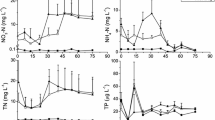
Abstract
This study has two main objectives, the first being the determination of net phytoplankton primary production to explain the phytoplankton’s function in a wetland carbon cycle, while the second objective is to relate this function with the phytoplankton assemblage composition. The annual variation in the phytoplankton production was monitored monthly for more than a year (2007–2008) in the semiarid eutrophic, hydrologically-perturbed “Tablas de Daimiel” National Park wetland. The phytoplankton fraction considered in this study comprised all organisms between the size 3 and 100 μm. The total biomass of phytoplankton was obtained by counting algae and calculating their volume, while net primary production and respiration were quantified by in situ incubations with the Winkler method. The respiration ranged from undetectable to 0.07 mgO2 l−1 h−1; net photosynthesis reached 0.20 mgO2 l−1 h−1. Net primary production was maximum at the end of the warm period (October 2007), and other peaks occurred at the start of summer (July 2007) or spring (March 2008). When maximum production took place, phytoplankton was mainly composed of small fast-growing chlorophytes (Tetraselmis cf. fontiana or Chlamydomonas sp.), in addition to some of the large, S-strategist algae (Peridinium umbonatum, Microcystis flos-aquae, Euglena sp.). The phytoplankton metabolism in “Tablas de Daimiel” was autotrophic as a whole due to changing contributions of algal groups. Only chlorophyte biomass was statistically related to net primary production. The conclusion reached is that this shallow eutrophic semiarid wetland possessed an annual net autotrophic production of phytoplankton fraction resulting from the small, fast-growing algae enhanced by hydrological perturbations that interrupted the autogenic course of S strategists.




Similar content being viewed by others
References
Angeler, D., M. Álvarez-Cobelas, C. Rojo & S. Sánchez-Carrillo, 2000. The significance of water inputs to plankton biomass and trophic relationships in a semi-arid freshwater wetland (central Spain). Journal of Plankton Research 22: 2075–2093.
Álvarez-Cobelas, M. & C. Rojo, 1994. Spatial, seasonal and long term variability of phytoplankton photosynthesis in lakes. Journal of Plankton Research 16: 1691–1716.
Álvarez-Cobelas, M., S. Cirujano & S. Sánchez-Carrillo, 2001. Hydrological and botanical man-made changes in the Spanish wetland of Las Tablas de Daimiel. Biological Conservation 97: 89–97.
Bondavalli, C., R. E. Ulanowicz & A. Bodani, 2000. Insights into the processing of carbon in the South Florida Cypress Wetlands: a whole-ecosystem approach using network analysis. Journal of Biogeography 27: 697–710.
Calijuri, M. C. & A. C. A. Dos Santos, 2001. Temporal variations in phytoplankton primary production in a tropical reservoir (Barra Bonita, SP-Brazil). Hydrobiologia 445: 11–26.
Carignan, R., D. Planas & C. Vis, 2000. Production and respiration in oligotrophic Shield lakes. Limnology and Oceanography 45: 189–199.
Craft, B. C. & P. W. Casey, 2000. Sediment and nutrient accumulation in floodplain and depressional freshwater wetlands of Georgia, USA. Wetlands 20: 323–332.
Cronk, J. K. & W. J. Mitsch, 1994. Aquatic metabolism in four newly constructed freshwater wetlands with different hydrological inputs. Ecological Engineering 3: 449–468.
Davison, E. A. & P. Artaxo, 2004. Globally significant changes in biological processes of the Amazon Basin: results of the Large-scale Biosphere-Atmosphere Experiment. Global Change Biology 10: 519–529.
Duarte, C. M. & Y. T. Prairie, 2005. Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 8: 862–870.
Egge, J. K., T. F. Thingstad, A. Engel, R. G. J. Bellerby & U. Riebesell, 2007. Primary production during nutrient-induced blooms at elevated CO2 concentrations. Biogeosciences Discussions 4: 4385–4410.
Falkowski, P. G. & J. A. Raven, 1997. Aquatic Photosynthesis. Blackwell Science, Oxford.
Fogg, G. E. & B. Thake, 1987. Algal Cultures and Phytoplankton Ecology, 3rd ed. The University of Wisconsin Press, Madison.
Hopkinson, C. S., 1992. A comparison of ecosystem dynamics in freshwater wetlands. Estuaries 15: 549–562.
Krupatkina, D. K., Z. Z. Finenko & A. A. Shalapyonok, 1991. Primary production and size-fractionated structure of the Black Sea phytoplankton in the winter-spring period. Marine Ecology-Progress Series 73: 25–31.
Lehman, P. W., 2007. The influence of phytoplankton community composition on primary productivity along the riverine to freshwater tidal continuum in the San Joaquin river, California. Estuaries and Coasts 30: 82–93.
Lund, J. W. G., C. Kipling & E. D. Le Cren, 1958. The inverted microscope method of estimating algal numbers and statistical basis of estimations by counting. Hydrobiologia 11: 143–170.
Middelburg, J. J., J. Nieuwenhuize, J. K. Lubberts & O. van de Plassche, 1997. Organic carbon isotope systematics of coastal marshes. Estuarine, Coastal and Shelf Science 45: 681–687.
Mitsch, W. J. & J. G. Gosselink, 2001. Wetlands, 3rd ed. John Wiley & Sons, New York.
Olrik, K., 1994. Phytoplankton Ecology. Miljøprojekt 251. Danish Environmental Protection Agency, Copenhagen.
Overmann, J. & M. M. Tilzer, 1989. Control of primary productivity and the significance of photosynthetic bacteria in a meromictic kettle lake. Mittlerer Buchensee, West Germany. Aquatic Science 51: 261–278.
Padisák, J., L. O. Crossetti & L. Nasselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia Hydrobiologia 621: 1–19.
Reynolds, C., 1988a. The Ecology of the Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C., 1988b. Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshwater Biology 14: 111–142.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Robarts, D. R., D. B. Donald & M. T. Arts, 1995. Phytoplankton primary production of three temporary northern prairie wetlands. Canadian Journal of Fisheries and Aquatic Sciences 52: 897–902.
Rodrigo, M. A., C. Rojo, M. Segura & J. Larrosa, 2009. Mechanisms of microalgae selection during the assembly of a planktonic community. Aquatic Ecology 43: 61–72.
Rojo, C. & M. Álvarez-Cobelas, 1993. Hypertrophic phytoplankton and the intermediate disturbance hypothesis. Hydrobiologia 249: 43–57.
Rojo, C., E. Ortega-Mayagoitia, M. A. Rodrigo & M. Álvarez-Cobelas, 2000a. Phytoplankton structure and dynamics in a semiarid wetland, the National Park Las Tablas de Daimiel (Spain). Archiv für Hydrobiologie 148: 397–419.
Rojo, C., E. Ortega-Mayagoitia & M. Álvarez-Cobelas, 2000b. Lack of pattern among phytoplankton assemblages. Or, what does the exception to the rule mean? Hydrobiologia 424: 133–139.
Rott, E., 1981. Some results of phytoplankton counting intercalibrations. Schweizerische Zeitschrift für Hydrologie 43: 34–62.
Sánchez-Carrillo, S. & M. Álvarez-Cobelas, 2001. Nutrient dynamics and eutrophication patterns in a semiarid wetland: the effects of fluctuating hydrology. Water, Air and Soil Pollution 131: 97–118.
Sandgren, C. D., 1988. Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Tilstone, G. H., F. G. Figueiras & L. M. Lorenzo, 2003. Phytoplankton composition, photosynthesis and primary production during different hydrographic conditions at the Northwest Iberian upwelling system. Marine Ecology-Progress Series 252: 89–104.
Waiser, M. J. & R. D. Robarts, 2004. Net heterotrophy in productive prairie wetlands with high DOC concentrations. Aquatic Microbial Ecology 34: 279–290.
Wetzel, R. G. & G. E. Likens, 2000. Limnological Analysis, 3rd ed. Springer-Verlag, New York.
Williams, P. J. L. & J. E. Robertson, 1991. Overall planktonic oxygen and carbon dioxide metabolisms: the problem of reconciling observations and calculations of photosynthetic quotients. Journal of Plankton Research 13: 153–169.
Acknowledgments
The authors would like to thank the Spanish Ministry of the Environment and Ministry of the Science for projects P-81/05 and CGL2006-02891. This research was also supported by Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (Excelencia project P07-CVI-02598). Moreover, the study has been possible; thanks to the fellowship awarded to María Mercedes Barón Rodriguez by the University of Valencia. We are indebted to colleagues from the Aquatic Ecology Group (the Institute of Natural Resources, CSIC) for their help in the field and for the laboratory work, and to Dr Nuria Navarro (Univ. Rey Juan Carlos, Madrid, Spain) for the loan of the titration equipment. This study was presented as a contributed article at the Bat Sheva de Rothschild seminar on Phytoplankton in the Physical Environment—The 15th Workshop of the International Association of Phytoplankton Taxonomy and Ecology, IAP (Israel).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. Zohary, J. Padisák & L. Naselli-Flores / Phytoplankton in the Physical Environment: Papers from the 15th Workshop of the International Association for Phytoplankton Taxonomy and Ecology (IAP), held at the Ramot Holiday Resort on the Golan Heights, Israel, 23–30 November 2008
Rights and permissions
About this article
Cite this article
Rojo, C., Barón-Rodríguez, M.M., Álvarez-Cobelas, M. et al. Sustained primary production with changing phytoplankton assemblages in a semiarid wetland. Hydrobiologia 639, 55–62 (2010). https://doi.org/10.1007/s10750-009-0017-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0017-1