishes: Depth as a cue to avoid predators. Estuaries 27: 966–976.
Espadero, A.D.A., Y. Nakamura, W.H. Uy, P. Tongnunui, and M. Horinouchi. 2020. Tropical intertidal seagrass beds: An overlooked foraging habitat for fishes revealed by underwater videos. Journal of Experimental Marine Biology and Ecology 526: 151353.
Evans, S.M., K.J. Griffin, R.A.J. Blick, A.G.B. Poore, and A. Vergés. 2018. Seagrass on the brink: Decline of threatened seagrass posidonia australis continues following protection. PLoS ONE 13: e0190370.
Friess, D.A., K. Rogers, C.E. Lovelock, K.W. Krauss, S.E. Hamilton, S.Y. Lee, R. Lucas, J. Primavera, A. Rajkaran, and S. Shi. 2019. The state of the world’s mangrove forests: Past, present, and future. Annual Review of Environment and Resources 44: 89–115.
Froese, R., and D. Pauly. 2021. FishBase. www.fishbase.org. Accessed July 2022.
Garside, C.J., and M.J. Bishop. 2014. The distribution of the European shore crab, Carcinus maenas, with respect to mangrove forests in southeastern Australia. Journal of Experimental Marine Biology and Ecology 461: 173–178.
Gilby, B.L., A.D. Olds, R.M. Connolly, N.A. Yabsley, P.S. Maxwell, I.R. Tibbetts, D.S. Schoeman, and T.A. Schlacher. 2017a. Umbrellas can work under water: Using threatened species as indicator and management surrogates can improve coastal conservation. Estuarine, Coastal and Shelf Science 199: 132–140.
Gilby, B.L., A.D. Olds, N.A. Yabsley, R.M. Connolly, P.S. Maxwell, and T.A. Schlacher. 2017b. Enhancing the performance of marine reserves in estuaries: just add water. Biological Conservation 210 (Part A): 1–7.
Gilby, B.L., A.D. Olds, R.M. Connolly, P.S. Maxwell, C.J. Henderson, and T.A. Schlacher. 2018. Seagrass meadows shape fish assemblages across estuarine seascapes. Marine Ecology Progress Series 588: 179–189.
Gilby, B.L., A.D. Olds, C.K. Duncan, N.L. Ortodossi, C.J. Henderson, and T.A. Schlacher. 2020. Identifying restoration hotspots that deliver multiple ecological benefits. Restoration Ecology 28: 222–232.
Glazner, R., J. Blennau, and A.R. Armitage. 2020. Mangroves alter predator-prey interactions by enhancing prey refuge value in a mangrove-marsh ecotone. Journal of Experimental Marine Biology and Ecology 526: 151336.
Goodridge Gaines, L.A., A.D. Olds, C.J. Henderson, R.M. Connolly, T.A. Schlacher, T.R. Jones, and B.L. Gilby. 2020. Linking ecosystem condition and landscape context in the conservation of ecosystem multifunctionality. Biological Conservation 243: 108479.
Goodridge Gaines, L.A., C.J. Henderson, J.D. Mosman, A.D. Olds, H.P. Borland, and B.L. Gilby. 2022. Seascape context matters more than habitat condition for fish assemblages in coastal ecosystems. Oikos 2022 (8): e09337.
Gorman, A.M., R.S. Gregory, and D.C. Schneider. 2009. Eelgrass patch size and proximity to the patch edge affect predation risk of recently settled age 0 cod (Gadus). Journal of Experimental Marine Biology and Ecology 371: 1–9.
Grober-Dunsmore, R., S.J. Pittman, C. Caldow, M.S. Kendall, and T.K. Frazer. 2009. A landscape ecology approach for the study of ecological connectivity across tropical marine seascapes. In Ecological connectivity among tropical coastal ecosystems, ed. I. Nagelkerken, 493–530. Dordrecht: Springer Netherlands.
Gross, C., C. Donoghue, C. Pruitt, C. Trimble Alan, and L. Ruesink Jennifer. 2017. Taxonomic and functional assessment of mesopredator diversity across an estuarine habitat mosaic. Ecosphere 8: e01792.
Hadwen, W.L., G.L. Russell, and A.H. Arthington. 2007. Gut content- and stable isotope-derived diets of four commercially and recreationally important fish species in two intermittently open estuaries. Marine and Freshwater Research 58: 363–375.
Hammerschlag, N., A.B. Morgan, and J.E. Serafy. 2010. Relative predation risk for fishes along a subtropical mangrove-seagrass ecotone. Marine Ecology Progress Series 401: 259–267.
Heck, K.L., Jr., and R.J. Orth. 2006. Predation in seagrass beds. In Seagrasses: Biology, Ecology and Conservation, 537–550. Dordrecht: Springer.
Heck, K.L., and T.A. Thoman. 1981. Experiments on predator-prey interactions in vegetated aquatic habitats. Journal of Experimental Marine Biology and Ecology 53: 125–134.
Henderson, C.J., A.D. Olds, S.Y. Lee, B.L. Gilby, P.S. Maxwell, R.M. Connolly, and T. Stevens. 2017. Marine reserves and seascape context shape fish assemblages in seagrass ecosystems. Marine Ecology Progress Series 566: 135–144.
Henderson, C.J., B.L. Gilby, T.A. Schlacher, R.M. Connolly, M. Sheaves, N. Flint, H.P. Borland, and A.D. Olds. 2019. Contrasting effects of mangroves and armoured shorelines on fish assemblages in tropical estuarine seascapes. ICES Journal of Marine Science 76: 1052–1061.
Henderson, C.J., B.L. Gilby, T.A. Schlacher, R.M. Connolly, M. Sheaves, P.S. Maxwell, N. Flint, H.P. Borland, T.S.H. Martin, B. Gorissen, and A.D. Olds. 2020a. Landscape transformation alters functional diversity in coastal seascapes. Ecography 43: 138–148.
Henderson, C.J., B.L. Gilby, T.A. Schlacher, R.M. Connolly, M. Sheaves, P.S. Maxwell, N. Flint, H.P. Borland, T.S.H. Martin, and A.D. Olds. 2020b. Low redundancy and complementarity shape ecosystem functioning in a low-diversity ecosystem. Journal of Animal Ecology 89: 784–794.
Hori, M., T. Suzuki, Y. Monthum, T. Srisombat, Y. Tanaka, M. Nakaoka, and H. Mukai. 2009. High seagrass diversity and canopy-height increase associated fish diversity and abundance. Marine Biology 156: 1447–1458.
Hosack, G.R., B.R. Dumbauld, J.L. Ruesink, and D.A. Armstrong. 2006. Habitat associations of estuarine species: Comparisons of intertidal mudflat, seagrass (Zostera marina), and oyster (Crassostrea gigas) habitats. Estuaries and Coasts 29: 1150–1160.
Hovel, K.A., and M.S. Fonseca. 2005. Influence of seagrass landscape structure on the juvenile blue crab habitat-survival function. Marine Ecology Progress Series 300: 179–191.
Hovel, K.A., and R.N. Lipcius. 2001. Habitat fragmentation in a seagrass landscape: Patch size and complexity control blue crab survival. Ecology 82: 1814–1829.
Hyndes, G.A., I. Nagelkerken, R.J. McLeod, R.M. Connolly, P.S. Lavery, and M.A. Vanderklift. 2014. Mechanisms and ecological role of carbon transfer within coastal seascapes. Biological Reviews 89: 232–254.
Irlandi, E.A., W.G. Ambrose, and B.A. Orlando. 1995. Landscape ecology and the marine environment: How spatial configuration of seagrass habitat influences growth and survival of the bay scallop. Oikos 72: 307–313.
Jaxion-Harm, J., and M.R. Speight. 2012. Algal cover in mangroves affects distribution and predation rates by carnivorous fishes. Journal of Experimental Marine Biology and Ecology 414–415: 19–27.
Johnson, K.D., J.H. Grabowski, and D.L. Smee. 2014. Omnivory dampens trophic cascades in estuarine communities. Marine Ecology Progress Series 507: 197–206.
Jones, T.R., C.J. Henderson, A.D. Olds, R.M. Connolly, T.A. Schlacher, B.J. Hourigan, L.A. Goodridge Gaines, and B.L. Gilby. 2020. The mouths of estuaries are key transition zones that concentrate the ecological effects of predators. Estuaries and Coasts 44: 1557–1567.
Kathiresan, K., and B.L. Bingham. 2001. Biology of mangroves and mangrove ecosystems. Advances in Marine Biology 40: 81–251.
Kennish, M.J. 2002. Environmental threats and environmental future of estuaries. Environmental Conservation 29: 78–107.
Laegdsgaard, P., and C. Johnson. 2001. Why do juvenile fish utilise mangrove habitats? Journal of Experimental Marine Biology and Ecology 257: 229–253.
Laurel, B.J., R.S. Gregory, and J.A. Brown. 2003. Predator distribution and habitat patch area determine predation rates on Age-0 juvenile cod Gadus spp. Marine Ecology Progress Series 251: 245–254.
Lobry, J., V. David, S. Pasquaud, M. Lepage, B. Sautour, and E. Rochard. 2008. Diversity and stability of an estuarine trophic network. Marine Ecology Progress Series 358: 13–25.
Loreau, M. 2004. Does functional redundancy exist? Oikos 104: 606–611.
Macia, A., K.G.S. Abrantes, and J. Paula. 2003. Thorn fish Terapon jarbua (Forskål) predation on juvenile white shrimp Penaeus indicus H. Milne Edwards and brown shrimp Metapenaeus monoceros (Fabricius): The effect of turbidity, prey density, substrate type and pneumatophore density. Journal of Experimental Marine Biology and Ecology 291: 29–56.
Martino, E.J., and K.W. Able. 2003. Fish assemblages across the marine to low salinity transition zone of a temperate estuary. Estuarine, Coastal and Shelf Science 56: 969–987.
Nagelkerken, I., S.J.M. Blaber, S. Bouillon, P. Green, M. Haywood, L.G. Kirton, J.O. Meynecke, J. Pawlik, H.M. Penrose, A. Sasekumar, and P.J. Somerfield. 2008. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquatic Botany 89: 155–185.
Nagelkerken, I., A.M. De Schryver, M.C. Verweij, F. Dahdouh-Guebas, G. van der Velde, and N. Koedam. 2010. Differences in root architecture influence attraction of fishes to mangroves: A field experiment mimicking roots of different length, orientation, and complexity. Journal of Experimental Marine Biology and Ecology 396: 27–34.
Nanjo, K., Y. Nakamura, M. Horinouchi, H. Kohno, and M. Sano. 2011. Predation risks for juvenile fishes in a mangrove estuary: A comparison of vegetated and unvegetated microhabitats by tethering experiments. Journal of Experimental Marine Biology and Ecology 405: 53–58.
Nanjo, K., H. Kohno, Y. Nakamura, M. Horinouchi, and M. Sano. 2014. Effects of mangrove structure on fish distribution patterns and predation risks. Journal of Experimental Marine Biology and Ecology 461: 216–225.
Olds, A.D., B.A. Frohloff, B.L. Gilby, R.M. Connolly, N.A. Yabsley, P.S. Maxwell, C.J. Henderson, and T.A. Schlacher. 2018. Urbanisation supplements ecosystem functioning in disturbed estuaries. Ecography 41: 2104–2113.
Oliver, T.H., M.S. Heard, N.J.B. Isaac, D.B. Roy, D. Procter, F. Eigenbrod, R. Freckleton, A. Hector, C.D.L. Orme, O.L. Petchey, V. Proença, D. Raffaelli, K.B. Suttle, G.M. Mace, B. Martín-López, B.A. Woodcock, and J.M. Bullock. 2015. Biodiversity and resilience of ecosystem functions. Trends in Ecology & Evolution 30: 673–684.
Orth, R.J., K.L. Heck, and J. van Montfrans. 1984. Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7: 339–350.
Pessanha, A.L.M., N.S. Sales, C.S. da Silva Lima, F.J.K. Clark, L.G. de Lima, D.E.P.C. de Lima, and G.J.S. Brito. 2021. The occurrence of fish species in multiple habitat types in a tropical estuary: Environmental drivers and the importance of connectivity. Estuarine, Coastal and Shelf Science 262: 107604.
Peterson, C.H., and R. Black. 1994. An experimentalist’s challenge - when artifacts of intervention interact with treatments. Marine Ecology Progress Series 111: 289–298.
Peterson, B.J., K.R. Thompson, J.H. Cowan Jr., and K.L. Heck Jr. 2001. Comparison of predation pressure in temperate and subtropical seagrass habitats based on chronographic tethering. Marine Ecology Progress Series 224: 77–85.
Primavera, J.H. 1997. Fish predation on mangrove-associated penaeids: The role of structures and substrate. Journal of Experimental Marine Biology and Ecology 215: 205–216.
QGIS Development Team. 2022. QGIS geographic information system. Open Source Geospatial Foundation.
Queensland Government. 2019. Regional ecosystem mapping, ed. Queensland Government. Brisbane, Australia.
R Core Team. 2022. R: A Langauge and Environment for Statistical Computing. Vienna, Austria.
Reiss, K., N.N. Fieten, P.L. Reynolds, and B.K. Eriksson. 2019. Ecosystem function of subarctic Zostera marina meadows: Influence of shoot density on fish predators and predation rates. Marine Ecology. Progress Series (halstenbek) 630: 41–54.
Richmond, C.E., D.L. Breitburg, and K.A. Rose. 2005. The role of environmental generalist species in ecosystem function. Ecological Modelling 188: 279–295.
Sanchez-Jerez, P., B.M. Gillanders, and M.J. Kingsford. 2002. Spatial variation in abundance of prey and diet of trumpeter (Pelates sexlineatus: Teraponidae) associated with Zostera capricorni seagrass meadows. Austral Ecology 27: 200–210.
Sheaves, M. 2009. Consequences of ecological connectivity: The coastal ecosystem mosaic. Marine Ecology-Press Series 391: 107–115.
Sheaves, M., J. Sheaves, K. Stegemann, and B. Molony. 2014. Resource partitioning and habitat-specific dietary plasticity of two estuarine sparid fishes increase food-web complexity. Marine and Freshwater Research 65: 114–123.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2015. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries and Coasts 38: 401–414.
Sheridan, P., and C. Hays. 2003. Are mangroves nursery habitat for transient fishes and decapods? Wetlands 23: 449–458.
Silliman, B.R., and M.D. Bertness. 2002. A trophic cascade regulates salt marsh primary production. Proceedings of the National Academy of Sciences of the United States of America 99: 10500–10505.
Skilleter, G.A., Y. Zharikov, B. Cameron, and D.P. McPhee. 2005. Effects of harvesting callianassid (ghost) shrimps on subtropical benthic communities. Journal of Experimental Marine Biology and Ecology 320: 133–158.
Skilleter, G.A., N.R. Loneragan, A. Olds, Y. Zharikov, and B. Cameron. 2017. Connectivity between seagrass and mangroves influences nekton assemblages using nearshore habitats. Marine Ecology Progress Series 573: 25–43.
Smith, T.M., and J.S. Hindell. 2005. Assessing effects of diel period, gear selectivity and predation on patterns of microhabitat use by fish in a mangrove dominated system in SE Australia. Marine Ecology Progress Series 294: 257–270.
Smith, T.M., J.S. Hindell, G.P. Jenkins, R.M. Connolly, and M.J. Keough. 2011. Edge effects in patchy seagrass landscapes: The role of predation in determining fish distribution. Journal of Experimental Marine Biology and Ecology 399: 8–16.
Sridharan, B., and N. Namboothri. 2015. Factors affecting distribution of fish within a tidally drained mangrove forest in the Andaman and Nicobar Islands, India. Wetlands Ecology and Management 23: 909–920.
Stuart-Smith, R.D., G.J. Edgar, J.F. Stuart-Smith, N.S. Barrett, A.E. Fowles, N.A. Hill, A.T. Cooper, A.P. Myers, E.S. Oh, J.B. Pocklington, and R.J. Thomson. 2015. Loss of native rocky reef biodiversity in Australian metropolitan embayments. Marine Pollution Bulletin 95: 324–332.
Taylor, M.D., A. Becker, and M.B. Lowry. 2018. Investigating the functional role of an artificial reef within an estuarine seascape: A case study of yellowfin bream (Acanthopagrus australis). Estuaries and Coasts 41: 1782–1792.
Webley, J., K. McInnes, D. Teixeira, A. Lawson, and R. Quinn. 2015. Statewide recreational fishing survey 2013–14. Brisbane: Queensland Department of Agriculture and Fisheries.
Whitfield, A.K. 2017. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Reviews in Fish Biology and Fisheries 27: 75–110.
Whitfield, A.K., and T.D. Harrison. 2021. Fish species redundancy in estuaries: A major conservation concern in temperate estuaries under global change pressures. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 979–983.
Whitfield, A.K., G.N. Grant, R.H. Bennett, and P.D. Cowley. 2018. Causes and consequences of human induced impacts on a ubiquitous estuary-dependent marine fish species. Reviews in Fish Biology and Fisheries 28: 19–31.
Wilson, K.A. 1989. Ecology of mangrove crabs: Predation, physical factors and refuges. Bulletin of Marine Science 44: 263–273.
Wilson, K.A., K.W. Able, and K.L. Heck Jr. 1990. Predation rates on juvenile blue crabs in estuarine nursery habitats: Evidence for the importance of macroalgae (Ulva lactuca). Marine Ecology. Progress Series (halstenbek) 58: 243–251.
Winfree, R., J.W. Fox, N.M. Williams, J.R. Reilly, and D.P. Cariveau. 2015. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecology Letters 18: 626–635.
Zarco-Perello, S., and S. Enríquez. 2019. Remote underwater video reveals higher fish diversity and abundance in seagrass meadows, and habitat differences in trophic interactions. Scientific Reports 9: 6596–6596.
Acknowledgements
The authors thank Tyson Jones and Cody James for their assistance with fieldwork and analysing the videos.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors acknowledge and thank Healthy Land and Water for funding this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ronald Baker
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mosman, J.D., Gilby, B.L., Olds, A.D. et al. Multiple Fish Species Supplement Predation in Estuaries Despite the Dominance of a Single Consumer. Estuaries and Coasts 46, 891–905 (2023). https://doi.org/10.1007/s12237-023-01184-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01184-z