Abstract
Increased sympathetic and reduced parasympathetic nerve activity is associated with disease progression and poor outcomes in patients with chronic heart failure. The demonstration that markers of autonomic imbalance and vagal dysfunction, such as reduced heart rate variability and baroreflex sensitivity, hold prognostic value in patients with chronic heart failure despite modern therapies encourages the research for neuromodulation strategies targeting the vagus nerve. However, the approaches tested so far have yielded inconclusive results. This review aims to summarize the current knowledge about the role of the parasympathetic nervous system in chronic heart failure, describing the pathophysiological background, the methods of assessment, and the rationale, limits, and future perspectives of parasympathetic stimulation either by drugs or bioelectronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The autonomic nervous system (ANS) plays a key role in the neuroendocrine control of the body by adapting vegetative functions to support homeostasis.
In healthy conditions, the sympathetic (SNS) and parasympathetic nervous systems (PSNS) participate in cardiovascular control in a complementary and, at least partially, opposite fashion. Of note, while the heart receives both innervations, the resistance vessels of the systemic circulation are exclusively controlled by the SNS.
On the other hand, autonomic imbalance is a determinant of cardiovascular disorders, such as chronic heart failure (CHF). In the acute setting, chemoreflex activation and baroreflex deactivation induce PSNS withdrawal and SNS overactivation as compensatory mechanisms to maintain perfusion and respiratory efficiency. Over the long term, SNS predominance is maladaptive and, also by activating the renin–angiotensin–aldosterone system (RAAS), favors salt and water retention, cardiac remodeling, and life-threatening arrhythmias [1].
Accordingly, several markers of autonomic dysfunction retain prognostic significance [2], while counteracting SNS improves survival in patients with CHF and reduced left ventricular ejection fraction (LVEF) [3]. On the other hand, because of the contradictory findings from clinical trials, the role of neurohormonal systems seems less consistent in patients with CHF and preserved LVEF, as reviewed in [4]. Indeed, also the usefulness of beta-blockers has been questioned in these patients, since they may contribute to chronotropic incompetence and, consequently, to exercise intolerance [5]. Notwithstanding, a growing body of evidence has shown that autonomic imbalance characterizes a significant subset of CHF patients also in the case of preserved LVEF, contributing to disease progression and poor outcomes [4, 6, 7]. Interestingly, in this context, the interaction between the ANS and immune system (i.e., the neuroimmune cross-talk) seems to play a crucial role, contributing to the activation of pro-inflammatory, pro-oxidative, and pro-fibrotic cascades [8].
Therefore, autonomic imbalance and its detrimental consequences persist in many CHF patients despite therapeutic advances, fostering research for the development of further neuromodulation strategies [9]. Various approaches have targeted the SNS, mainly involving denervation, with inconclusive results [10]. Stimulating the PSNS represents a valuable alternative. Indeed, through either direct (i.e., on cardiac cells) or indirect (i.e., on pro-inflammatory pathways) effects, increasing cholinergic signaling may be beneficial to patients with CHF across the whole LVEF spectrum [11].
Nevertheless, the off-target effects of cholinergic drugs and the uncertain efficacy of bioelectronic devices have so far prevented their clinical translation [10, 12]. Transcutaneous vagus nerve stimulation (tVNS) is an emerging opportunity to achieve neuromodulation non-invasively but is still to be tested in large-scale trials [13, 14].
In this review, we aim to summarize the current knowledge about the role of the PSNS in CHF pathophysiology, describing the methods to evaluate vagal cardiovascular control and unraveling the rationale, limits, and perspectives of stimulating PSNS to improve outcomes.
Cardiovascular parasympathetic control
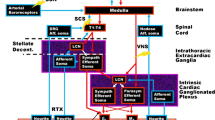
Cardiovascular autonomic control relies on a series of reflex arcs composed of specialized peripheral receptors, efferent and afferent arms, and integrative centers [15].
Efferent axons of the ANS are organized into preganglionic and postganglionic fibers; for the SNS, the preganglionic axons are long and the postganglionic axons are short, whereas the reverse is true for the PSNS. Preganglionic neurons of the SNS are located within the lateral horn of the thoracolumbar spinal cord, whereas for the PSNS, these are located within the sacral spinal cord and in the brainstem. With respect to the heart, preganglionic neurons of the PSNS are located in the ventrolateral region of the nucleus ambiguous and in the dorsal motor nucleus and project within the vagus nerve [16]. These axons originate bilaterally within the caudal ventrolateral medulla and exit the brain via the jugular foramina [17].
The vagus nerve is a mixed nerve, composed of afferent (80–90%) and efferent (10–20%) fibers [18]. Though organized in different fascicles, at least in the cervical tract, the topography of vagal fibers in humans is still an object of study [18]. From the cervical and thoracic tracts of the vagus nerve arise the cardiac branches, which converge to the cardiac ganglia, being part of the intrinsic cardiac nervous system (ICNS) [18]. Within the ICNS, postganglionic neurons are organized in clusters, constituting functional circuits with sympathetic neurons and interneurons [19, 20]. Out of over 800 cardiac ganglia, seven subplexuses have been identified in humans: the dorsal and ventral right atrial plexuses, the dorsal and ventral left atrial plexuses, the middle dorsal plexus, and the right and left coronary plexuses [21]. Though functionally intertwined, atrial plexuses mainly modulate chronotropic and dromotropic functions, while coronary plexuses modulate ventricle contractility [21].
From the ICNS arise visceral afferent fibers, carrying mechanical, chemical, and nociceptive signals [22], whose somata are located in the nodose ganglion or the C6–T6 dorsal root ganglia [23]. The transduction properties of these fibers depend on their location but often display multimodal properties [24]. Furthermore, the activation of these fibers may result in either negative (i.e., inhibiting PSNS and activating SNS) or positive (i.e., activating PSNS and inhibiting SNS) responses [25].
Beyond cardiac fibers, the vagus nerve contains afferences from pulmonary arteries, aortic (left), and brachiocephalic artery (right) walls, carrying information from chemoreceptors and mechanoreceptors [26]. Though its physiological role is controversial, the auricular branch of the vagus nerve supplies sensory innervation to the acoustic meatus, the conchae, and the tragus. After traversing the temporal pyramid and engaging connections with the facial and glossopharyngeal nerves, these fibers reach the jugular ganglion [26].
Vagal afferents project to the nucleus tractus solitarius (NTS) and the area postrema of the medulla. The NTS has direct and indirect connections with cortical and subcortical structures (e.g., limbic areas, rostral ventrolateral medulla, intermediate lateral medulla, locus coeruleus) [18, 27] and is deemed responsible for modulating efferent pathways by integrating afferent signals [28].
Role of acetylcholine on the heart
In cardiac ganglia, vagal fibers release acetylcholine (ACh), which binds to nicotinic receptors on postganglionic neurons, releasing ACh, which binds to muscarinic (M) receptors on cardiomyocytes. Growing evidence suggests that cardiac ganglia are integrative centers, in which parasympathetic and sympathetic signals modulate synaptic transmission, with contributions of interneurons and glial cells [29].
On cardiomyocytes, ACh induces negative chronotropic, dromotropic, inotropic, and bathmotropic effects [30], mediated by M2 and, less abundantly, M3 receptors [31]. The activation of M2 receptors results in (1) inhibition of adenylyl-cyclase, decreasing the activity of L-type Ca2+ channels, with negative dromotropic and inotropic effects [32], and “funny” current, with negative chronotropic effect [33, 34], and (2) stimulation of Giβγ subunit, activating the inwardly rectifying ACh-sensitive potassium channels (KACh), with negative chronotropic, dromotropic, and bathmotropic effects [35]. Stimulation of M3 receptors, via a Gq protein, activates phospholipase C [36] reducing sinoatrial node firing [37]. Finally, M1 receptors modulate cardiac control with opposite effects to those of M2 receptors [38] and modulate norepinephrine release from sympathetic terminals [39].
Through all these actions, the PSNS exerts beneficial effects on cardiac function. Indeed, by reducing cytoplasmatic Ca2+ concentration, hyperpolarizing cardiac cells, and decreasing sympathetic activity, ACh reduces the risk of ventricular arrhythmias [40]. Furthermore, cholinergic stimulation contributes to the inhibition of pro-hypertrophic, pro-fibrotic, and pro-apoptotic cascades, by reducing the activation of the mitogen-activated protein kinase and transforming growth factor-β pathways and activating the phosphoinositide 3-kinase/Akt signaling [41, 42]. Beyond these direct effects of cardiomyocytes, ACh influences cardiac function also indirectly, by modulating the immune system, through the cholinergic anti-inflammatory pathway [43]. Although the precise mechanisms are unclear, vagal efferences seem to promote the homing of cholinergic T-cells in the spleen. These cells release ACh, which binds α7-nicotinic receptors on macrophages, favoring their shift to the anti-inflammatory phenotype and reducing the secretion of tumor-necrosis-factor-α and other inflammatory cytokines [44]. While the physiological impact of this reflex on cardiac function is unknown, its dysfunction, secondary to sympathovagal imbalance, may be crucial in disease conditions such as CHF [45]. Indeed, increased circulating levels of inflammatory cytokines and immune cell infiltrates have been reported in patients with CHF, in which they have been associated with clinical severity and risk of adverse events [46, 47].
Notably, all the effects of ACh are characterized by an instantaneous (“rapid-off”) modulation due to the presence of acetylcholinesterase (AChE) in the synaptic cleft, which is responsible for the dynamic transduction properties of PSNS activity [48].
Methods of assessment of the parasympathetic nervous system
Muscle sympathetic nerve activity (MSNA) in accessible peripheral nerves is considered the gold standard for assessing sympathetic function [49]. However, given that cardiac sympathetic axons cannot be accessed by microelectrodes, measuring norepinephrine spillover into cardiac veins is the only means by which cardiac sympathetic drive can be assessed in humans [50]. Notably, there is a strong correlation between MSNA and noradrenaline spillover to the heart [51].
As for the PSNS, the responses to Ewing’s battery, heart rate variability (HRV), and baroreflex sensitivity (BRS) are used as indirect markers [52].
Ewing’s battery
Developed to assess diabetes-related ANS dysfunction [53], the Ewing’s battery is also used in patients with CHF [54] and consists of evaluating heart rate and blood pressure responses to specific challenges (namely, Valsalva maneuver, standing-up, deep breathing, and sustained handgrip). According to the results of each test, a score is calculated to identify autonomic dysfunction [53].
Heart rate variability
HRV refers to the fluctuations in the time between consecutive heartbeats, reflecting the ability of the cardiovascular system to adapt to endogenous/exogenous changes (Fig. 1) [55]. A comprehensive description of HRV measures is provided elsewhere [55, 56]. Briefly, in resting conditions, heart rate shows beat-to-beat changes following linear patterns, due to ANS modulation, influenced by visceral feedback and triggered by changes in respiratory activity, vascular tone, body temperature, hormones, and circadian fluctuations.
HRV can be measured in its time and frequency domain [55, 57, 58]. Among the time-domain measures, the standard deviation of normal-to-normal (NN) intervals (SDNN) expresses the overall variability of heart rate. While the root mean square of successive NN interval differences (rMSSD) and the percentage of successive NN intervals that differ more than 50 ms (pNN50) reflect vagal modulation, no time-domain measure specifically expresses SNS modulation [55, 57,58,59].
The frequency-domain analysis relies on spectral methods to decompose the whole variability of a signal into frequency bands [60]. For heart rate, the main spectral components are high frequency (HF, 0.15–0.4 Hz), low frequency (LF, 0.04–0.15 Hz), and very low frequency (VLF, 0.003–0.04 Hz) [61]. While HF expresses vagal modulation mainly linked to respiratory sinus arrhythmia, both SNS and PSNS contribute to the LF component, centered on the 0.1-Hz oscillations, observable also at the vascular level (i.e., Meyer waves). Body temperature, hormones, and altered respiratory patterns (periodic breathing) contribute to VLF [61].
Several other HRV parameters have been proposed, including non-linear and entropy indices [56].
Baroreflex testing
Arterial baroreflex modulates autonomic response to blood pressure changes. The reflex arc consists of stretch-sensitive receptors, mainly located in the carotid sinus in humans, an afferent arm to the brain via the glossopharyngeal nerve, and efferent vagal and sympathetic pathways [62]. Since the baroreflex-mediated modulation of sinus node activity mostly relies on rapid vagal signaling, BRS is considered a surrogate of PSNS function [15].
The use of pharmacological challenges to evoke controlled changes in blood pressure has been used to assess BRS [63, 64], showing prognostic value [65, 66]. To avoid the effects of drugs on end-organ function, different methods to assess the “spontaneous” BRS have been developed. The sequence method relies on the identification of the sequences of consecutive heartbeats with a parallel increase/decrease in heartbeats and blood pressure [67]. Other methods rely on the cross-correlations between the spectral components of heartbeats and blood pressure [67]. Though each of these measures showed predictive value, a simple ratio between the standard deviations of NN intervals and systolic blood pressure was the most accurate and reproducible among six methods [68] and a strong outcome predictor in a large cohort of CHF patients (Fig. 2).
Clinical and prognostic significance of baroreflex sensitivity in patients with chronic heart failure. Abnormal baroreflex sensitivity (BRS) is frequently (36%) observed in patients with chronic heart failure (CHF) patients (n = 267), in which it is associated with functional impairment, lower heart rate variability, and a higher risk of cardiac death [9]
Finally, a further possibility relies on the appliance of positive or negative pressure to the neck to assess the cardiac and vascular consequences of baroreceptors loading/unloading [69].
Vagus nerve microneurography recording
While MSNA is the gold standard method to assess SNS function, only recently, the first recordings from the human vagus nerve were obtained through ultrasound-guided insertion of a tungsten microelectrode into fascicles of the vagus nerve in the neck (Fig. 3) [70]. This allowed the identification and classification of tonically active neurons directed to the sinoatrial node [70] and to document the cardiac and respiratory modulation of multiunit nerve activity [71]. Though preliminary, these findings pave the way for future experiments to study vagus nerve functions in health and disease. Indeed, given that it is possible to identify intrafascicular sites exhibiting cardiac rhythmicity, it will be very interesting to use this technique to identify changes in afferent and efferent vagal activity in CHF, for example.
Microneurography recording of the vagus nerve. A tungsten microelectrode is inserted into the dorsolateral cervical region (A). Ultrasound guidance allows for the identification of the vagus nerve among muscular and vascular cervical structures (B). Direct visualization of the microelectrode through the probe allows for its precise direction toward the vagus nerve area, while avoiding vascular structures. CCA common carotid artery, ICA internal carotid artery, IJV internal jugular vein
Parasympathetic dysfunction in heart failure
The neurohormonal model is a cornerstone of CHF pathophysiology. Accordingly, the pharmacological antagonism of SNS and RAAS has shown significant prognostic benefits and is a pillar of CHF treatment in patients with reduced LVEF [72].
Notably, sympathovagal imbalance is only partially reversed by current therapies. Indeed, increased MSNA and reduced HRV, sustained by feedback resetting, sleep disorders, and abnormal central control, feature in many CHF patients [73]. While increased MSNA showed a linear relation with disease severity and outcomes, the significance of abnormal vagal control is more controversial because of the absence of a gold standard marker [73]. Accordingly, most of the evidence derives from studies assessing HRV and/or BRS, which mirror the PSNS influence on the sinus node.
All the measures of HRV are depressed in CHF patients and related to disease severity and outcomes. Of note, patients with CHF and preserved LVEF show an intermediate phenotype between patients with reduced LVEF and healthy controls [74,75,76].
The underlying mechanisms are still to be completely understood. While the reduction in HF reflects blunted vagal modulation [77], also VLF [78] and LF components are often decreased or absent in CHF patients, proportionally to disease severity [77], and predict mortality [79]. Baroreflex desensitization and abnormal central control, also associated with cardiorespiratory interactions, are possible explanations [80, 81]. Decreased arterial compliance, alterations in sensory transduction, abnormal central mediation of the reflex, and efferent neurotransmission may be the mechanisms of reduced baroreflex function, while increased angiotensin-II and aldosterone signaling, as well as oxidative stress, are the proposed molecular pathways [82,83,84,85,86].
Beyond BRS, other sites of vagal dysfunction may be identified, from the generation of central outflow to ganglionic and postganglionic synapses to neurotransmission efficiency. In this respect, the presence of antibodies against the M2 receptors has been documented in patients with dilated cardiomyopathy [87] and associated with cardiac remodeling in rat models of CHF [88]. Nevertheless, a series of landmark studies showed that, despite the reduction of vagal tone and the response to electrical vagus nerve stimulation, the number and activity of cardiac M2 receptors are preserved or upregulated in CHF, while AChE is downregulated, probably as compensatory mechanisms [89,90,91]. On the other hand, an abnormal transmission at the ganglionic level was identified and proposed as a key mechanism [92].
Future studies, e.g., through direct vagal recordings, are expected to shed light on the complex mechanisms behind PSNS dysfunction in CHF patients.
Targeting parasympathetic dysfunction in heart failure
Targeting the PSNS represents an unmet need in CHF patients (Fig. 4). Indeed, beyond restoring neurohormonal balance, improving cholinergic stimulation also exerts anti-inflammatory actions, which is beneficial in CHF across the whole LVEF spectrum [11] and particularly in patients with preserved LVEF, in whom the efficacy of beta-blockers has been questioned [4, 8]. Nevertheless, the clinical trials conducted so far have not confirmed the expectations derived from preclinical and preliminary clinical studies (Tables 1, 2, and 3) [10].
Vagal dysfunction as a therapeutic target in patients with chronic heart failure. Although different approaches have been already tested to improve vagal cardiovascular control in patients with chronic heart failure (CHF), no specific treatment has entered the clinical scenario so far. BRS baroreflex sensitivity, HRV heart rate variability, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-B-type natriuretic peptide, NYHA New York Health Association. Created with BioRender.com
Pharmacological approaches
Many of the drugs recommended for CHF treatment improve sympathovagal balance [93,94,95,96]. On the other hand, no molecule acting specifically on the cholinergic pathway is approved for this purpose (Table 1).
As reviewed elsewhere [97], AChE inhibitors showed protective effects in CHF rodents, by increasing HRV and BRS and reducing SNS activity [97]. Since some AChE inhibitors are used for neurological conditions (donepezil in the treatment of Alzheimer’s disease and pyridostigmine of myasthenia gravis), their cardiovascular effects have been investigated.
In two randomized, cross-over, double-blind studies, oral pyridostigmine improved heart rate recovery in 20 (30 mg, single dose) and 23 (45 mg, 3 t.i.d., for 1 day) CHF patients, respectively [98, 99]. In another study, pyridostigmine (30 mg, 3 t.i.d. for 2 days) lowered the incidence of premature ventricular beats and increased rMSSD and pNN50 in 20 CHF patients [100]. In a randomized, double-blind study, enrolling 21 CHF patients with heart rate > 70 bpm, pyridostigmine (30 mg, 3 t.i.d. for 6 months) reduced heart rate, natriuretic peptide levels, and inflammatory markers and improved symptoms and exercise capacity [101]. While the high rate of systemic effects (mainly gastrointestinal) due to systemic cholinergic stimulation may limit compliance to AChE inhibitors, the risk of long-term effects, including QT prolongation, is unknown in CHF patients [97]. While no studies have specifically investigated the effect of these molecules on clinical endpoints in patients with CHF, in an observational study on patients with Alzheimer’s disease without a history of CHF, the use of AChE inhibitors was associated with a significantly lower risk of new-onset CHF and cardiovascular death compared to propensity-score-matched non-users [102]. Similarly, in another observational study, patients with dementia treated with AChE inhibitors showed a lower risk of major adverse cardiovascular events, including heart failure-related hospitalization, compared with controls [103].
A potential alternative to AChE inhibitors is represented by two antimuscarinic agents, namely, scopolamine and pirenzepine, whose low-dose administration is associated with vagotonic effects, due to a greater affinity for M1 receptors, favoring ACh binding to M2 receptors and reducing norepinephrine release [104, 105]. The application of a scopolamine patch for 24 h in 21 CHF patients increased RR interval and HRV [106]. Similar findings were replicated in a randomized, cross-over, double-blind study, in which transdermal scopolamine increased HRV and BRS in 15 CHF patients [107]. Also, the intravenous administration of pirenzepine in 15 CHF patients increased SDNN and HF power [104]. The effects of these molecules were compared in a single-blind, placebo-controlled, cross-over trial in 20 post-myocardial infraction patients. While both drugs increased HRV and BRS, pirenzepine use yielded a lower rate of adverse effects (only 5% of patients reported nausea vs. 50% of patients reporting dry mouth, drowsiness, blurred vision, or nausea on scopolamine) [108].
Despite these findings, no study has investigated the efficacy of these molecules on clinical endpoints.
Vagus nerve stimulation
Initially approved for drug-resistant epilepsy, VNS relies on an implantable neurostimulator activating cervical vagus fibers via a pulse-delivering electrode [109].
After the promising findings of preclinical research and preliminary human studies [110,111,112], VNS was tested in large-scale clinical trials, namely, the CardioFit [113], the ANTHEM-HF [114], the NECTAR-HF [115], the INOVATE-HF [116], and the ANTHEM-HFpEF study [117]. Despite some improvement in qualitative endpoints (e.g., symptoms and quality of life), no significant benefits were observed in terms of cardiac remodeling, neurohormonal activation, hospitalizations, and mortality [118]. Moreover, many patients experienced some discomfort secondary to VNS, while the risk of either procedural or long-term device-related complications raised safety concerns [118].
Although technological reasons (e.g., delivered currents, stimulation frequency, duty cycles) may have contributed to such results, the clinical efficacy of VNS has been questioned and the ANTHEM-HFrEF, designed to test a new-generation device, has been stopped prematurely for financial reasons (NCT03425422).
Transcutaneous vagus nerve stimulation
A series of anatomical studies have shown that afferent fibers of the auricular branch of the vagus nerve may be found on the surface of the external ear [119]. The electrical stimulation of these fibers increased activity in central areas involved in autonomic control, including the ipsilateral NTS, dorsal raphe, locus coeruleus, contralateral parabrachial area, amygdala, and nucleus accumbens [119].
The cardiovascular consequences of tVNS have been studied almost exclusively in healthy individuals. While short-term tVNS increased HRV and BRS [120, 121], heterogeneous protocols and stimulation parameters contributed to the mixed findings reported by other works [122]. A single study showed that tVNS may reduce MSNA [123].
Based on the encouraging findings derived from preclinical studies of ischemic CHF models, in which tVNS improved cardiac remodeling and lowered arrhythmic risk [124, 125], tVNS was tested in patients with acute myocardial infarction, showing a reduction in ischemia–reperfusion injury [126]. Furthermore, in 50 patients with CHF and preserved LVEF, 1-h daily tVNS for 3 months improved left ventricular longitudinal strain and quality of life and reduced inflammatory markers [14], confirming and extending preclinical findings [127].
Further studies are ongoing to evaluate the effects of tVNS on various endpoints in CHF patients (Table 2).
Baroreflex activation therapy
Considering the pathophysiological and prognostic significance of decreased BRS in CHF, enhancing this reflex has a solid rationale for improving outcomes [12]. Indeed, the direct activation of the afferent arm of baroreflex could maximize cardiovascular benefits, limiting off-target effects. Baroreflex activation therapy (BAT) involves a subcutaneous pulse generator with an extravascular lead placed on the carotid sinus. Developed to treat resistant hypertension, BAT increases markers of vagal control and reduces MSNA, with anti-remodeling cardiac effects [128, 129].
The clinical efficacy of BAT was tested in two randomized, open-label clinical trials, enrolling patients with CHF and reduced LVEF symptomatic despite therapies. In the Barostim HOPE4HF study (n = 146), BAT improved the distance walked, quality of life, symptoms, and neurohormonal activation [130]. Similar findings were obtained in the BeAT-HF (n = 408), leading to the approval of the device for clinical use [131]. Considering these findings, the use of BAT in selected patients was acknowledged in CHF guidelines [132]. While the safety and efficacy of BAT on symptoms and quality of life were confirmed in the postmarket phase of the BeAT-HF (n = 323, median follow-up 3.6 years/patient), the risk of cardiovascular mortality and hospitalizations did not differ between BAT and control [133].
An alternative to BAT is the implantation of an endovascular device into the carotid sinus to amplify the normal pulse-driven activation of carotid baroreceptors [134]. Developed for hypertensive patients, the Mobius HD device was tested in a small study (HF-FIM) enrolling 29 CHF patients, improving the distance walked, quality of life, LVEF, and natriuretic peptides [134].
A similar approach, but targeting aortic baroreceptors, is under investigation (NCT02633644).
Spinal cord stimulation
The electrical stimulation of the dorsal spinal cord represents the most ancient bioelectronic neuromodulation strategy, first proposed for neuropathic pain and refractory angina [135]. Though the precise mechanisms remain unknown, this approach improved vagal tone and reduced SNS activity [135]. On the base of preclinical evidence [136, 137], spinal cord stimulation was tested in two trials enrolling patients with CHF and reduced LVEF. While it was effective on symptoms, quality of life, and cardiac remodeling in the SCS-HEART study (n = 22) [138], no significant benefits were observed in the larger (n = 66), randomized, and single-blind DEFEAT-HF study [139]. Differences in surgical approach and current delivery contributed to this discrepancy.
Non-pharmacological and non-bioelectronic approaches
Exercise training represents one of the most efficacious ways to improve sympathovagal balance (Table 3). In 17 patients with advanced CHF, an 8-week exercise training improved HRV and norepinephrine spillover [140]. Furthermore, in patients with a history of myocardial infarction, a 4-week training period increased BRS, lowering cardiac mortality during follow-up [141]. The improvement in sympathovagal balance hence represents a key mechanism behind the benefits provided by physical training in CHF patients.
Yoga and meditation have beneficial effects on autonomic cardiovascular control, too. The slow breathing that characterizes these practices reduced heart rate and increased HRV and BRS in healthy subjects [142], while a downward modulation of chemoreflex sensitivity was described as well [143].
A few studies have evaluated these approaches in patients with CHF [144]. Among 19 CHF patients, meditation (two 1-h training sessions, followed by 30-min b.i.d. sessions for 12 weeks) reduced norepinephrine levels and improved quality of life and ventilatory efficiency, but did not affect oxygen consumption and LVEF [145]. Similar benefits were also attributed to yoga [146] and tai chi [147].
Conclusions and perspectives
Despite therapeutic advances, the prognosis of CHF patients remains poor, with autonomic imbalance contributing to disease progression and life-threatening events. Implementing the current therapeutic armamentarium with novel neuromodulation strategies may prove valuable. Although enhancing vagal control holds promise, tailored pharmacological strategies remain underexplored, while technological uncertainties and conflicting findings have hampered the transition of bioelectronic devices into clinical scenarios.
Several issues should therefore be addressed [148]. A return to physiology seems mandatory to deepen our understanding of PSNS function in healthy and disease conditions. In this respect, direct recordings from the human vagus nerve offer a unique chance to optimize neuromodulation devices, shifting toward closed-loop protocols aligned with physiology to maximize benefits and minimize off-target effects. Battery duration, biocompatibility, and miniaturization are other obstacles to overcome.
In the meantime, abandoning the possibility of pharmacological PSNS stimulation, despite promising preliminary findings, appears a missed opportunity. Encouraging future studies to examine the efficacy of pirenzepine or scopolamine in CHF patients on modern treatments is hence warranted.
Finally, improving patient selection is paramount. Although neglected in the main trials conducted so far, assessing residual autonomic dysfunction, even by HRV and/or BRS, is crucial to identifying patients who could benefit from neuromodulation. Indeed, this is expected to maximize treatment effectiveness, limit biological and economic costs, and even decrease the number of patients needed for enrollment in clinical trials.
Abbreviations
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- ANS:
-
Autonomic nervous system
- BAT:
-
Baroreflex activation therapy
- BRS:
-
Baroreflex sensitivity
- CHF:
-
Chronic heart failure
- CNS:
-
Central nervous system
- HF:
-
High frequency
- HRV:
-
Heart rate variability
- ICNS:
-
Intrinsic cardiac nervous system
- LF:
-
Low frequency
- LVEF:
-
Left ventricular ejection fraction
- MSNA:
-
Muscle sympathetic nerve activity
- NTS:
-
Nucleus tractus solitarius
- PSNS:
-
Parasympathetic nervous system
- pNN50:
-
Percentage difference between adjacent NN intervals > 50 ms
- rMSSD:
-
Root mean square of successive RR interval
- SDNN:
-
Standard deviation of NN intervals
- SNS:
-
Sympathetic nervous system
- tVNS:
-
Transcutaneous VNS
- VLF:
-
Very low frequency
- VNS:
-
Vagus nerve stimulation
References
Hadaya J, Ardell JL (2020) Autonomic modulation for cardiovascular disease. Front Physiol 11:617459. https://doi.org/10.3389/fphys.2020.617459
Guzzetti S, Magatelli R, Borroni E, Mezzetti S (2001) Heart rate variability in chronic heart failure. Auton Neurosci Basic Clin 90:102–105. https://doi.org/10.1016/S1566-0702(01)00274-0
Dickerson LW, Rodak DJ, Fleming TJ et al (1998) Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J Auton Nerv Syst 70:129–141. https://doi.org/10.1016/s0165-1838(98)00048-4
Castiglione V, Gentile F, Ghionzoli N et al (2023) Pathophysiological rationale and clinical evidence for neurohormonal modulation in heart failure with preserved ejection fraction. Card Fail Rev 9:e09. https://doi.org/10.15420/cfr.2022.23
Palau P, Seller J, Domínguez E et al (2021) Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol 78:2042–2056. https://doi.org/10.1016/j.jacc.2021.08.073
Kaye DM, Nanayakkara S, Wang B et al (2022) Characterization of cardiac sympathetic nervous system and inflammatory activation in HFpEF patients. JACC Basic Transl Sci 7:116–127. https://doi.org/10.1016/j.jacbts.2021.11.007
Takeda R, Hissen SL, Akins JD et al (1979) (2024) Sympathetic neural control at rest and during the cold pressor test in patients with heart failure with preserved ejection fraction. Hypertens Dallas Tex 81:917–926. https://doi.org/10.1161/HYPERTENSIONAHA.123.21918
Kittipibul V, Fudim M (2022) Tackling inflammation in heart failure with preserved ejection fraction: resurrection of vagus nerve stimulation? J Am Heart Assoc 11:e024481. https://doi.org/10.1161/JAHA.121.024481
Giannoni A, Gentile F, Buoncristiani F et al (2022) Chemoreflex and baroreflex sensitivity hold a strong prognostic value in chronic heart failure. JACC Heart Fail 10:662–676. https://doi.org/10.1016/j.jchf.2022.02.006
van Bilsen M, Patel HC, Bauersachs J et al (2017) The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 19:1361–1378. https://doi.org/10.1002/ejhf.921
Roy A, Guatimosim S, Prado VF et al (2015) Cholinergic activity as a new target in diseases of the heart. Mol Med Camb Mass 20:527–537. https://doi.org/10.2119/molmed.2014.00125
Gentile F, Passino C, Emdin M, Giannoni A (2022) Baroreflex activation therapy in heart failure: targeting the right patient. Eur J Heart Fail 24:1674–1676. https://doi.org/10.1002/ejhf.2627
Dasari TW, Csipo T, Amil F et al (2021) Effects of low-level tragus stimulation on endothelial function in heart failure with reduced ejection fraction. J Card Fail 27:568–576. https://doi.org/10.1016/j.cardfail.2020.12.017
Stavrakis S, Elkholey K, Morris L et al (2022) Neuromodulation of inflammation to treat heart failure with preserved ejection fraction: a pilot randomized clinical trial. J Am Heart Assoc 11:e023582. https://doi.org/10.1161/JAHA.121.023582
Shivkumar K, Ajijola OA, Anand I et al (2016) Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 594:3911–3954. https://doi.org/10.1113/JP271870
Hopkins DA, Bieger D, deVente J, Steinbusch WM (1996) Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res 107:79–96. https://doi.org/10.1016/s0079-6123(08)61859-2
Plecha DM, Randall WC, Geis GS, Wurster RD (1988) Localization of vagal preganglionic somata controlling sinoatrial and atrioventricular nodes. Am J Physiol 255:R703-708. https://doi.org/10.1152/ajpregu.1988.255.5.R703
Ottaviani MM, Vallone F, Micera S, Recchia FA (2022) Closed-loop vagus nerve stimulation for the treatment of cardiovascular diseases: state of the art and future directions. Front Cardiovasc Med 9:866957. https://doi.org/10.3389/fcvm.2022.866957
Carlson MD, Geha AS, Hsu J et al (1992) Selective stimulation of parasympathetic nerve fibers to the human sinoatrial node. Circulation 85:1311–1317. https://doi.org/10.1161/01.cir.85.4.1311
Gatti PJ, Johnson TA, Massari VJ (1996) Can neurons in the nucleus ambiguus selectively regulate cardiac rate and atrio-ventricular conduction? J Auton Nerv Syst 57:123–127. https://doi.org/10.1016/0165-1838(95)00104-2
Zandstra TE, Notenboom RGE, Wink J et al (2021) Asymmetry and heterogeneity: part and parcel in cardiac autonomic innervation and function. Front Physiol 12:665298. https://doi.org/10.3389/fphys.2021.665298
Ardell JL, Andresen MC, Armour JA et al (2016) Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 594:3877–3909. https://doi.org/10.1113/JP271869
Hopkins DA, Armour JA (1989) Ganglionic distribution of afferent neurons innervating the canine heart and cardiopulmonary nerves. J Auton Nerv Syst 26:213–222. https://doi.org/10.1016/0165-1838(89)90170-7
Paintal AS (1953) A study of right and left atrial receptors. J Physiol 120:596–610. https://doi.org/10.1113/jphysiol.1953.sp004920
Campagna JA, Carter C (2003) Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology 98:1250–1260. https://doi.org/10.1097/00000542-200305000-00030
Lumbers ER, McCloskey DI, Potter EK (1979) Inhibition by angiotensin II of baroreceptor-evoked activity in cardiac vagal efferent nerves in the dog. J Physiol 294:69–80. https://doi.org/10.1113/jphysiol.1979.sp012915
Thayer JF, Loerbroks A, Sternberg EM (2011) Inflammation and cardiorespiratory control: the role of the vagus nerve. Respir Physiol Neurobiol 178:387–394. https://doi.org/10.1016/j.resp.2011.05.016
Bonaz B, Sinniger V, Pellissier S (2016) Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol 594:5781–5790. https://doi.org/10.1113/JP271539
Fedele L, Brand T (2020) The intrinsic cardiac nervous system and its role in cardiac pacemaking and conduction. J Cardiovasc Dev Dis 7:54. https://doi.org/10.3390/jcdd7040054
Fukuda K, Kanazawa H, Aizawa Y et al (2015) Cardiac innervation and sudden cardiac death. Circ Res 116:2005–2019. https://doi.org/10.1161/CIRCRESAHA.116.304679
Caulfield MP, Birdsall NJ (1998) International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279–290
Osterrieder W, Brum G, Hescheler J et al (1982) Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 298:576–578. https://doi.org/10.1038/298576a0
DiFrancesco D, Ducouret P, Robinson RB (1989) Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science 243:669–671. https://doi.org/10.1126/science.2916119
DiFrancesco D, Tromba C (1987) Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch 410:139–142. https://doi.org/10.1007/BF00581906
Logothetis DE, Kurachi Y, Galper J et al (1987) The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325:321–326. https://doi.org/10.1038/325321a0
Dhein S, van Koppen CJ, Brodde OE (2001) Muscarinic receptors in the mammalian heart. Pharmacol Res 44:161–182. https://doi.org/10.1006/phrs.2001.0835
Abramochkin DV, Tapilina SV, Sukhova GS et al (2012) Functional M3 cholinoreceptors are present in pacemaker and working myocardium of murine heart. Pflugers Arch 463:523–529. https://doi.org/10.1007/s00424-012-1075-1
Sharma VK, Colecraft HM, Wang DX et al (1996) Molecular and functional identification of m1 muscarinic acetylcholine receptors in rat ventricular myocytes. Circ Res 79:86–93. https://doi.org/10.1161/01.res.79.1.86
Lechner SG, Mayer M, Boehm S (2003) Activation of M1 muscarinic receptors triggers transmitter release from rat sympathetic neurons through an inhibition of M-type K+ channels. J Physiol 553:789–802. https://doi.org/10.1113/jphysiol.2003.052449
Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS (2008) Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 118:863–871. https://doi.org/10.1161/CIRCULATIONAHA.107.760405
Wang S, Han H, Pan Z et al (2012) Choline inhibits angiotensin II-induced cardiac hypertrophy by intracellular calcium signal and p38 MAPK pathway. Naunyn Schmiedebergs Arch Pharmacol 385:823–831. https://doi.org/10.1007/s00210-012-0740-4
Zhao L, Chen T, Hang P et al (2019) Choline attenuates cardiac fibrosis by inhibiting p38MAPK signaling possibly by acting on M3 muscarinic acetylcholine receptor. Front Pharmacol 10:1386. https://doi.org/10.3389/fphar.2019.01386
Pavlov VA, Wang H, Czura CJ et al (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9:125–134
Alen NV (2022) The cholinergic anti-inflammatory pathway in humans: state-of-the-art review and future directions. Neurosci Biobehav Rev 136:104622. https://doi.org/10.1016/j.neubiorev.2022.104622
Czura CJ, Tracey KJ (2005) Autonomic neural regulation of immunity. J Intern Med 257:156–166. https://doi.org/10.1111/j.1365-2796.2004.01442.x
Rocha-Resende C, da Silva AM, Prado MAM, Guatimosim S (2021) Protective and anti-inflammatory effects of acetylcholine in the heart. Am J Physiol Cell Physiol 320:C155–C161. https://doi.org/10.1152/ajpcell.00315.2020
Bozkurt B, Coats AJ, Tsutsui H et al (2021) Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail S1071–9164(21):00050–00056. https://doi.org/10.1016/j.cardfail.2021.01.022
Nakahara T, Kawada T, Sugimachi M et al (1998) Cholinesterase affects dynamic transduction properties from vagal stimulation to heart rate. Am J Physiol 275:R541-547. https://doi.org/10.1152/ajpregu.1998.275.2.R541
Urbancsek R, Forgács IN, Papp TB et al (2020) Theory and history of the study of muscle sympathetic nerve activity. Orv Hetil 161:1190–1199. https://doi.org/10.1556/650.2020.31780
Esler M, Lambert G, Brunner-La Rocca HP et al (2003) Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand 177:275–284. https://doi.org/10.1046/j.1365-201X.2003.01089.x
Wallin BG, Esler M, Dorward P et al (1992) Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol 453:45–58. https://doi.org/10.1113/jphysiol.1992.sp019217
Gerritsen J, TenVoorde BJ, Dekker JM et al (2003) Measures of cardiovascular autonomic nervous function: agreement, reproducibility, and reference values in middle age and elderly subjects. Diabetologia 46:330–338. https://doi.org/10.1007/s00125-003-1032-9
Ewing DJ, Martyn CN, Young RJ, Clarke BF (1985) The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8:491–498. https://doi.org/10.2337/diacare.8.5.491
Tjeerdsma G, Szabó BM, van Wijk LM et al (2001) Autonomic dysfunction in patients with mild heart failure and coronary artery disease and the effects of add-on beta-blockade. Eur J Heart Fail 3:33–39. https://doi.org/10.1016/s1388-9842(00)00119-7
Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065. https://doi.org/10.1161/01.CIR.93.5.1043
Sassi R, Cerutti S, Lombardi F et al (2015) Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol 17:1341–1353. https://doi.org/10.1093/europace/euv015
Kleiger RE, Stein PK, Bigger JT (2005) Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc 10:88–101. https://doi.org/10.1111/j.1542-474X.2005.10101.x
Bilchick KC, Berger RD (2006) Heart rate variability. J Cardiovasc Electrophysiol 17:691–694. https://doi.org/10.1111/j.1540-8167.2006.00501.x
Kleiger RE, Miller JP, Bigger JT, Moss AJ (1987) Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59:256–262. https://doi.org/10.1016/0002-9149(87)90795-8
Pola S, Macerata A, Emdin M, Marchesi C (1996) Estimation of the power spectral density in nonstationary cardiovascular time series: assessing the role of the time-frequency representations (TFR). IEEE Trans Biomed Eng 43:46–59. https://doi.org/10.1109/10.477700
Tiwari R, Kumar R, Malik S et al (2021) Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev 17:e160721189770. https://doi.org/10.2174/1573403X16999201231203854
Donald DE, Shepherd JT (1980) Autonomic regulation of the peripheral circulation. Annu Rev Physiol 42:429–439. https://doi.org/10.1146/annurev.ph.42.030180.002241
Milic M, Sun P, Liu F et al (2009) A comparison of pharmacologic and spontaneous baroreflex methods in aging and hypertension. J Hypertens 27:1243–1251. https://doi.org/10.1097/HJH.0b013e32832a6e1b
Smith JJ, Porth CM, Erickson M (1994) Hemodynamic response to the upright posture. J Clin Pharmacol 34:375–386. https://doi.org/10.1002/j.1552-4604.1994.tb04977.x
La Rovere MT, Bigger JT, Marcus FI et al (1998) Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet Lond Engl 351:478–484. https://doi.org/10.1016/s0140-6736(97)11144-8
La Rovere MT, Pinna GD, Maestri R et al (2009) Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol 53:193–199. https://doi.org/10.1016/j.jacc.2008.09.034
La Rovere MT, Pinna GD, Raczak G (2008) Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc 13:191–207. https://doi.org/10.1111/j.1542-474X.2008.00219.x
Bernardi L, De Barbieri G, Rosengård-Bärlund M et al (2010) New method to measure and improve consistency of baroreflex sensitivity values. Clin Auton Res Off J Clin Auton Res Soc 20:353–361. https://doi.org/10.1007/s10286-010-0079-1
Taylor JA, Halliwill JR, Brown TE et al (1995) “Non-hypotensive” hypovolaemia reduces ascending aortic dimensions in humans. J Physiol 483(Pt 1):289–298. https://doi.org/10.1113/jphysiol.1995.sp020585
Ottaviani MM, Wright L, Dawood T, Macefield VG (2020) In vivo recordings from the human vagus nerve using ultrasound-guided microneurography. J Physiol 598:3569–3576. https://doi.org/10.1113/JP280077
Patros M, Ottaviani MM, Wright L et al (2022) Quantification of cardiac and respiratory modulation of axonal activity in the human vagus nerve. J Physiol 600:3113–3126. https://doi.org/10.1113/JP282994
Packer M (1992) The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 20:248–254. https://doi.org/10.1016/0735-1097(92)90167-l
Floras JS, Ponikowski P (2015) The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 36:1974–1982b. https://doi.org/10.1093/eurheartj/ehv087
Arora R, Krummerman A, Vijayaraman P et al (2004) Heart rate variability and diastolic heart failure. Pacing Clin Electrophysiol PACE 27:299–303. https://doi.org/10.1111/j.1540-8159.2004.00431.x
Seravalle G, Quarti-Trevano F, Dell’Oro R et al (2019) Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens 37:443–448. https://doi.org/10.1097/HJH.0000000000001856
Badrov MB, Mak S, Floras JS (2021) Cardiovascular autonomic disturbances in heart failure with preserved ejection fraction. Can J Cardiol 37:609–620. https://doi.org/10.1016/j.cjca.2020.12.006
Guzzetti S, Cogliati C, Turiel M et al (1995) Sympathetic predominance followed by functional denervation in the progression of chronic heart failure. Eur Heart J 16:1100–1107. https://doi.org/10.1093/oxfordjournals.eurheartj.a061053
Ponikowski P, Chua TP, Amadi AA et al (1996) Detection and significance of a discrete very low frequency rhythm in RR interval variability in chronic congestive heart failure. Am J Cardiol 77:1320–1326. https://doi.org/10.1016/s0002-9149(96)00199-3
Galinier M, Pathak A, Fourcade J et al (2000) Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J 21:475–482. https://doi.org/10.1053/euhj.1999.1875
van de Borne P, Montano N, Pagani M et al (1997) Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation 95:1449–1454. https://doi.org/10.1161/01.cir.95.6.1449
Bristow MR, Ginsburg R, Minobe W et al (1982) Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211. https://doi.org/10.1056/NEJM198207223070401
Dunlap ME, Kinugawa T, Sica DA, Thames MD (2019) Cardiopulmonary baroreflex control of renal sympathetic nerve activity is impaired in dogs with left ventricular dysfunction. J Card Fail 25:819–827. https://doi.org/10.1016/j.cardfail.2019.08.012
Grassi G, Quarti-Trevano F, Esler MD (2021) Sympathetic activation in congestive heart failure: an updated overview. Heart Fail Rev 26:173–182. https://doi.org/10.1007/s10741-019-09901-2
Becker BK, Tian C, Zucker IH, Wang H-J (2016) Influence of brain-derived neurotrophic factor-tyrosine receptor kinase B signalling in the nucleus tractus solitarius on baroreflex sensitivity in rats with chronic heart failure. J Physiol 594:5711–5725. https://doi.org/10.1113/JP272318
Li Y-L, Zhang D, Tu H, Muelleman RL (2016) Altered ENaC is associated with aortic baroreceptor dysfunction in chronic heart failure. Am J Hypertens 29:582–589. https://doi.org/10.1093/ajh/hpv141
Tu H, Zhang D, Li Y-L (2019) Cellular and molecular mechanisms underlying arterial baroreceptor remodeling in cardiovascular diseases and diabetes. Neurosci Bull 35:98–112. https://doi.org/10.1007/s12264-018-0274-y
Caforio ALP, Mahon NJ, Tona F, McKenna WJ (2002) Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail 4:411–417. https://doi.org/10.1016/s1388-9842(02)00010-7
Liu HR, Zhao RR, Jiao XY et al (2002) Relationship of myocardial remodeling to the genesis of serum autoantibodies to cardiac beta(1)-adrenoceptors and muscarinic type 2 acetylcholine receptors in rats. J Am Coll Cardiol 39:1866–1873. https://doi.org/10.1016/s0735-1097(02)01865-x
Vatner DE, Sato N, Galper JB, Vatner SF (1996) Physiological and biochemical evidence for coordinate increases in muscarinic receptors and Gi during pacing-induced heart failure. Circulation 94:102–107. https://doi.org/10.1161/01.cir.94.1.102
Dunlap ME, Bibevski S, Rosenberry TL, Ernsberger P (2003) Mechanisms of altered vagal control in heart failure: influence of muscarinic receptors and acetylcholinesterase activity. Am J Physiol Heart Circ Physiol 285:H1632-1640. https://doi.org/10.1152/ajpheart.01051.2002
Bibevski S, Dunlap ME (2011) Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev 16:129–135. https://doi.org/10.1007/s10741-010-9190-6
Bibevski S, Dunlap ME (1999) Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation 99:2958–2963. https://doi.org/10.1161/01.cir.99.22.2958
Mortara A, La Rovere MT, Pinna GD et al (2000) Nonselective beta-adrenergic blocking agent, carvedilol, improves arterial baroreflex gain and heart rate variability in patients with stable chronic heart failure. J Am Coll Cardiol 36:1612–1618. https://doi.org/10.1016/s0735-1097(00)00900-1
Miller AJ, Arnold AC (2019) The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin Auton Res Off J Clin Auton Res Soc 29:231–243. https://doi.org/10.1007/s10286-018-0572-5
Abstract 13303: snfluence of ARNI on cardiac autonomic nervous system | Circulation. https://www.ahajournals.org/doi/10.1161/circ.146.suppl_1.13303. Accessed 22 Jan 2024
Balcıoğlu AS, Çelik E, Şahin M et al (2022) Dapagliflozin improves cardiac autonomic function measures in type 2 diabetic patients with cardiac autonomic neuropathy. Anatol J Cardiol 26:832–840. https://doi.org/10.5152/AnatolJCardiol.2022.1934
Khuanjing T, Palee S, Chattipakorn SC, Chattipakorn N (2020) The effects of acetylcholinesterase inhibitors on the heart in acute myocardial infarction and heart failure: From cells to patient reports. Acta Physiol Oxf Engl 228:e13396. https://doi.org/10.1111/apha.13396
Androne AS, Hryniewicz K, Goldsmith R et al (2003) Acetylcholinesterase inhibition with pyridostigmine improves heart rate recovery after maximal exercise in patients with chronic heart failure. Heart Br Card Soc 89:854–858. https://doi.org/10.1136/heart.89.8.854
Serra SM, Costa RV, Teixeira De Castro RR et al (2009) Cholinergic stimulation improves autonomic and hemodynamic profile during dynamic exercise in patients with heart failure. J Card Fail 15:124–129. https://doi.org/10.1016/j.cardfail.2008.10.018
Behling A, Moraes RS, Rohde LE et al (2003) Cholinergic stimulation with pyridostigmine reduces ventricular arrhythmia and enhances heart rate variability in heart failure. Am Heart J 146:494–500. https://doi.org/10.1016/S0002-8703(03)00319-3
Villacorta AS, Villacorta H, Caldas JA et al (2019) Effects of heart rate reduction with either pyridostigmine or ivabradine in patients with heart failure: a randomized, double-blind study. J Cardiovasc Pharmacol Ther 24:139–145. https://doi.org/10.1177/1074248418799364
Hsieh M-J, Chen D-Y, Lee C-H et al (2022) Association between cholinesterase inhibitors and new-onset heart failure in patients with Alzheimer’s disease: a nationwide propensity score matching study. Front Cardiovasc Med 9:831730. https://doi.org/10.3389/fcvm.2022.831730
Rampa L, Santangelo R, Gaspardone C et al (2023) Potential cardiologic protective effects of acetylcholinesterase inhibitors in patients with mild to moderate dementia. Am J Cardiol 200:162–170. https://doi.org/10.1016/j.amjcard.2023.05.041
Hayano T, Shimizu A, Ikeda Y et al (1999) Paradoxical effects of pirenzepine on parasympathetic activity in chronic heart failure and control. Int J Cardiol 68:47–56. https://doi.org/10.1016/s0167-5273(98)00335-0
Venkatesh G, Fallen EL, Kamath MV et al (1996) Double blind placebo controlled trial of short term transdermal scopolamine on heart rate variability in patients with chronic heart failure. Heart Br Card Soc 76:137–143. https://doi.org/10.1136/hrt.76.2.137
La Rovere MT, Mortara A, Pantaleo P et al (1994) Scopolamine improves autonomic balance in advanced congestive heart failure. Circulation 90:838–843. https://doi.org/10.1161/01.cir.90.2.838
Casadei B, Conway J, Forfar C, Sleight P (1996) Effect of low doses of scopolamine on RR interval variability, baroreflex sensitivity, and exercise performance in patients with chronic heart failure. Heart Br Card Soc 75:274–280. https://doi.org/10.1136/hrt.75.3.274
Pedretti RF, Colombo E, Sarzi Braga S et al (1995) Effects of oral pirenzepine on heart rate variability and baroreceptor reflex sensitivity after acute myocardial infarction. J Am Coll Cardiol 25:915–921. https://doi.org/10.1016/0735-1097(94)00468-6
Schwartz PJ, De Ferrari GM, Sanzo A et al (2008) Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail 10:884–891. https://doi.org/10.1016/j.ejheart.2008.07.016
Chen M, Zhou X, Yu L et al (2016) Low-level vagus nerve stimulation attenuates myocardial ischemic reperfusion injury by antioxidative stress and antiapoptosis reactions in canines. J Cardiovasc Electrophysiol 27:224–231. https://doi.org/10.1111/jce.12850
De Ferrari GM (2014) Vagal stimulation in heart failure. J Cardiovasc Transl Res 7:310–320. https://doi.org/10.1007/s12265-014-9540-1
Chen M, Yu L, Zhou X et al (2015) Low-level vagus nerve stimulation: an important therapeutic option for atrial fibrillation treatment via modulating cardiac autonomic tone. Int J Cardiol 199:437–438. https://doi.org/10.1016/j.ijcard.2015.07.083
De Ferrari GM, Crijns HJGM, Borggrefe M et al (2011) Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32:847–855. https://doi.org/10.1093/eurheartj/ehq391
Premchand RK, Sharma K, Mittal S et al (2014) Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 20:808–816. https://doi.org/10.1016/j.cardfail.2014.08.009
Zannad F, De Ferrari GM, Tuinenburg AE et al (2015) Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J 36:425–433. https://doi.org/10.1093/eurheartj/ehu345
Gold MR, Van Veldhuisen DJ, Hauptman PJ et al (2016) Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol 68:149–158. https://doi.org/10.1016/j.jacc.2016.03.525
Kumar HU, Nearing BD, Mittal S et al (2023) Autonomic regulation therapy in chronic heart failure with preserved/mildly reduced ejection fraction: ANTHEM-HFpEF study results. Int J Cardiol 381:37–44. https://doi.org/10.1016/j.ijcard.2023.03.030
Zafeiropoulos S, Ahmed U, Bikou A et al (2023) Vagus nerve stimulation for cardiovascular diseases: is there light at the end of the tunnel? Trends Cardiovasc Med S1050–1738(23):00064–00066. https://doi.org/10.1016/j.tcm.2023.07.003
Butt MF, Albusoda A, Farmer AD, Aziz Q (2020) The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat 236:588–611. https://doi.org/10.1111/joa.13122
Antonino D, Teixeira AL, Maia-Lopes PM et al (2017) Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimulat 10:875–881. https://doi.org/10.1016/j.brs.2017.05.006
Bretherton B, Atkinson L, Murray A et al (2019) Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging 11:4836–4857. https://doi.org/10.18632/aging.102074
Soltani D, Azizi B, Sima S et al (2023) A systematic review of the effects of transcutaneous auricular vagus nerve stimulation on baroreflex sensitivity and heart rate variability in healthy subjects. Clin Auton Res Off J Clin Auton Res Soc 33:165–189. https://doi.org/10.1007/s10286-023-00938-w
Clancy JA, Mary DA, Witte KK et al (2014) Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimulat 7:871–877. https://doi.org/10.1016/j.brs.2014.07.031
Wang Z, Yu L, Wang S et al (2014) Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail 7:1014–1021. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001564
Yu L, Wang S, Zhou X et al (2016) Chronic intermittent low-level stimulation of tragus reduces cardiac autonomic remodeling and ventricular arrhythmia inducibility in a post-infarction canine model. JACC Clin Electrophysiol 2:330–339. https://doi.org/10.1016/j.jacep.2015.11.006
Yu L, Huang B, Po SS et al (2017) Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC Cardiovasc Interv 10:1511–1520. https://doi.org/10.1016/j.jcin.2017.04.036
Elkholey K, Niewiadomska M, Morris L et al (2022) Transcutaneous vagus nerve stimulation ameliorates the phenotype of heart failure with preserved ejection fraction through its anti-inflammatory effects. Circ Heart Fail 15:e009288. https://doi.org/10.1161/CIRCHEARTFAILURE.122.009288
Schmidli J, von Allmen RS (1946) Mohaupt MG (2014) Electrical carotid baroreceptor stimulation. Wien Med Wochenschr 164:508–514. https://doi.org/10.1007/s10354-014-0329-2
Babar N, Giedrimiene D (2022) Updates on baroreflex activation therapy and vagus nerve stimulation for treatment of heart failure with reduced ejection fraction. Cardiol Res 13:11–17. https://doi.org/10.14740/cr1330
Abraham WT, Zile MR, Weaver FA et al (2015) Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail 3:487–496. https://doi.org/10.1016/j.jchf.2015.02.006
Zile MR, Abraham WT, Weaver FA et al (2015) Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction: safety and efficacy in patients with and without cardiac resynchronization therapy. Eur J Heart Fail 17:1066–1074. https://doi.org/10.1002/ejhf.299
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Zile MR, Lindenfeld J, Weaver FA et al (2024) Baroreflex activation therapy in patients with heart failure and a reduced ejection fraction: long-term outcomes. Eur J Heart Fail. https://doi.org/10.1002/ejhf.3232
Piayda K, Sievert K, Sievert H et al (2022) Endovascular baroreflex amplification with the MobiusHD device in patients with heart failure and reduced ejection fraction: interim analysis of the first-in-human results. Struct Heart J Heart Team 6:100086. https://doi.org/10.1016/j.shj.2022.100086
Upadhyay GA, Singh JP (2016) Spinal cord stimulation for heart failure in the DEFEAT-HF study: lost battle or lasting opportunities? JACC Heart Fail 4:137–139. https://doi.org/10.1016/j.jchf.2015.11.007
Issa ZF, Zhou X, Ujhelyi MR et al (2005) Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation 111:3217–3220. https://doi.org/10.1161/CIRCULATIONAHA.104.507897
Liu Y, Yue W-S, Liao S-Y et al (2012) Thoracic spinal cord stimulation improves cardiac contractile function and myocardial oxygen consumption in a porcine model of ischemic heart failure. J Cardiovasc Electrophysiol 23:534–540. https://doi.org/10.1111/j.1540-8167.2011.02230.x
From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B et al (2018) Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke Off J Int Stroke Soc 13:612–632. https://doi.org/10.1177/1747493018778713
Zipes DP, Neuzil P, Theres H et al (2016) Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: the DEFEAT-HF study. JACC Heart Fail 4:129–136. https://doi.org/10.1016/j.jchf.2015.10.006
Coats AJ, Adamopoulos S, Radaelli A et al (1992) Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 85:2119–2131. https://doi.org/10.1161/01.cir.85.6.2119
La Rovere MT, Bersano C, Gnemmi M et al (2002) Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106:945–949. https://doi.org/10.1161/01.cir.0000027565.12764.e1
Anasuya B, Deepak KK, Jaryal AK, Narang R (2020) Effect of slow breathing on autonomic tone & baroreflex sensitivity in yoga practitioners. Indian J Med Res 152:638–647. https://doi.org/10.4103/ijmr.IJMR_559_19
Spicuzza L, Gabutti A, Porta C et al (2000) Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet Lond Engl 356:1495–1496. https://doi.org/10.1016/S0140-6736(00)02881-6
Viveiros J, Chamberlain B, O’Hare A, Sethares KA (2019) Meditation interventions among heart failure patients: an integrative review. Eur J Cardiovasc Nurs 18:720–728. https://doi.org/10.1177/1474515119863181
Curiati JA, Bocchi E, Freire JO et al (2005) Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med N Y N 11:465–472. https://doi.org/10.1089/acm.2005.11.465
Krishna BH, Pal P, G K P, et al (2014) Effect of yoga therapy on heart rate, blood pressure and cardiac autonomic function in heart failure. J Clin Diagn Res JCDR 8:14–16. https://doi.org/10.7860/JCDR/2014/7844.3983
Gu Q, Wu S-J, Zheng Y et al (2017) Tai chi exercise for patients with chronic heart failure: a meta-analysis of randomized controlled trials. Am J Phys Med Rehabil 96:706–716. https://doi.org/10.1097/PHM.0000000000000723
Giannoni A, Gentile F, Passino C (2022) Bioelectronic medicine and its applications in cardiology. Eur Heart J 43:4453–4455. https://doi.org/10.1093/eurheartj/ehac343
Acknowledgements
Figure 4 has been created with BioRender.com.
Funding
Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Francesco Gentile: conceptualization (lead) and writing original draft (lead); Giulia Orlando: writing original draft (lead); Sabrina Montuoro: writing original draft (supporting); Yu Fu Ferrari Chen: writing original draft (supporting); Vaughan Macefield: review and editing (equal); Claudio Passino: review and editing (equal); Alberto Giannoni: review and editing (equal); Michele Emdin: conceptualization (lead), review, and editing (lead).
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gentile, F., Orlando, G., Montuoro, S. et al. Treating heart failure by targeting the vagus nerve. Heart Fail Rev (2024). https://doi.org/10.1007/s10741-024-10430-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s10741-024-10430-w