Abstract
Cardiovascular disease is a major non-communicable disease globally, with increasing prevalence, posing a significant public health challenge. It is the leading non-obstetric cause of perinatal morbidity and mortality, with a substantial number of cardiac fatalities occurring in individuals without any known pre-existing cardiovascular disease. Peripartum cardiomyopathy is a type of de novo heart failure that occurs in pregnant women in the late stages of pregnancy or following delivery. Despite extensive research, diagnosing and managing peripartum cardiomyopathy remains challenging, resulting in significant morbidity and mortality. Recent advancements and novel approaches have been made to better understand and manage peripartum cardiomyopathy, including molecular and non-molecular biomarkers, genetic predisposition and risk prediction, targeted therapies, multidisciplinary care, and improved patient education. This narrative review provides a comprehensive overview and new perspectives on peripartum cardiomyopathy, covering its epidemiology, updated pathophysiological mechanisms, diagnosis, management, and future research directions for healthcare professionals, researchers, and clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cardiovascular disease (CVD) is the leading non-communicable disease worldwide, and its burden is rising, becoming a significant global public health issue [1,2,3]. It is the primary non-obstetric cause of perinatal morbidity and mortality, with up to 78% of cardiac fatalities having no known pre-existing CVD [2,3,4]. Peripartum cardiomyopathy (PPCM) is a pregnancy-associated heart failure (HF) that mainly affects childbearing women, resulting in increased disability-adjusted life years and constitutes a significant financial strain to the healthcare systems, especially in low- and middle-income countries [5,6,7,8,9,10,11,12,13]. Globally, the mortality rates of PPCM range from 7 to 15% [6, 14,15,16,17]. PPCM diagnosis and management continue to pose significant challenges despite extensive research. Notably, there have been several recent substantial advancements and novel approaches to understanding and managing PPCM [5,6,7]. These include molecular and non-molecular biomarkers, genetic predisposition, risk prediction, targeted therapies, multidisciplinary care, and improved patient education. This narrative review provides a comprehensive understanding of PPCM, including its history, mechanisms, diagnosis, and management, and highlights future research directions for healthcare professionals, researchers, and clinicians.
History of definition of peripartum cardiomyopathy
Peripartum cardiomyopathy, also known as postpartum cardiomyopathy or pregnancy-related HF, has had various clinical definitions and diagnostic criteria across regional and national health organizations [5, 12, 15,16,17,18,19]. In the late eighteenth century, Virchow and colleagues noted a relationship between pregnancy and HF [20,21,22]. Fraser (1935) demonstrated that HF was one of the leading causes of maternal mortality, not due to obstetric complications [23, 24]. Subsequently, in 1936, Hull and Hidden were the first to document pregnancy-related HF in New Orleans, labelling the condition “toxic” postpartal heart disease [25]. The term PPCM was introduced by Demakis and Rahimtoola in 1971 [26].
The National Heart Lung and Blood Institute (NHLBI) and The Office of Rare Diseases workshop coined the first universally accepted definition of PPCM in 2000. The definition comprised of four criteria: the development of cardiac failure in the last month of pregnancy or 5 months after delivery in the absence of an identifiable cause of cardiac failure, the absence of recognizable structural heart disease before the last month of pregnancy, and left ventricular dysfunction demonstrated by classic echocardiographic criteria, such as left ventricular ejection fraction < 45%, Motion-mode fractional shortening < 30%, or left ventricular end-diastolic dimension > 2.7 cm/m2 [26, 27].
In 2010, the Study group of Postpartum Cardiomyopathy of the European Society of Cardiology (ESC) proposed the following definition: “PPCM is an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found. It is a diagnosis of exclusion. The left ventricle may not be dilated, but the ejection fraction is nearly always reduced below 45%” [28, 29]. Bauersachs and colleagues, in the ESC position paper 2019, simplify the definition to a criterion of three: HF secondary to left ventricular systolic dysfunction with LVEF < 45%, occurrence towards the end of pregnancy or in the months following delivery, and no other identifiable cause of heart failure. The left ventricle does not necessarily need to be dilated [5].
Clinicians and researchers worldwide currently use the ESC 2019 simplified case definition of PPCM [5, 30]. A broader proposed definition includes two additional groups: early PPCM, diagnosed as early as the first month of pregnancy until the ninth month, and late PPCM, diagnosed from 6 to 12 months post-delivery [31]. The current definition aims to clarify PPCM, enabling early diagnosis and management, thus reducing feto-maternal morbidity and mortality.
Epidemiology
Peripartum cardiomyopathy is a universal condition with an unknown true incidence and prevalence with substantial variance in rates observed between and within countries. Worldwide, the estimated incidence rate is 1:2000, ranging from 1:300 in rural Haiti to 1:20,000 in Japan [6, 12, 16,17,18,19]. The reason for the variations between geographic locations is unknown but might be associated with ethnicity and socioeconomic factors [13]. According to a study conducted by Brar and colleagues in the USA, the condition’s overall incidence rate was 1 in 4025 live births [32]. The study also revealed that African Americans had the highest incidence rate, seven times greater than Hispanics and almost three times higher than Caucasians [8, 32]. Sub-Saharan Africa has the highest disease burden globally, with Nigeria having the highest incidence rates (1:100 live births) among the Hausa-Fulani community in Kano [8, 32]. This was believed to be linked to the cultural practice of sleeping on hot mud beds and consuming high-salt pap after childbirth. However, Sanderson and associates, with the use of echocardiography, noted that a significant number of patients were misdiagnosed as PPCM but had high-output heart failure with preserved ejection fraction. Furthermore, in the PEACE registry, this practice did not reach statistical significance [13, 33, 34].
South Africa has emerged as a global lighthouse in PPCM clinical research. In 1995, in Durban, South Africa, Desai reported a PPCM prevalence rate of 1:1000 live births, while an earlier study in Johannesburg by Seftel and colleagues described a rate of 1:3000 [35, 36]. In a 4-year cohort of 38 patients followed-up at Klerksdorp/Tshepong Hospital from 2011 to 2014, Sigauke and colleagues reported an incidence rate of 1: 1000, which mirrors rates reported in the Heart of Soweto Study in Johannesburg, which included approximately 200 women with PPCM [14, 37, 38]. However, it is worth noting that previous studies have been limited by small sample sizes and conducted in single centers. The exact incidence of PPCM in other continents, namely, Asia, Australia, and Europe, remains unknown, and ongoing worldwide registries like the EURObservational Research Programme and local epidemiological studies will answer some questions [6, 7].
Peripartum cardiomyopathy is prevalent across all age groups in women; nonetheless, it demonstrates greater prevalence and is linked to more adverse outcomes among women at the lower and upper ends of the reproductive age spectrum (< 20 and > 35 years) [5, 7, 8, 13, 16, 32]. The mean age in worldwide large registries and studies ranges from 28.9 to 33 years (IPAC study, PEACE registry, Global ESC EORP and German study) (7–9,13). The global variance can be attributed to differences in ethnicity, environment, and timely access to diagnosis. There is a higher prevalence of undiagnosed diseases in older women, which may act as confounders [5, 39,40,41]. Contrary to previous reports, the PEACE study in Nigeria noted a high incidence of PPCM among younger women [13]. However, the study’s small sample size, lack of power, and geographic and economic variation could explain the discrepancy. Approximately 19% of cases are diagnosed during the final month of pregnancy, while 75% of PPCM patients are diagnosed within the first month after delivery. Out of this group, 45% are diagnosed during the first week following childbirth [32, 42].
The global incidence rate of peripartum cardiomyopathy (PPCM) is expected to be determined through the standardized case definition, increased disease awareness, improved accessibility to echocardiography, and various ongoing registries. Due to the acute nature of PPCM, the prevalence rate is closely associated with the incidence rate.
Aetio-pathophysiology
The exact aetio-pathophysiology of PPCM is unknown [12, 16, 17]. Numerous clinical studies and national registries have extensively investigated various factors and mechanisms in the pathophysiology of PPCM. Among these mechanisms, the vasculo-hormonal theory is believed to be the most plausible explanation for the etiology of PPCM (Table 1) [16, 17]. The vascular model of PPCM focuses on the cardiac inability to adapt to the haemodynamic changes and stresses that occur during pregnancy and the peripartum period, resulting in cardiomyocyte release of vasoactive molecules that eventually cause angiogenic imbalance and vascular dysfunction. The “two-hit” hypothesis suggests that in genetically susceptible individuals, multiple insults to the myocardium before or during pregnancy trigger an inflammatory response during gestation and postpartum, leading to impaired myocardial function [16,17,18,19]. There is a temporal mismatch between the peak of haemodynamic stress in the second trimester and the onset of PPCM in the third or fourth trimester (12 weeks postpartum), suggesting the involvement of other probable factors. These factors include viral infection, autoimmunity, hormonal imbalance, and nutritional deficiencies like selenium [17].
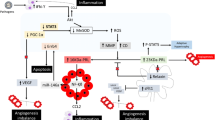
Lately, there has been a paradigm shift in the critical processes hypothesized in the pathophysiology of PPCM [5, 17, 43]. Vascular angiogenic imbalance, increased oxidative stress, and inflammation are the principal mechanisms supported by in vivo and ex vivo (knock-out STAT mice with PPCM) evidence [44,45,46]. The development of PPCM through vascular impairment arises from two separate pathophysiological pathways involving the pituitary and placenta, resulting in an angiogenic imbalance (Fig. 1). The first pathway involves 16 kDa prolactin (PRL), nuclear factor-kappa B(NFκB), and microRNA-146a. The second pathway is regulated by the balance between the placental-derived anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt1) and pro-angiogenic factor vascular endothelial growth factor (VEGF) [16, 17, 43].
Novel pathophysiological mechanisms of peripartum cardiomyopathy. Oxidative stress triggered by pregnancy, genetic susceptibility, infections, and autoimmune factors promotes the release of cathepsin-D from the cardiomyocyte, which promotes the proteolytic cleavage of 23 kDa prolactin to an anti-angiogenic form known as 16 kDa prolactin (also known as vasoinhibin) leading to angiogenic imbalance and, subsequently, endothelial dysfunction and cardiomyocyte apoptosis. kDa, kilodalton; miRNA-146a, micro-ribonucleic acid 146a; MnSOD, manganese superoxide dismutase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ROS, reactive oxygen species; sFLt-1, soluble Fms-like tyrosine kinase 1; STAT3, signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor. Created with BioRender.com
In the first pathway, the increased oxidative stress state during pregnancy reduces the signal transducer and activator of the transcription 3 (STAT3) factor. This reduction curtails the expression of the reactive oxygen species (ROS) scavenger, manganese superoxide dismutase (MnSOD) [44]. The decreased level of MnSOD results in the accumulation of ROS, leading to the escalation, activation, and secretion of peptidase cathepsin D from the cardiomyocytes into the circulation. During the peripartum period, the anterior pituitary gland secures elevated levels of the full-length lactation hormone 23 kDa PRL. Cathepsin D cleaves 23 kDa PRL in the cardiac vasculature into the cardiotoxic and vasculotoxic fragment 16 kDa PRL (vasoinhibin), which acts on urokinase-type plasminogen activator receptor (uPAR) to induce endothelial damage, impairing cardiomyocyte metabolism, resulting in apoptosis. Vasoinhibin is a driver of PPCM through the transcription factor, nuclear factor kappa-B (NFκB), that upregulates endothelial expression of microRNA-146a [5, 17, 19]. MicroRNA-146a, an endothelial secretome, causes cardiomyocyte dysfunction and apoptosis by blocking multiple pathways involving Erbb4, Nras and Notch1[19]. Vasoinhibin, in concert with interferon-γ (IFN-γ) derived from inflammation, induces cardiac hypertrophy orchestrated by protein kinase B (Akt) [44].
In the second pathway, the placental expression of the pro-angiogenic factor VEGF is downregulated through two mechanisms [16]. In the peripartum period, the placenta secretes several vasoactive molecules. The secretion of peroxisome proliferator-activated receptor gamma 1-alpha coactivator (PGC1α), a potent regulator of angiogenesis, is downregulated. Additionally, there is an increase in the production of the anti-angiogenic sFlt1, which leads to inadequate upregulation of VEGF, worsening the angiogenic milieu of PPCM [12, 19]. Studies have shown that the suppression of PGC1α in murine hearts is associated with sFlt1-induced cardiomyopathy. Elevated levels of sFlt1 have also been linked to disease severity and a poor prognosis [16, 46]. During this period, hormonal levels of progesterone and activin A increase while levels of vasoprotective relaxin 2 decrease. Progesterone regulates cardiac energetics by suppressing glucose metabolism, cardiomyocyte hypertrophy, and dysfunction [17]. Activin A, a transforming growth factor-beta (TGF-β) superfamily member, promotes cardiomyocyte inflammation, fibrosis, and remodelling in PPCM, resulting in injury and dysfunction. Activin A levels may become part of the diagnostic and prognostic toolkit for managing PPCM, an emerging area of interest [47,48,49].
Several risk factors are associated with a preponderance of disease progression, rendering the host vulnerable to PPCM. Traditionally, predisposing risk factors have been stratified as possible, probably, and emerging [14]. These factors include multi-parity, twin gestation, extended breastfeeding, increased maternal age, selenium deficiency, smoking, alcohol, illicit drug use, low body mass index (BMI) < 18.5 kg/m2 (underweight), African ancestry, family history of disease, and gestational hypertensive disorders [13, 14, 18]. Individuals who have experienced PPCM in the past are likely to experience a relapse or deterioration in subsequent pregnancies [42].
Recent research has focused on emerging risk factors, especially gestational hypertensive disorders such as gestational hypertension, pre-eclampsia, and eclampsia [29]. This disease group is associated with significant cardiovascular morbidity and mortality in addition to PPCM [19, 43]. In a cohort of patients with hypertensive heart failure of pregnancy (HHFP) and idiopathic PPCM, significant differences in clinical presentation and outcomes were noted, with the possibility that HHFP may not fit the case definition of PPCM; suffice to say this has not translated to clinical research and practice [50, 51]. Many cohorts of PPCM patients have noted a high prevalence of hypertensive disorders and pre-eclampsia in pregnancy, suggesting that this group of diseases predisposes them to develop PPCM. Notably, gestational hypertensive heart disease and PPCM present a clinical conundrum as they manifest similar symptomology, yet they have divergent management, including anti-hypertensive and anti-failure therapies [52].
In 1963, Pierce and colleagues were the first to report family clustering of PPCM cases and the possibility of genetic predisposition in the pathogenesis [53]. Subsequently, multiple studies have supported the role of genetics in up to 15% of patients [54]. Fett and colleagues in Haiti reported cases of PPCM in a mother and her daughter, further supporting the role of genetics in the manifestation of the disease [55]. Additional evidence of familial occurrence includes variable prevalence in different regions and ethnic groups [56]. Dewi and Nugroho categorized genetically associated PPCM into three groups: PPCM as a subgroup of familial dilated cardiomyopathy, PPCM associated with titin (TTN), and PPCM in women with carrier X-linked cardiomyopathy. Worldwide, the giant sarcomeric protein-coding gene with titin (TTN) truncating mutation has been the predominant genetic contributor to developing PPCM, among others, beta-myosin heavy chain, myosin-binding protein C (MYBPC3), lamin A/C, or sodium voltage-gated channel alpha subunit 5 (SCN5A) [57, 58]. X-linked cardiomyopathy includes Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and lysosomal-associated membrane protein (LAMPs) gene mutations [56, 57].
The “two-hit hypothesis” theory proposes that in gene-positive, phenotype-negative women without clinical symptoms before pregnancy, the physiological stress caused by the anti-vascular and hormonal effects of pregnancy and delivery may unmask the concealed cardiomyopathy [57]. Although further research is warranted, the advent of genome-wide association studies (GWAS) and molecular techniques has provided a better understanding of how genetics, proteomes, non-coding ribonucleic acids (RNA), heat shock proteins, and chaperones may play a role in the pathogenesis of PPCM [58,59,60]. However, more than 90% of individuals with genetic variants do not develop PPCM, suggesting the implication of extra-genetic factors [15].
It is important to note that the exact mechanisms and triggers of PPCM are not yet fully understood despite the valuable insights provided by the theories above. Nevertheless, ongoing research is diligently working to unravel the intricate interplay of various factors contributing to PPCM. The goal is to improve diagnosis and treatment approaches confidently.
Biomarkers of peripartum cardiomyopathy (molecular vs. non-molecular)
Patients with PPCM usually present in acute HF and are investigated similarly to other cardiomyopathies as it is a diagnosis of exclusion [5, 61,62,63]. This daunting task delays the diagnosis, hence the need for biomarkers to tease out PPCM from its mimics, such as HHP, myocarditis, pulmonary embolus, and pre-existing dilated cardiomyopathy. Biomarkers offer a cost-effective, non-invasive, and easily measurable method for diagnosing PPCM and establishing prognosis while also providing crucial information on complex pathophysiology [60, 64]. It is crucial to research biomarker screens during the antepartum and postpartum periods due to the distinct kinetic patterns of specific hormones, growth factors, and enzymes [5, 29, 65]. In this review, biomarkers are categorized as molecular and non-molecular.
Molecular biomarkers
Recently, numerous novel macromolecules have been evaluated for the diagnosis, prognostication, and treatment of HF in the general population, with the potential of being extrapolated to PPCM [66, 67]. These novel HF biomarkers are stratified according to their role in the pathogenesis of PPCM, namely, cardiomyocyte inflammation, fibrosis, hypertrophy, and apoptosis (Fig. 2).
Summary of the clinical utility of biomarkers in peripartum cardiomyopathy. Several established and novel biomarkers involved in the pathophysiology of peripartum cardiomyopathy are used in diagnosing, prognosis, and monitoring treatment response. The green tick indicates the purpose for which a biomarker is used in clinical practice. Fas/Apo1, Fas cell surface death receptor/ apoptosis antigen 1; IL-4/6, interleukin 4 and 6; INF-y, interferon-gamma; microRNA-146a, microribonucleic acid-146a; NT-proBNP, N-terminal Pro B-type natriuretic peptide; Ox-LDL, oxidized low-density lipoproteins; sFlt-1, soluble Fms-like tyrosine kinase 1; sST2 soluble suppression of tumourigenicity-2; TGF- β, transforming growth factor- beta; TNF-α, tumor necrosis factor-alpha. Created with BioRender.com
Biomarkers such as troponins, brain natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP), and mid-regional pro-atrial natriuretic peptides (MR-proANP) are routinely used in the diagnosis and monitoring of HF, including PPCM [15, 62, 63]. Natriuretic peptides (NP) are produced in response to atrial stretch and have high specificity for HF in general [15, 68]. Natriuretic peptides, especially NT-proBNP, are well-established in the diagnosis of PPCM in the acute setting as they have a very high negative predictive value when using low cut-off levels (i.e., BNP < 100 pg/ml, NT-proBNP < 300 pg/ml, and MR-proANP < 120 pmol/l) [5, 62, 63]. Hoevelmann and colleagues demonstrated that NT-proBNP > 900 pg/ml at diagnosis was associated with a poor prognosis for left ventricular recovery in a cohort of 42 women [69]. The elevation in NP and troponin levels lacks specificity for PPCM and may also be present in other acute chest syndromes, both cardiac and non-cardiac, including myocardial ischemia, tachyarrhythmia, pulmonary embolism, and preeclampsia [15].
Numerous biomarkers have been identified as potential indicators of PPCM, including microRNA-146a, cathepsin D, 16 kDa-PRL, interferon-γ, asymmetric dimethylarginine (ADMA), and sFlt1. Ongoing evaluation is being conducted to assess their accuracy in diagnosing PPCM. Additionally, other biomarkers such as soluble suppression of tumorigenicity 2 (sST2), Galectin 3 (Gal-3), relaxin, VEGF, growth-differentiation factor-15 (GDF-15), adrenomedullin (ADM), long noncoding RNA, and heat shock proteins are currently under investigation for their clinical significance in routine medical practice. These biomarkers can potentially improve diagnostic capabilities and advance medical care in PPCM management.[16,17,18,19, 43, 70,71,72].
Non-molecular biomarkers
This category of biomarkers includes physical characteristics, histological and imaging (radiological) parameters measured as indicators of normal biological processes, pathological processes, or responses to therapeutic intervention [73]. The diagnosis of PPCM requires a high index of suspicion as it can present anywhere in the continuum, from insidious nonspecific symptoms of fatigue, malaise, and congestion, which can be confused as regular physiological changes of pregnancy to dramatic, acute, severe decompensated cardiac failure in extremis [5]. The clinician must obtain a detailed history and focused physical examination from the outset. Libhaher and colleagues noted that common physical characteristics like hypotension and resting tachycardia were predictors of poor outcomes [74]. Subjective but useful functional status tools such as the New York Heart Association functional status (NYHA FC), Kansas City Cardiomyopathy questionnaire, and 6-min walk test can be utilized to track disease progression and monitor the well-being of patients [62, 63, 75]. Non-molecular biomarkers offer substantial benefits owing to their ease of accessibility and seamless integration into standard healthcare protocols, rendering them a practical and feasible option.
Electrocardiography
A 12-lead electrocardiogram (ECG) is recommended for diagnostic workup in patients with PPCM, as it is inexpensive, safe, and widely available.[5, 61,62,63]. Tibazarwa and colleagues reported that more than 90% of PPCM patients at presentation have non-specific ECG abnormalities, which include sinus tachycardia, aberrant conduction, commonly left bundle branch block (LBBB), atrial fibrillation, repolarisation, abnormalities (T wave inversion), and prolonged corrected QT interval (QTc) [76, 77]. In a cohort of 66 PPCM patients, Hoevelmann and colleagues reported that sinus arrhythmia at diagnosis was associated with good outcomes [78]. On the contrary, sinus tachycardia and prolonged QTc interval at presentation were noted to be a predictor of poor prognosis [77,78,79].
Cardiac imaging
Cardiovascular imaging in PPCM is classified as non-ionizing (echocardiography) and ionizing (chest radiography (CXR), cardiac magnetic resonance (CMR), computed tomography (CT), ventilation/perfusion (V/Q) scan, invasive coronary angiography (ICA), and nuclear medicine imaging (single-photon emission computed tomography (SPECT)/positron emission tomography (PET) [5, 80]. Multi-modality cardiovascular imaging plays a crucial role in the diagnosis, risk stratification, prognostication, and therapeutic guidance on follow-up of PPCM (Table 2) [81].
Non-ionizing cardiac imaging
Echocardiography
Echocardiography refers to all cardiac ultrasound imaging techniques and is the preferred imaging modality in PPCM as it is widely available [5, 15, 30, 61]. Transthoracic echocardiography (TTE) is cheap, safe during pregnancy and lactation, reproducible, and provides real-time cardiac structural and functional assessment necessary to diagnose PPCM promptly [72, 82]. Normal pregnancy adaptive structural changes include mild chamber dilation, LV wall thickening, elevated cardiac output, and increased LV filling pressures. LV systolic dysfunction with an ejection fraction of < 45% with or without chamber dilation is a criterion for the current diagnosis of PPCM [5]. Complications such as functional mitral regurgitation, pulmonary hypertension, and intramural thrombus can be identified in TTE [16, 19, 83]. Myocardial strain analysis, notably global longitudinal strain (GLS), has been validated as a diagnostic and prognostic parameter in cardiac failure [84, 85].
Kiran and colleagues, in a cohort of 43 PPCM patients, reported low right ventricular (RV) fractional area change (RVFAC 31.4% with 86% accuracy) and high left atrial volume index (LAVi > 29.6 ml/m2, with 72% accuracy) at presentation to be independent predictors of adverse events [86]. The EORP PPCM registry confirmed RV dysfunction’s prognostic significance [87, 88]. In addition, LVEF < 30% and LV dilation (left ventricular end-diastolic diameter (LVEDD) ≥ 60 mm) are associated with poor outcomes [87, 89].
Cardiac magnetic resonance (CMR)
Cardiovascular magnetic resonance imaging is the gold standard radiation-free modality for assessing cardiac structure and function and myocardial tissue characterization [90]. The main advantage is that CMR has a higher spatial and temporal resolution, outstanding accuracy, reproducibility, independence from the acoustic window, and higher sensitivity for detecting LV thrombus [80, 90,91,92,93]. Cardiac MRI is an excellent technique for ruling out other de novo heart failure diagnoses in the peripartum period to complement echocardiography, especially in cases with inconclusive or uncertain results [5, 15, 94]. Late gadolinium enhancement (LGE) signifies myocardial fibrosis or scarring. It is usually found in the mid-myocardial or subepicardial segments on the lateral wall but can also be present in other areas in a diffuse or patchy pattern in PPCM. LGE can help rule out diagnoses such as myocarditis [19, 81, 95, 96]. In a cohort of 10 patients, Arora and colleagues reported that LGE was associated with poor outcomes [91]. CMR is not widely available and is expensive. There is a shortage of clinicians with interpretation expertise, and gadolinium-based studies should be avoided during the first trimester [92]. In PPCM, though CMR offers unparalleled insights into the cardiac structure and function, aiding in risk stratification, confirming the diagnosis, prognosticating and monitoring therapeutic success, it is currently not used in routine clinical practice [92,93,94].
Ionizing cardiac imaging
Chest radiography (CXR)
Chest radiography is one of the most accessible and reproducible initial investigations in PPCM [82]. Notable radiological changes include cardiomegaly and pulmonary infiltrates varying with the degree of pulmonary venous hypertension, depicting the severity of mean capillary wedge pressure. In PPCM, the utility of CXR at presentation supports the diagnosis, stratifies the extent of heart failure, and excludes other acute chest syndromes [5].
Computer tomography (CT)
Cardiac tomography is not the first-line imaging modality for evaluating peripartum cardiomyopathy (PPCM) but can be used for this purpose [5]. Its role includes enhancing echocardiography when the diagnosis is unclear, assessing cardiac structure and function, and detecting intracardiac and extracardiac complications such as intramural thrombus and pulmonary embolus [80, 81]. Furthermore, it can be used to assess coronary artery disease and aid in distinguishing PPCM from other types of cardiomyopathies and myocarditis by providing detailed images of myocardial tissue characteristics. Cardiac CT has advantages due to its non-invasiveness, reproducibility, independence from poor acoustic windows, and high spatial resolution. It can be beneficial in cases where surgical intervention is necessary by providing detailed anatomical information. If necessary, iodinated contrast agents can be used during pregnancy and breastfeeding. However, it is important to note that computer tomography is seldom used in routine PPCM care bundles due to radiation and cost [5, 18, 80]. Transthoracic echocardiography (TTE) and cardiac MRI are often preferred because they do not involve radiation and can provide detailed functional and structural data sets [81].
Although cardiac CT is not the primary imaging method for peripartum cardiomyopathy, it can be valuable in specific clinical situations requiring detailed anatomical information or when complications are suspected. Its use should be carefully considered due to the risks of radiation exposure, especially during the peripartum period [81].
Nuclear imaging
Nuclear imaging techniques such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), and cardiac scintigraphy or myocardial perfusion imaging (MPI) have limited use in diagnosing PPCM [5]. They are mainly used to distinguish PPCM from ischaemic cardiomyopathy [80, 81]. A ventilation/perfusion (V/Q) scan is particularly useful in identifying or ruling out pulmonary embolism, which has similar symptoms to PPCM. V/Q scans involve lower radiation exposure compared to CT pulmonary angiography, making them safer for postpartum women [80]. However, it is important to note that even low doses of radiation exposure can be concerning, especially during pregnancy. Additionally, these techniques can be costly and require expertise in nuclear cardiology to accurately interpret results [81].
Although it has limitations, nuclear imaging can be beneficial in certain situations for thoroughly evaluating PPCM. It can be crucial in ruling out conditions like pulmonary embolism and coronary artery disease, which have symptoms similar to PPCM. This ensures that PPCM can be managed appropriately and effectively.
Endomyocardial biopsy
Endomyocardial biopsy is not routinely used to diagnose and predict PPCM unless a cardiac transplant is considered. It is useful when myocarditis or other rare metabolic or storage cardiac myocyte diseases are suspected [5, 93].
The use of molecular and non-molecular biomarkers in PPCM provides valuable information for diagnosing, prognosticating, and managing disease. Cardiac biomarkers like BNP/NT-proBNP and troponins play a pivotal role in diagnosis, while inflammatory, oxidative stress and prolactin-related markers offer insights into the disease’s mechanisms and potential treatment options. These biomarkers’ comprehensive comprehension and application can significantly enhance patient care and outcomes in PPCM.
Management
The clinical management of PPCM is a daunting task without disease-specific, evidence-based data; the therapeutic strategy mirrors other forms of HF with left ventricular systolic dysfunction directed by international guidelines [5, 15,16,17,18,19, 61,62,63, 97]. A multi-disciplinary approach is crucial as PPCM has peculiarities and nuances necessitating the concerted input from cardiologists, obstetricians, obstetric-medicine physicians, intensivists, cardiac surgeons, anaesthesiologists, neonatologists, and nurses [5, 15, 96,97,98]. In stable PPCM, Bauersachs and colleagues recommend the BOARD scheme therapeutic approach (bromocriptine, oxygen, anticoagulation, relaxants, and diuretics) [99].
Most patients present with de novo decompensated HF, characterized by exertional dyspnea that eventually progresses to rest dyspnea and fluid overload [61]. Generally, mechanical ventilatory and circulatory support may be required in cardiopulmonary compromise with inotropic support [5, 15, 61,62,63]. Urgent delivery with caesarean section should be instituted [82].
The peripartum period is highly oxidative, and STAT3 expression is protective [44]. When inotropic support is required, noradrenaline and levosimendan are preferred to dobutamine, a beta 1-adrenergic receptor agonist. Dobutamine is controversial as it has been associated with worse outcomes due to persistent beta 1-adrenergic receptor activation [5, 15, 100, 101]. Stepal and colleagues noted that chronic beta 1-adrenergic receptor stimulation was associated with heart failure in both postpartum and in mice with cardiomyocyte-specific STAT3 depletion and a proof of concept in the German registry cohort [101].
In chronic PPCM in the postpartum period, optimal guideline-directed medical therapy (GDMT) consists of the four heart failure pillars of disease-modifying drugs, namely renin–angiotensin–aldosterone (RAAS) inhibitors (angiotensin converting enzyme inhibitors (ACEI)/ angiotensin receptor/neprilysin receptor inhibitors (ARNI) and mineralocorticoid receptor antagonist (MRA)), sodium-glucose cotransporter-2 (SGLT2) inhibitors, and beta-blockers (Fig. 3) [15, 61,62,63, 82]. The historic hierarchical introduction of HF therapy is an area of guideline development, as a rapid commencement of all four drug classes is recommended, preferably before hospital discharge [96,97,98]. Additionally, ivabradine, vericiguat, hydralazine, and isosorbide dinitrate can be added to select PPCM patients [61,62,63, 96]. RAAS inhibitors are not recommended during pregnancy due to fetotoxicity. Instead, a combination of hydralazine and nitrates may be used.
Pharmacological therapy in peripartum cardiomyopathy. Drug therapy of PPCM during pregnancy includes the following: beta-1 receptor selective blockers (metoprolol is preferred while atenolol should be avoided); diuretics such as furosemide and hydrochlorothiazides should be used only in the presence of pulmonary congestion as they may decrease blood flow to the placenta; and anticoagulation therapy such as LMWH or vitamin k antagonists can be used at prophylactic dose or at therapeutic dose in the presence of intracardiac or systemic thrombo-embolism according to the stage of pregnancy. ACE-I, ARNI, and MRA are contraindicated during pregnancy. Drug therapy post-partum includes the aforementioned therapies in addition to ACE-I/ARNI, MRA, vasodilators, SGLT2-I, and bromocriptine. ACE-I, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor/ neprilysin inhibitor; HCTZ, hydrochlorothiazide; LMWH, low-molecular-weight heparin; MRA, mineralocorticoid receptor antagonist; SGLT2-I, sodium-glucose cotransporter-2 inhibitors. Created with BioRender.com
SGLT2 inhibitors, ivabradine, and vericiguat are not recommended during pregnancy and in breastfeeding mothers due to the paucity of safety data [16, 19, 96]. Advanced heart failure therapies in PPCM include cardiac resynchronisation therapy, implantable cardioverter defibrillator, left ventricular assist device, and cardiac transplant. These therapies are commonly used in high-income countries but are not readily available in middle- and low-income countries, even though these countries bear the most significant disease burden [12, 16, 96]. Anticoagulation is usually recommended in patients with intramural thrombus, venous thromboembolic disease, and atrial fibrillation. In PPCM patients with very low EF, prophylactic anticoagulation should be considered [5, 37, 82, 99]. Wearable cardiac defibrillators are recommended for newly diagnosed PPCM with LVEF < 35% for risk of sudden cardiac death due to ventricular tachycardia [5].
Bromocriptine is a dopamine agonist that blocks prolactin production. The drug is recommended in PPCM patients with severe left ventricular systolic dysfunction or those unwilling to breastfeed [9, 61, 82]. Based on evidence from recent studies, the ESC HF guidelines recommend adding bromocriptine to treat acute PPCM, especially in severe cases. However, the AHA/ACC HF guidelines have not approved using bromocriptine in PPCM because it is not FDA-approved for that purpose [5, 15,16,17,18,19, 51, 96, 102,103,104,105,106]. Patients taking bromocriptine are advised to take preventative anticoagulants because bromocriptine is pro-thrombotic (Regitz) [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107]. The ongoing REBIRTH (Impact of Bromocriptine on Clinical Outcomes for Peripartum Cardiomyopathy) study in North America is designed to address the safety and efficacy of bromocriptine [5, 17].
It is recommended that all patients diagnosed with peripartum cardiomyopathy (PPCM) undergo genetic testing if resources permit, especially those with a genetic predisposition. This includes patients with a family history of PPCM, a known cardiomyopathy mutation, or specific ethnic backgrounds. The process involves genetic counselling, DNA extraction, targeted sequencing of known cardiomyopathy-related genes, and genome or exome sequencing. Subsequently, any identified mutations are interpreted. Individuals with positive genetic results should undergo post-test genetic counselling, and their relatives should be screened to identify at-risk individuals who may require cardiac follow-up [56].
Patient education
Patient education is crucial for the integrated management of PPCM for several reasons (Fig. 4) [5, 108,109,110,111]. It raises awareness, enabling early detection of medical conditions by imparting knowledge about risk factors, medication adherence, and disease monitoring. Moreover, it facilitates patients’ access to support groups and resources for family planning, compliance with follow-up care, and adoption of pregnancy precautions [109, 110]. However, a pressing need exists for enhanced utilization and further research on patient education in PPCM management. The establishment of cardiac-obstetric clinics staffed with proficient multi-disciplinary healthcare professionals who are well-versed in the intricacies of PPCM is imperative [98]. Patient education empowers women to make well-informed decisions about their health, facilitate self-monitoring of their condition, adhere to treatment plans, and seek timely medical care as needed [5, 61,62,63, 110]. This underutilized resource is pivotal for delivering comprehensive care and support to individuals affected by PPCM.
The integrated care of peripartum cardiomyopathy. The integration of biomarkers, genetic profiling, conventional and disease-specific guideline-directed therapy, patient education, and a multi-disciplinary team contributes to early diagnosis and individualized care of peripartum cardiomyopathy, which leads to improved fetomaternal and safety outcomes. NT-proBNP, N-terminal pro b-type natriuretic peptide; sFLt-1, soluble Fms-like tyrosine kinase 1; PPCM, peripartum cardiomyopathy; TTN, titin protein. Created with BioRender.com
Novel therapeutic targets
Peripartum cardiomyopathy management has a tremendous unmet need for active research and investigation into potential therapeutic targets [5, 71, 75, 112,113,114,115]. In the dynamic field of PPCM research, it is crucial to recognize that while numerous therapeutic targets have shown promise in early studies, only some of these therapies are guaranteed to translate from the bench to the bedside [71]. Table 3 illustrates the potential novel therapeutic targets in PPCM [113,114,115,116,117,118,119,120,121].
Serum proteomic profiling (SPP) in PPCM is an emerging area of research that aims to identify specific protein biomarkers associated with the disease by analyzing the protein composition of blood samples to uncover unique patterns, signatures, or themes that may help in diagnosis, understanding pathophysiology, and guiding treatment strategies [113, 114].
A study conducted by Kodogo et al. utilized an untargeted SPP to analyze patients diagnosed with PPCM. This study is significant as it revealed 15 upregulated and 14 downregulated proteins, establishing a significant association between PPCM and a combination of adiponectin, quiescin sulfhydryl oxidase 1, inter-α-trypsin inhibitor heavy chain, and NT-proBNP [113]. These findings indicate salient biological themes related to immune response, inflammation, and coagulation and provide valuable insights into the pathophysiology of PPCM. Furthermore, Lovell and colleagues observed analogous perturbations in inflammation and lipid metabolism utilizing proteomic methodologies, further validating the findings of the Kodogo study [114]. These advancements have unveiled the intricate and diverse mechanisms underlying the pathophysiology of PPCM, warranting further research.
Clinical outcomes
Maternal outcomes
PPCM is potentially life-threatening if not diagnosed early and managed appropriately [5, 61]. Heart failure can be progressive, leading to reduced effort tolerance, poor quality of life, and even death. Maternal complications include hypoxia, thromboembolism, arrhythmias, hospital readmission, and misdiagnosis as preeclampsia [5, 110]. Traditionally, maternal outcomes were known to follow the rule of thirds, where a third recovered, remained stable, and deteriorated [22]. In recent years, the increased disease awareness combined with the advent of HF anti-remodelling therapy improved outcomes, with nearly half recovering, a quarter remaining stable, and another quarter eventually deteriorating [19, 122,123,124,125,126,127,128]. In PPCM patients on GDMT who fully recover, the continuation of anti-remodelling therapy is recommended [5, 124]. Halliday and colleagues, in a pilot trial, though underpowered, showed that in heart failure patients with improved or recovered ejection fraction, 40% relapsed on discontinuation of therapy [11].
Counselling regarding subsequent pregnancies is of utmost importance for all PPCM patients, emphasizing the potential for relapse, exacerbation of heart failure, and mortality, notwithstanding an improved or recovered ejection fraction [109,110,111]. The treatment armamentarium for heart failure has undergone a paradigm shift with the introduction of SGLT2 inhibitors [62, 63, 98]. This advancement is anticipated to enhance further the clinical outcomes of peripartum cardiomyopathy (PPCM). Further investigation is necessary to examine the clinical outcomes of patients undergoing novel and robust heart failure therapy.
Fetal outcomes
Fetal complications may be due to fetal distress secondary to maternal hypoxia or placental hypoperfusion caused by reduced cardiac output induced by excessive diuresis or reduction in blood pressure from aggressive afterload reduction [122,123,124]. Prematurity, stillbirth, and neonatal death are also among some of the fetal complications [126]. There is a lack of data on neonatal outcomes in patients with PPCM, as they are seldom reported [5,6,7, 126].
Predictors of clinical outcome
In the initial 6 months following the diagnosis of PPCM, ventricular remodelling occurs alongside an exponential recovery of left ventricular function [89, 90]. The role of baseline LVEF and dimension as predictors of mortality has been a subject of debate, with conflicting findings across various studies [84]. Previous studies might have failed to demonstrate significant differences due to insufficient data or statistical power to establish a clear association between baseline LVEF or dimension and mortality in PPCM patients [86, 128]. However, recent research suggests that these baseline parameters may hold predictive value in predicting outcomes in PPCM [67, 72, 76, 84]. Higher NYHA functional class, Fas/Apo-1, prolonged hospitalization and admission to the intensive care unit (ICU) at diagnosis were linked to increased mortality risk [86]. At the same time, novel findings have identified young age (< 20 years) and extreme body mass index (< 18.5 kg/m2 and > 30 kg/m2) at diagnosis as independent predictors of mortality [13, 16, 126]. Hoevelmann and colleagues observed that prolonged QTc and sinus tachycardia at baseline on ECG also predict poor outcomes among PPCM patients at 6 and 12 months, respectively [78]. Several contemporary studies have highlighted RV dysfunction and reduced LV global longitudinal and circumferential strain patterns as indicators of adverse PPCM prognosis [14, 129]. Patients with a history of previous PPCM are susceptible to relapse or deterioration during SSP [7, 42, 125, 128, 130,131,132]. In a cohort of 34 SSP PPCM patients, Hilfiker-Kleiner and colleagues reported an overall relapse rate of 53% and a mortality rate of 12% [132]. The study also noted that persistently reduced LV systolic function was associated with high mortality and a lower recovery rate. In patients with recovered systolic function, exercise stress tests with adequate contractile reserve may identify individuals at low risk of PPCM in subsequent pregnancies. Currently, novel risk prediction scores are being developed for PPCM to identify high-risk individuals, expedite diagnosis, forecast outcomes, and provide guidance for effective management [133,134,135].
Conclusion
Peripartum cardiomyopathy (PPCM) is a potentially life-threatening condition, but its global prevalence and incidence are still unknown due to misdiagnosis and underreporting. The pathophysiology of PPCM is complex, and it remains a diagnosis of exclusion despite increased research. The only well-established biomarkers for diagnosis and guiding therapy are natriuretic peptides pro-BNP/NT-proBNP. However, other potential biomarkers such as sFlt, 16 kDa PRL, micro-RNA, SPP, and multi-modality cardiac imaging require further research. Clinical management follows conventional heart failure therapy, with recent focus on ARNI and SGLT2 inhibitors. Bromocriptine is currently the only disease-specific therapy, with additional efficacy and safety studies underway. Most women with the integration of PPCM care recover, and future research should concentrate on identifying disease-specific biomarkers and developing risk stratification models.
Data availability
Not applicable—the current paper is a review.
Abbreviations
- AHA/ACC:
-
American Heart Association/American College of Cardiology
- CVD:
-
Cardiovascular disease
- ESC:
-
European Society of Cardiology
- HF:
-
Heart failure
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- MnSOD:
-
Manganese superoxide dismutase
- NYHA:
-
New York Heart Association
- PGC1ᾳ:
-
Peroxisome proliferator-activated receptor gamma 1-alpha coactivator
- PPCM:
-
Peripartum cardiomyopathy
- PRL:
-
Prolactin
- ROS:
-
Reactive oxygen species
- RV:
-
Right ventricle
- sFlt1:
-
Soluble fms-like tyrosine kinase-1
- SGLT2i:
-
Sodium-glucose cotransporter-2 inhibitors
- SPP:
-
Serum proteomic profile
- SSP:
-
Subsequent pregnancy
- STAT3:
-
Signal transducer and activator of the transcription 3
- VEGF:
-
Vascular endothelial growth factor
References
Ogah OS, Adebiyi AA, Sliwa K (2019) Heart failure in sub-Saharan Africa. https://pdfs.semanticscholar.org
Draper ES, Gallimore ID, Smith LK, et al (2019) Mothers and babies: reducing risk through audits and confidential enquiries across the UK (MBRRACE-UK). Available from https://www.npeu.ox.ac.uk/downloads/files/mbrrace-uk/reports/MBRRACE-UK
Schutte JM, Steegers EAP, Schuitemaker NEW et al (2016) Rise in maternal mortality in the Netherlands. Br J Obstet Gynaecol 117:399–406
Elkayam U, Goland S, Pieper PG, Silverside CK (2016) High-risk cardiac disease in pregnancy: part I. J Am Coll Cardiol 68:396–410
Bauersachs J, König T, Van der Meer P, Petrie MC et al (2019) Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 21:827–843
Sliwa K, Mebazaa A, Hlifiker-Kleiner D et al (2017) Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM). EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the heart failure association of the european society of cardiology study group on peripartum cardiomyopathy. Eur J Heart Failure 19(9):1131–1141
Sliwa K, Petrie MC, Vaan der Meer P et al (2020) Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J 41(39):3787–3797. https://doi.org/10.1093/eurheartj/ehaa455
McNamara DM, Elkayam U, Alharethi R et al (2015) IPAC investigators. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am College Cardiol 66:905–914
Hilfiker-Kleiner D, Haghikia A, Berliner D et al (2017) Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomised study. Eur Heart J 38:2671–2679
Strasserking FE, Musho J, Heimburger DC et al (2022) Peripartum cardiomyopathy: characteristics and outcomes among women seen at a referral hospital in Lusaka. Zambia Int J Cardiol Heart Vasc 42:101104
Gambahaya ET, Hakim J, Kao D et al (2017) Peripartum cardiomyopathy among cardiovascular patients referred for echocardiography at Parirenyatwa Teaching Hospital, Harare. Zimbabwe Cardiovasc J Africa 28(1):8–12
Karaye KM, Shehu MN, Ngantcha M et al (2023) Peripartum cardiomyopathy: a review article. West Afr J Med 40(1):104–113
Karaye KM, Ishaq NA, Sai’du H, Balarabe SA, Ahmed BG, Adamu UG et al (2021) Peripartum cardiomyopathy in Nigeria (PEACE) registry investigators. Disparities in clinical features and outcomes of peripartum cardiomyopathy in high versus low prevalent regions in Nigeria. ESC Heart Fail 8:3257–3267
Blauwet LA, Libhaber E, Forster O et al (2013) Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart 99:308–313
Sliwa K, Bauresachs J, Arany Z et al (2021) Peripartum cardiomyopathy: from genetics to management. Eur Heart J 42:3094–3102
Viljoen C, Hoevelmann J (2023) Sliwa K (2023) Peripartum cardiomyopathy: risk factors and predictors of outcome. Curr Opinion Cardiol 38(3):223–232. https://doi.org/10.1097/HCO.0000000000001037
Arany Z (2024) Peripartum cardiomyopathy. New England J Med 390:154–164. https://doi.org/10.1056/NEJMra2306667
Albakri A (2018) Peripartum cardiomyopathy: a review of literature on clinical status and meta-analysis of diagnosis and clinical management. J Integr Cardiol 4(3):1–12
Azibani F, Sliwa K (2018) Peripartum cardiomyopathy: an update. Curr Heart Fail Rep 15:297–306
Ritchie C (1849) Clinical contribution to the pathology, diagnosis and treatment of certain chronic heart diseases. Edinburgh Med Surg J 2:333
Porak C (1880) De L’influence réciproque de la grossesse et des maladies du Coeur. Dissertation, Medical Faculty of Paris
Pyatt JR, Dubey G (2011) Peripartum cardiomyopathy: current understanding, comprehensive management review and new developments. Postgrad Med J 87(1023):34–39
Walsh JJ, Burch GE (1961) Postpartal heart disease. Arch Intern Med 108(6):817–822
Skaluba SJ, Berkson DM (2001) Peripartum cardiomyopathy: case report and literature review. Congest Heart Fail 7(2):88–92
Hull E, Hafkesbring E (1937) Toxic postpartal heart disease. New Orleans Med Surg J 89:550–557
Demakis JG, Rahimtoola SH (1971) Peripartum cardiomyopathy. Circulation 44(5):964–968
Pearson GD, Veille JC, Rahimtoola S et al (2000) Peripartum cardiomyopathy. J Am Med Assoc 283(9):1183–1188
Sliwa K, Hilfiker-Kleiner D, Petrie MC et al (2010) Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12:767–778
Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J (2015) Peripartum cardiomyopathy: current state management and future perspectives. Eur J Heart Fail 36:1090–1097
Davies MB, Arany Z, McNamara D et al (2019) Peripartum cardiomyopathy. J Am Coll Cardiol 75(2):209–221
Wu VCC, Chen T, Yeh J et al (2017) Clinical outcomes of peripartum cardiomyopathy: a 15-year nationwide population-based study in Asia. Medicine 96(43):1–8
Brar SS, Khan SS, Sandhu GK et al (2007) Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 100(2):302–304
Isezuo SA, Abubakar SA (2007) Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethnic Disease 17:228–233
Sanderson JE, Adesanya CO, Anjorin FI, Parry EHO (1979) Postpartum cardiac failure-heart failure due to volume overload? Am Heart J 97:613–621
Desai D, Moodley J, Naidoo D (1995) Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa, and a review of the literature. Trop Doct 25:118–123
Seftel H, Susser M (1961) Maternity and myocardial failure in African women. Br Heart J 23:4352
Sigauke FR (2015) An investigation into the clinical outcomes of peripartum cardiomyopathy at Klerksdorp/Tshepong Hospital Complex. Dissertation, University of KwaZulu Natal http://hdl.handle.net/10413/16052
Forster OAEM (2007) Peripartum cardiomyopathy – an autoimmune disease? Dissertation, University of Witwatersrand http://hdl.handle.net/10539/5011
Gentry MB, Dias JK, Luis A et al (2010) African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol 55(7):654–659. https://doi.org/10.1016/j.jacc.2009.09.043
Sinkey RG, Rajapreyar IN, Szychowski JM et al (2022) Racial disparities in peripartum cardiomyopathy: eighteen years of observations. J Matern Fetal Neonatal Med 35(10):1891–1898
Mielniczuk LM, Williams K, Davis DR et al (2006) Frequency of peripartum cardiomyopathy. Am J Cardiol 97(12):1765–1768. https://doi.org/10.1016/j.amjcard.2006.01.039
Elkayam U, Akhter MW, Singh H et al (2005) Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation 111(16):2050–5. https://doi.org/10.1161/01.CIR.0000162478.36652.7E
Hilfiker-Kleiner D, Sliwa K (2014) Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 11:364–370
Hilfiker-Kleiner D, Kaminski K, Podewski E et al (2007) A cathepsin D-cleaved 16kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128:589–600
Mebazaa A, Seronde MF, Gayat E et al (2017) Imbalanced angiogenesis in peripartum cardiomyopathy: diagnostic value of placental growth factor. Circ J 18(11):1654–1661
Patten I, Rana S, Shahul S et al (2012) Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485:333–338
Roh JD, Yu A, Rana S et al (2021) Shared senescence pathophysiology in pre-eclampsia and peripartum cardiomyopathy. Circulation 144:A12940. https://doi.org/10.1126/scitranslmed.adi0077
Roh JD, Castro C, Yu A et al (2024) Placental senescence pathophysiology is shared between peripartum ccardiomyopathy and preeclampsia in mouse and human. Sci Transl Med 16(743):eadi0077
Shahul S, Ramadan H, Nizamuddin J et al (2018) Activin A and late postpartum cardiac dysfunction among women with hypertensive disorders of pregnancy. Hypertension 72:188–193
Ntusi NBA, Badri M, Gumedze F, Sliwa K, Mayosi BM (2015) Pregnancy-associated heart failure: a comparison of clinical presentation and outcome between hypertensive heart failure of pregnancy and idiopathic peripartum cardiomyopathy. PLoS ONE 10(8):e0133466
Barasa A, Rosengren A, Sandström TZ, Ladfors L, Schaufelberger M (2017) Heart failure in late pregnancy and postpartum: incidence and long-term mortality in Sweden 1997–2010. J Cardiac Fail 23(5):370–378. https://doi.org/10.1016/j.cardfail.2016.12.011
Firoz T, Magee LA, MacDonell K et al (2014) Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum. Br J Obstet Gynaecol 121(10):1210–1218. https://doi.org/10.1111/1471-0528.12737
Pierce JA, Price BO, Joyce JW (1963) Familial occurrence of postpartal heart failure. Arch Intern Med 111:651–655. https://doi.org/10.1001/archinte.1963.03620290117016
Haghikia A, Podewski E, Libhaber E et al (2013) Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Research Cardiology 108(4):366. https://doi.org/10.1007/s00395-013-0366-9
Fett JD, Ansari AA, Sundstrom JB, Combs GF (2002) Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol 86(2–3):311–316
Lee S, Cho GJ, Park GU et al (2018) Incidence, risk factors, and clinical characteristics of peripartum cardiomyopathy in South Korea. Circ Heart Fail 11(4):e004134. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004134
Dewi IP, Nugroho J (2021) Genetics of peripartum cardiomyopathy. Gynaecol Obstet Reprod Med 27(3). https://doi.org/10.21613/GORM.2021.1072
Spracklen TF, Chakafana G, Schwartz PJ et al (2021) Genetics of peripartum cardiomyopathy: current knowledge, future directions and clinical implications. Genes 12(1):103. https://doi.org/10.3390/genes12010103
Chakafana G, Spracklen TF, Kamuli S et al (2021) Heat shock proteins: potential modulators and candidate biomarkers of peripartum cardiomyopathy. Front Cardiovasc Med 8:633013. https://doi.org/10.3389/fcvm.2021.633013
Shrivastava A, Haase T, Zeller T et al (2020) Biomarkers for heart failure prognosis: proteins, genetic scores and non-coding RNAs. Front Cardiovasc Med 7:601364. https://doi.org/10.3389/fcvm.2020.60136
Bauersachs J, Arrigo M, Hilfiker-Kleiner D et al (2016) Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the heart failure association of the european society of cardiology study group on peripartum cardiomyopathy. Eur J Heart Failure 18:1096. https://doi.org/10.1002/ejhf.586
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 42(36):3599–3726
Heidenreich P, Bozkurt B, Aguilar D et al (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145:895–1032. https://doi.org/10.1161/CIR.0000000000001063
Nasrien EI, Januzzi LJ (2018) The future of biomarker-guided therapy for heart failure after guiding evidence-based therapy using biomarker intensified treatment in heart failure (GUIDE-IT) study. Curr Heart Fail Rep 15:37–43
Ersbøll AS, Goetze JP, Johansen M et al (2021) Biomarker and their relation to cardiac function late after peripartum cardiomyopathy. J Cardiac Fail 27(2):168–175
Biasucci LM, Maino A, Grimaldi MC et al (2021) Novel biomarkers in heart failure: new insights in pathophysiology and clinical perspective. Journal Clinical Medicine 10:2771
Aspromonte N, Gulizia MM, Clerico A (2017) NMCO/ELAS/SIBioC consensus document: biomarkers in heart failure. Europ Heart J Suppl 19(Suppl. D):D102–D112. https://doi.org/10.1093/eurheartj/sux027
Roberts E, Ludman AJ, Dworynski K et al (2015) The diagnostic accuracy of the natriuretic peptides in heart failure: systemic review and diagnostic meta-analysis in the acute setting. BMJ 350:h910. https://doi.org/10.1136/bmj.h910
Hoevelmann J, Viljoen CA, Azibani F et al (2020) Prognostic value of NT-pro-BNP for myocardial recovery in peripartum cardiomyopathy. Eur Heart Journal 41(2):ehaa946.3185. https://doi.org/10.1093/ehjci/ehaa946.3185
Bernard V, Young J, Chanson P, Binartet N (2015) New insights in prolactin: pathological implications. Nat Rev Endocrinol 36:1–11
Halkein J, Tabruyn SP, Ricke-Hoch M et al (2013) MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Investig 123:2143–2154
Hosseinpour A, Hosseinpour H, Kheshti F, Abdollahifard S (2022) Attar A (2022) Prognostic value of various markers in recovery from peripartum cardiomyopathy: a systematic review and meta-analysis. Eur Soc Cardiol Heart Failure 9(5):3483–3495. https://doi.org/10.1002/ehf2.14085
Food and Drug Administration (2021) Biomarker qualification program. https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/biomarker-qualification-program
Libhaher E, Sliwa K, Bachelier K et al (2015) Low systolic blood pressure and resting as predictors of outcome in patients with PPCM. Int J Cardiol 190:376–382. https://doi.org/10.1016/j.ijcard.2015.04.081
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Tibazarwa K, Lee G, Mayosi B et al (2012) The 12-lead ECG in peripartum cardiomyopathy. Cardiovasc J Afr 23(6):322–329
Mbakwem AC, Bauersachs J, Viljoen C et al (2021) Peripartum cardiomyopathy investigators group. Electrocardiographic features and their echocardiographic correlates in peripartum cardiomyopathy: results from the ESC EORP PPCM registry. ESC Heart Failure 8:879–889
Hoevelmann J, Viljoen CA, Manning K et al (2018) The prognostic significance of the 12-lead ECG in peripartum cardiomyopathy. Int J Cardiol 276:177–184
Honigberg MC, Elkayam U, Rajagopalan N et al (2019) IPAC Investigators Electrocardiographic findings in peripartum cardiomyopathy. Clin Cardiol 42:524–529
Leo I, Nakou E, Marvao, et al (2022) Imaging in women with heart failure: sex-specific characteristics and current challenges. Cardiac Fail Rev. https://doi.org/10.15420/cfr.2022.17
Ricci F, Innocentiis C, Verrengia E et al (2020) The role of multimodality cardiovascular imaging in peripartum cardiomyopathy. Front Cardiovasc Med 7:4. https://doi.org/10.33892/fcvm.2020.00004
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J et al (2018) ESC Scientific Document Group, 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy: the Task Force for the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J 39(34):3165–3241
Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P (2000) Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/Apo-1. J Am Coll Cardiol 35:701–705
Sanusi M, Momin ES, Mannan V et al (2022) Using echocardiography and biomarkers to determine prognosis in peripartum cardiomyopathy: a systematic review. Cureus 14(6):e26130. https://doi.org/10.7759/cureus.26130
Sugahara M, Kagiyama N, Hasselberg NE et al (2019) IPAC Investigators. Global left ventricular strain at presentation is associated with subsequent recovery in patients with peripartum cardiomyopathy. J Am Soc Echocardiography 32:1565–1573
Kiran GR, Rajkumar C, Chandrasekhar P (2021) Clinical and echocardiographic predictors of outcomes in peripartum cardiomyopathy: a single centre, six-month follow-up study. India Heart Journal 73:319–324
Blauwet LA, Delgado-Montero A, Ryo K et al (2016) (2016) Right ventricular function in peripartum cardiomyopathy at presentation is associated with subsequent left ventricular recovery and clinical outcomes. Circ Heart Fail 9(5):e002756. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002756
Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE (2011) Left ventricular remodelling in heart failure. J Am Coll Cardiol 4(1):98–108
Liang Y, Xu Y, Li W et al (2020) Left ventricular function recovery in peripartum cardiomyopathy: a cardiovascular magnetic resonance study by myocardial T1 and T2 mapping. J Cardiovasc Magn Reson 22:2. https://doi.org/10.1186/s12968-019-0590-z
Aora NP, Mahajan N, Mohamad T (2022) Cardiac resonance in peripartum cardiomyopathy. Eur Heart J- Case Reports 6:1–6. https://doi.org/10.1093/ehjcr/ytac395
Ntusi NBA, Badri M, Gumedze F, Sliwa K, Mayosi BM (2015) Pregnancy-associated heart failure: a comparison of clinical presentation and outcome between hypertensive heart failure of pregnancy and idiopathic peripartum cardiomyopathy. PLoS ONE 10(8):e0133466. https://doi.org/10.1371/journal.pone.0133466
Mouquet F, Lions C, Groote De et al (2008) Characterisation of peripartum by cardiac magnetic resonance imaging. Eur Radiol 18:2765–2769
Aremu O, Jermy S, Samuels P et al (2022) Utility of cardiovascular imaging in pregnancy. South Africa Heart Journal 19(3):194–200
Petryka-Mazurkiewicz J, Kryczka K, Mazurkiewicz L et al (2021) Cardiovascular magnetic resonance in peripartum cardiomyopathy: comparison with idiopathic dilated cardiomyopathy. Diagnostics 11:1752
Chirillo F, Baritussio A, Cucchini U et al (2021) Challenges in the diagnosis of peripartum cardiomyopathy: a case series. Eur Heart J- Case Rep 5(2):ytab001. https://doi.org/10.1093/ehjcr/ytab001
Maddox TM, Januzzi JL, Allen LA et al (2024) 2024 ACC expert consensus decision pathway for the treatment of heart failure with reduced ejection fraction. JACC 83(15):1444–1488. https://doi.org/10.1016/j.jacc.2023.12.024
Lucà F, Colivicchi F, Parrini I et al (2023) The role of the pregnancy heart team in clinical practice. Front Cardiovasc Med 10:1135294. https://doi.org/10.3389/fcvm.2023.1135294
Carlson S, Schultz J, Ramu B et al (2023) Peripartum cardiomyopathy: risks, diagnosis, and management. J Multidiscip Healthc 16:1249–1258. https://doi.org/10.2147/JMDH.S372747
Arrigo M, Blet A, Mebazaa A (2017) Bromocriptine for the treatment of peripartum cardiomyopathy: welcome on BOARD. Eur Heart J 38(35):2680–2682. https://doi.org/10.1093/eurheartj/ehx428
Labbene I, Arrigo M, Tavares M et al (2016) Decongestive effects of levosimendan in cardiogenic shock induced by postpartum cardiomyopathy. Anaesthesia Crit Care Pain Med 36:39–42. https://doi.org/10.1016/j.accpm.2016.02.009
Stapel B, Kohlhaas M, Ricke-Hoch M et al (2017) Low STAT3 expression sensitizes to toxic effects of beta-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J 38:349–361. https://doi.org/10.1093/eurheartj/ehw086
Sliwa K, Blauwet L, Tibazarwa K et al (2010) Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation 121(13):1465–1473
Koenig T, Bauersachs J, Hilfiker-Kleiner D (2018) Bromocriptine for treating peripartum cardiomyopathy. Card Fail Rev 4(1):46–49
Tremblay-Gravel M, Marquis-Gravel G, Avram R et al (2019) The effect of bromocriptine on left ventricular functional recovery in peripartum cardiomyopathy: insights from the BRO-HF retrospective cohort study. ESC Heart Fail 6(1):27–36
Badianyana M, Das PK, Gaddameedi SR et al (2021) A systemic review of the utility of bromocriptine in acute periparipartum cardiomyopathy. Cureus 13(9):e18248. https://doi.org/10.7759/cureus.18248
Loewe C, Dragovic LJ (1998) Acute coronary artery thrombosis in a postpartum woman receiving bromocriptine. Am J Forensic Med Pathol 19:258–260
Hopp L, Haider B, Iffy L (1996) Myocardial infarction postpartum in patients taking bromocriptine to prevent breast engorgement. Int J Cardiol 57:227–232
Desplantie O, Tremblay-Gravel M, Avram R et al (2015) The medical treatment of new-onset peripartum cardiomyopathy: a systematic review of prospective studies. Can J Cardiol 31(12):1421–1426
Rosman L, Salmoirago-Blotcher E, Cahill J et al (2017) Depression and health behaviours in women with peripartum cardiomyopathy. Heart Lung 46(5):363–368
Carlin AJ, Alfirevic Z, Gyte GML (2010) Interventions for treating peripartum cardiomyopathy to improve outcomes for women and babies. Cochrane Database Syst Rev 9:1–22
Halliday B, Wassall R, Lota AS et al (2019) Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 393:61–73
Imamdin A, Lecour S, Opie LH et al (2016) Heat rate- a novel target for the treatment of peripartum cardiomyopathy? SA Heart Journal 13(4):304–307
Kodogo V, Viljoen C, Hoevelmann J et al (2023) Proteomic profiling in patients with peripartum cardiomyopathy: a biomarker study of the ESC EORP PPCM registry. JACC Heart Fail 11(12):1708–1725
Lovell JP, Bermea K, Yu J et al (2023) Serum proteomic analysis of peripartum cardiomyopathy reveals distinctive dysregulation of inflammatory and cholesterol metabolism pathways. J Am Coll Cardiol 11(9):1231–1242
Chaudhari K, Choudhary M, Chaudhari K et al (2022) Advancement in current therapeutic modalities in postpartum cardiomyopathy. Cureus 14(3):e22813. https://doi.org/10.7759/cureus.22813
Pheiffer C, Dias S, Jack B, Malaza N, Adam S (2021) Adiponectin as a potential biomarker for pregnancy disorders. Int J Mole Sci 22:1326. https://doi.org/10.3390/ijms22031326
Lei X, Qiu S, Yang G et al (2023) Adiponectin and metabolic cardiovascular diseases: therapeutic opportunities and challenges. Genes & Diseases 10:1525–1536
Beadle RM, Williams LK, Kuehl M et al (2015) Improvement in cardiac energetics by perhexiline in heart failure due to dilated cardiomyopathy. J Am Coll Cardiol Heart Failure 3:202–211
Ravi T, Henning H, Wiebke S et al (2016) Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 27(3):903–913
Feijóo-Bandín S, Aragón-Herrera A, Rodríguez-Penas D et al (2017) Relaxin-2 in cardiometabolic diseases: mechanisms of action and future perspectives. Front Physiol 8:599
Sliwa K, Skudicky D, Candy G (2002) The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Failure 4(3):305–9. https://doi.org/10.1016/s1388-9842(02)00008-9
Elkayam U, Tummala PP, Rao K et al (2001) Maternal and foetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 344(21):1567–1571. https://doi.org/10.1056/NEJM200105243442101
Al Riyami N, Al Khayari S, Al Zadjali R et al (2023) Incidence, risk factors, maternal and neonatal outcomes of peripartum cardiomyopathy (PPCM) in Oman. Global Heart 18(1):23. https://doi.org/10.5334/gh.1198
Singh S, Munikrishna M, Sheela SR (2020) Feto-maternal outcome in patients with peripartum cardiomyopathy: a 5-year study in a tertiary care hospital in Kolar district. Int J Reprod Contraception, Obstet Gynecol 9(5):1853. https://doi.org/10.18203/2320-1770.ijrcog20201526
Jackson AM, Macartney M, Brooksbank K et al (2023) A 20-year population study of peripartum cardiomyopathy. Eur Heart J 44:5128–5141. https://doi.org/10.1093/eurheartj/ehad626
Foster E, Sharma GK, Sander GE, et al (2019) Peripartum(postpartum) cardiomyopathy. Medscape. 2019. https://emedicine.medscape.com/article/153153-overview
Biteker M, Iihan D, Biteker G, Duman D, Bozkurt B et al (2012) Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur Heart Failure J 14:895–901
Sliwa K, Petrie MC, Hilfiker-Kleiner DM et al (2017) Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the heart failure association of the european society of cardiology study group on peripartum cardiomyopathy. Eur J Heart Fail 20:951–962
Hoevelmann J, Engel ME, Muller E et al (2022) A global perspective on the management and outcomes of peripartum cardiomyopathy: a systematic review and meta-analysis. Eur J Heart Fail 24(9):1719–1736
Sliwa K, Forster O, Zhanje F et al (2004) Outcome of subsequent pregnancy in patients with documented peripartum cardiomyopathy. Am J Cardiol 93(11):1441–1443
Masuku DS (2018) Maternal and fetal outcomes of subsequent pregnancy in patients with documented peripartum cardiomyopathy. Dissertation, University of Cape Town. http://hdl.handle.net/11427/29669
Hilfiker-Kleiner D, Haghikia A, Masuko D et al (2017) Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail 19(12):1723–1728. https://doi.org/10.1002/ejhf.808
Davis MB, Jarvie J, Gambahaya E et al (2021) Risk prediction for peripartum cardiomyopathy in delivering mothers: a validated risk model PPCM risk prediction model. J Cardiac Fail 27(2):159–167. https://doi.org/10.1016/j.cardfail.2020.12.022
Zhang Z, Zheng W, Chen W et al (2023) A new risk score for the assessment of outcomes for Chinese patients with peripartum cardiomyopathy. Heart Lung 60:81–86
Jackson AM, Goland S, Farhan H et al (2024) A novel score to predict left ventricular recovery in peripartum cardiomyopathy derived from the ESC EORP Peripartum Cardiomyopathy Registry. Eur Heart J 45(16):1430–1439. https://doi.org/10.1093/eurheartj/ehad888
Acknowledgements
The authors thank the University of Witwatersrand Department of Cardiology and Obstetrics staff in Charlotte Maxeke Johannesburg Academic, Helen Joseph and Rahima Moosa Mother and Child tertiary hospitals. BioRender software was used to create the figures in the manuscript.
Funding
Open access funding provided by University of the Witwatersrand.
Author information
Authors and Affiliations
Contributions
FS was responsible for the conception and writing of the first draft of the manuscript. HN and NT reviewed and edited the manuscript. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval is not applicable as the current paper is a review.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sigauke, F.R., Ntsinjana, H. & Tsabedze, N. Peripartum cardiomyopathy: a comprehensive and contemporary review. Heart Fail Rev (2024). https://doi.org/10.1007/s10741-024-10435-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10741-024-10435-5