Abstract
Exercise offers many physical and health benefits to people with heart failure (CHF), but aerobic training (AT) predominates published literature. Resistance training (RT) provides additional and complementary health benefits to AT in people with CHF; we aimed to elucidate specific health benefits accrued, the mechanism of effect and safety of RT. We conducted a systematic search for RT randomised, controlled trials in people with CHF, up until August 30, 2023. RT offers several benefits including improved physical function (peak VO2 and 6MWD), quality of life, cardiac systolic and diastolic function, endothelial blood vessel function, muscle strength, anti-inflammatory muscle markers, appetite and serious event rates. RT is beneficial and improves peak VO2 and 6MWD, partly restores normal muscle fibre profile and decreases inflammation. In turn this leads to a reduced risk or impact of sarcopenia/cachexia via effect on appetite. The positive impact on quality of life and performance of activities of daily living is related to improved function, which in turn improves prognosis. RT appears to be safe with only one serious event reported and no deaths. Nevertheless, few events reported to date limit robust analysis. RT appears to be safe and offers health benefits to people with CHF. RT modifies the adverse muscle phenotype profile present in people with CHF and it appears safe. Starting slowly with RT and increasing load to 80% of 1 repetition maximum (RM) appears to offer optimal benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most people with congestive heart failure (CHF) are severely de-conditioned so any exercise training delivery is likely to accrue benefits in both physical and mental health [1]. Resistance training (RT) offers many advantages to people with CHF, perhaps the most notable is restoration of muscle strength due to changes in fibre type and function. Although aerobic training is often the cornerstone of exercise rehabilitation in people with CHF, this form of training may not be optimal for altering muscle fibre deterioration in older people or those with CHF. As we age, RT is required to prevent a shift towards type II muscle fibre size decreases, even in healthy people [2]. This type II muscle fibre shift is even more pronounced in people with CHF, but in these patients there is also a shift towards reductions in the number and size of type I fibres [2]. These muscle fibre type changes may be driven by inflammatory mediators detected in the muscle but not systemic circulation [3].
Between 2007 and 2016, there may have been hesitancy around the use of RT in people with CHF especially in those with very low left ventricular ejection fractions (LVEF%). This may be explained by Haykowsky’s 2007 meta-analysis in people with CHF that suggested whilst AT was beneficial for LVEF, RT may lead to a reduction in LVEF of around 4.5% [4]. There were, however some limitations to this finding, namely it was based upon one study [5] that pre-dated modern imaging techniques and the study size was only 25 participants. In 2016, an updated meta-analysis, which included three new RT studies, abrogated these concerns [6]. Both fibre type changes and reduced LVEF% may contribute to lower peak VO2 in people with CHF. Peak VO2 remains strongly prognostic in people with CHF [7].
In 2021, we updated our 2016 meta-analysis and presented novel findings suggesting RT raised both peak VO2 and six-minute walk distance (6MWD) independently of LVEF% change [8]. Whilst the number of randomised, controlled studies of RT is relatively small compared to AT, there is evidence of cardiovascular benefit in people with CHF. There also exists, in the published literature, evidence that RT also provides benefits in terms of quality of life (QoL) [1], non-cardiac muscle strength [9], endothelial function [10], heart rate variability [11], insulin sensitivity [12], sleep quality [13] and serum BNP [14, 15]. These findings give rise to the options that RT remains perhaps under-utilised in people with CHF and the mechanisms of benefit are not well understood, although peak VO2 has been shown to improve prognosis [7]. Further comprehensive safety data for the use of RT in people with CHF are yet to be published.
This work aimed to provide a systematic review of the benefits and safety from published randomised, controlled trials of RT in people with CHF. Further, we also considered data from non-randomised controlled trials to identify mechanisms of effect from RT in people with CHF.
Methods
Search strategy
We began with our study by Fisher et al. which was a 2022 meta-analysis of 17 randomised, controlled trials (RCTs) on resistance training (RT) in people with heart failure [8]. Our 2022 work was a pooled analysis of 13 RCTs compared RT to sedentary control, 2 RCTs compared RT to aerobic training (AT) and 2 duplicate publications that provided some additional data beyond the primary publication.
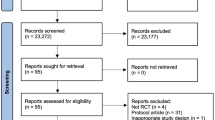
We updated the literature search August 2023. No new RCTs of RT in people with CHF were found, but several mechanistic papers proved useful and formed part of this body of work. We conducted the systematic literature search in PubMed, Web of Science and the Cochrane Library of Controlled Trials up until 30 September 2023. The search strategy included key words related to congestive heart failure and exercise training and related MeSH terms. This was supplemented by manually searching reference lists from systematic reviews and eligible studies for additional works. The strategy for database searches is documented here [8]. This search strategy was repeated for the additional data bases used: Web of Science and Cochrane Library of Controlled Trials.
Inclusion and exclusion criteria
Two authors (RS and NAS) independently assessed all identified articles for eligibility and joint consultation was used to resolve any disagreement.
Included studies
We included RCTs conducted on adult humans (over 18 years) that reported change in listed outcomes after exercise training. RT studies were considered provided the intervention was for a minimum of 3 weeks duration. Crossover studies were only excluded if the washout period was less than 2 weeks. There was no language restriction. We also drew upon comparative data on AT from several other reviews and meta-analyses [11, 16,17,18,19,20,21].
Excluded studies
Identified studies not reporting any of the required outcomes were excluded. Studies with participants with any medical condition that impaired RT participation were excluded.
Comparisons
Included studies compared RT intervention group(s) to a non-exposed, matched health status, control (usual care) group or a sham intervention group.
Outcome measures
The primary outcome measures were change in peak VO2, 6MWD, muscle fibre type, area and sphericity, cardiac systolic and diastolic function, forearm blood flow, endothelial function, heart rate variability, systemic or intramuscular inflammation, quality of life, energy intake and appetite, adverse events and safety of RT.
Data extraction
From each included study, we extracted the first author’s name, year of publication, country, study design, type of study population and participants’ baseline characteristics (including age, gender, number of participants and resting BP). In addition, the characteristics of training interventions (i.e. exercise program delivery venue and method, type of exercise, intensity, duration and frequency of the protocol) and the mean change and standard deviation of the desired outcome variables were recorded. Data extraction was conducted independently by two authors (RS and NAS) using a predesigned Excel data extraction sheet.
Statistical analyses
Data sets were organised and descriptive analyses performed using Excel 2016 for all included studies. As meta-analyses were completed for our previous works [6, 8], we calculated weighted means and standard deviations in STATA V.18 [22]. Weighted means were used for most outcome measures. We also estimated the minimal clinically important difference (MCID) for some outcomes using the change in standard deviation divided by 2 [23].
Risk of bias
Study quality and reporting for each study was evaluated utilising the TESTEX tool [24].
Results
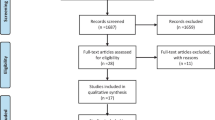
Our search found the original 17 randomised, controlled trials from our earlier publication and one new trial. Of the 18 trials, 14 compared RT to control, 2 compared RT to AT and 2 were duplicate publications (see Table 1). We also considered other types of papers to provide comparative data of other types of exercise (i.e. aerobic) so that RT could be benchmarked against other types of exercise for outcomes such as safety. We have listed sources of comparative data in the relevant sections below.
Physical fitness
We compared (see Fig. 1) the percentage change in peak VO2 between RT and AT at various intensities, and also sedentary control, using comparative data from Ismail et al. [38]. We found RT produced a similar peak VO2 change of 25.5 ± 1.65 ml·kg·min−1 to that previously observed with aerobic exercise at high intensity which was significantly greater than peak VO2 changes reported following vigorous, moderate or low intensity [38]. Non-exercising control participants’ peak VO2 decreased by 7%. We found a 9.5 ± 4.8% improvement in 6-min walk distance following RT, although control participants improved 5.5% (see Fig. 2). Further, we found the changes in both peak VO2 to be above the minimal clinically important difference (MCID) of the SD (1.65 ml·kg·min−1/2) at 0.825 ml/kg·min−1. We calculated the MCID or 6MWD to be 39 m, which is almost identical to previous work that demonstrated that a 6MWD increase of around 40 m is the MCID threshold for change in people with CHF [39].
Cardiac function
Pooled data from 7 studies showed systolic function, measured by LVEF, improved with RT by 24.8 ± 10.9% whilst non-exercising control participants with CHF improved by 6.1 ± 4.3% (see Fig. 3).
Data from one study suggested diastolic function, as measured by E/e’, decreased and thereby improved by − 18.8 ± 7.7% and by − 11.8 ± 6.5% in non-exercising control participants with CHF (see Fig. 4). Improved cardiac function is closely related to lower left atrial volume index and prevention of raised LV mass index, both indices are strong predictors of cardiac events [18].
Quality of life
Only two studies reported change in Minnesota living with heart failure questionnaire scores (MLWHFQ) in exercise versus control groups. The MLWHFQ is a measure of quality of life, which was improved (lower score) by almost 8.75 points (30%) in people who undertook RT. In contrast, there was a small reduction (higher score) in quality of life scores in the control group (see Fig. 5).
RT and inflammatory muscle markers of CHF
Work by Larsen showed significantly greater area of type I fibres in healthy people versus those with CHF, whilst type IIA fibres were more common in CHF. Thickness of type IIA and IIB was significantly greater in healthy people versus those with CHF [2]. Larsen also reported greater sphericity of type 2A muscle fibres in people with CHF versus healthy [2]. Further, two studies Gielen [3] and Larsen [2] showed that intramuscular levels of TNF-alpha were significantly higher in people with CHF versus those who are healthy. Larsen further showed that IL-1, IL-6 and the inducible form of nitric oxide synthase mRNA were significantly higher in people with CHF versus healthy [2]. Skeletal muscle IGF-1 levels are also increased with exercise to induce protein synthesis [3]. Exercise has anti-inflammatory/antioxidant effects and likely combats cachexia [19, 40].
Blood vessel function and heart rate variability
Previous work has identified that exercise has a systemic impact on remodelling of conduit arteries in humans and that RT may be advantageous in subjects with chronic heart failure in this regard [17]. Further, both forearm blood flow [17] and heart rate variability are improved in people with heart failure following RT, with evidenced by a reduced ratio low- to high-frequency spectral power [11].
Appetite, energy expenditure and cardiac cachexia
Andreae showed daily step counts were positively associated with better appetite in people with CHF which may abrogate adverse prognosis due to cardiac cachexia [20]. After 18-month follow-up, Andreae showed that people with CHF who were more active retained their physical function. The obesity paradox [41] explains the lower mortality rates observed in obese/overweight people with CHF due to cardiac cachexia. Both aerobic and RT exercise have been previously shown to improve appetite and energy intake in people with chronic kidney disease [21].
Safety of RT in CHF
Based upon the included studies of this review, no deaths during RT have been reported in people with CHF in over 8000 patient-hours of exercise. The only event of note is a single episode of ventricular arrhythmia reported in one study in a person undertaking RT [3].
All that can be concluded from existing data is that limited safety data currently exists for RT in CHF as most studies are small and < 12 weeks. Currently there exists an insufficient number of RT related events that precludes a meaningful statistical analysis. We conducted a power calculation that indicated around 200 events (deaths) would be required to detect a statistically significant difference for safety in RT versus control groups.
Other germane observations around event data collection are that there is clearly heterogenous and irregular event data collection. Some studies only report events that occur during RT exercise sessions; other studies collect and report event data during entire study duration period when people may be sleeping or resting. Another observation to make is that the terminology and definitions vary by study, for example the threshold for what constitutes a serious event is not standardised.
We cannot be unequivocally certain if RT is safe or unsafe without more event data, but no published evidence of serious events to date suggests RT is safe. In comparison, AT has an event rate of 1 in 3300 patient-hours for CHF [42]. The event rate for coronary artery disease is 1 in 62,000 patient-hours of AT and in a healthy population the event rate is 1 in 600,000 [43] (see Fig. 6). The literature on RT and CHF currently has data from 15 randomised, controlled trials (RCTs) which is relatively scarce compared to the 150 RCTs on AT and CHF.
Markers of event risk
During RT the rate pressure product (RPP) is possibly around 5% lower than in AT at moderate [44] intensity (55–70% age-predicted max HR or 40–60% peak VO2) and the intermittent nature of RT likely to reduce event risk. If one approximates heart rate (HR) and systolic blood pressure (SBP) during AT and RT as follows:
Aerobic Training: HR 130 beats·min−1 × (peak) SBP 160 mmHg = RPP 20,800.
Resistance Training: HR 110 × (peak) SBP 160 = RPP 17,600.
The slightly lower RPP during RT is likely to translate into a safer exercise modality than AT, especially when one compares the sustained HR and BP levels with the shorter peaks observed with RT. Additional evidence of cardiovascular event risk benefit lies with the improved heart rate variability (HRV) reported following a program of RT with the ratio of low-to-high-frequency spectral power reduced by 44% [11].
Study quality
Table 2 shows study quality and reporting for each study utilising the TESTEX tool.
The median TESTEX score was 11 out of 15 which previous work indicates overall good study quality [46]. Allocation concealment, intention to treat (ITT) analysis and physical activity monitoring in the control group were the only TESTEX criteria performed on less than 50% of the 24 included studies. Allocation concealment is very difficult in exercise studies and ITT is only relevant if > 15% of participants withdraw prematurely from the study.
Risk of bias
The overall risk of bias was low as median TESTEX score indicated overall good study quality. One study scores 5, but this relatively low score was attributable to the study being published in Chinese and an English version would have certainly yielded a higher score. One study scored 8 and three scored 9; the other 19 studies scored 10 or above, confirming generally low risk of bias.
Discussion
The need for RT for people with CHF
People with CHF are severely deconditioned [47], so there is large potential for improved peak VO2. Intuitively any form of exercise training is likely to benefit peak VO2. In contrast, in a person with CHF no regular exercise training or physical activity will lead to further deterioration in peak VO2 and this is often linked with sarcopenia and cachexia [15, 40]. As RT and AT appear to provide benefit via some shared, and some different, physiological pathways, a combination of RT and AT is likely to be optimal [48]. The fibre shift observed in people with CHF will, intuitively, require new type II fibres to possess oxidative capacity [49]. In turn this suggests a combined RT and AT training prescription is optimal.
RT exercise prescription recommendations
Previous and current general RT prescription recommendations for CHF
Meyer [50] previously stated that dynamic RT exercise is well tolerated in chronic stable CHF if:
-
1.
Initial contraction intensity is low.
-
2.
Small muscle groups are involved.
-
3.
Work phases are kept short.
-
4.
Small number of repetitions per set is performed.
-
5.
Work/rest ratio is > or = 1:2.
Meyer also suggested that with tolerance, contraction intensity can be increased [50]. Meyer proposed that following 12 weeks of RT, maximal strength could be improved by 15 to 50% [50]. Recent work by Paluch et al. [13] made recommendations for resistance training prescription for high-risk cardiovascular disease patients which included:
-
1.
Intensity = <40% of 1RM
-
2.
Number of repetitions = 15–20
-
3.
Weekly frequency = ≥2 days per week
-
4.
Planned rest days between sessions
-
5.
Muscle adaptation = endurance
-
6.
Resistance training modes = bodyweight, bands, machines and free weights
There appears to be some discrepancy between Meyer’s recommendations and those by Paluch and others. Meyer argued for a small number of repetitions to be performed, whereas Paluch et al. argued for 15–20 repetitions.
Most CHF RT studies have used less than 80% of 1RM which produced a standardised mean difference (SMD) of 0.76 [8]. Yet in 2005 Levinger et al. used a training intensity of 80% of 1RM or greater and their results produced an SMD of 1.07 [9], although overlap in measures of variance mean these SMDs were not significantly different. This finding raises a question about the current intensity guidelines and if they are too conservative? Could better functional outcomes be gained for CHF patients if they were provided with a periodised program that guided them towards using higher percentages of 1RM.
A recent systematic review and network meta-analysis by Chen and others, asked if ‘moderate resistance training is adequate for older adults with sarcopenia?’ [51] as sarcopenia is a common comorbidity for people with CHF [52]. Chen et al. stratified training intensity into three categories via the use of a description (light to moderate, moderate and moderate to vigorous), percentage of 1RM ranges (< 49%, 50–69% and 70–84%) and RPE ranges (using both a modified Borg scale of 1–10 and a traditional Borg scale of 6–20) [51]. This review concluded that moderate to vigorous (70–84% of 1RM, modified Borg RPE 7–8 or traditional Borg 14–17) intensity resistance training yielded greater muscle mass, lower extremity strength and physical performance than less intense training options [51]. The finding that higher intensity resistance training offers greater benefits to people with CHF [9] and concurrent sarcopenia [51] perhaps warrants changes to the current recommendations. We would perhaps add that RT should be complemented by AT as newly developed muscle fibres will require adequate O2 delivery. Further, as people with HF and preserved ejection fraction (HFpEF) tend to be older and or frail than those with reduced ejection fraction (HFrEF), we recommend early RT in those with HFpEF [47].
Specific RT considerations
Consideration needs to be given to program design with particular focus on training intensity and its classification. A patient’s understanding of training intensity is very important as training at the wrong intensity may lead to poor outcomes and excessive fatigue. Yet there are strategies available to increase a patient’s understanding of training intensity and mitigate the fatigue associated with resistance training whilst still reaping the benefits of increasing muscular strength. Often in people with chronic disease, a submaximal or non-failure resistance training protocol could be employed to reduce the fatigue associated with training to repetition failure, as non-failure training has been shown to be similarly effective for increasing strength as training to repetition failure [53] whilst minimising resistance training related fatigue and lowering training session intensity. To help patients understand different training intensities, a rate of perceived exertion (RPE) scale can be used, such as the OMNI-Resistance Exercise Scale [54,55,56]. However, another RPE scale, which has also been designed for resistance training, allows for patients to understand training intensities and non-failure training as the scale incorporates repetitions in reserve (RIR) [57]. The use of RIR can allow for individuals to autoregulate training intensity [57, 58]. RIR provides an overview of the Resistance Exercise-Specific RPE Scale and its relationship with RIR. For example, an RPE score on this scale of 8 out of 10 would indicate that 2 more repetitions could be completed at the end of a set (Table 3).
Resistance Exercise-Specific RPE Scale designed by Zourdos et al. [57].
An RPE scale incorporating RIR and therefore a non-failure protocol allows for the overall intensity of the session to be adjusted or autoregulated by the patient. For instance, a patient could be programmed to complete a set on the leg press machine at 70% of 1RM with 2–5 repetitions in reserve allowing for autoregulation by the client.
Some clinical exercise physiologists may prefer to avoid 1RM testing as they believe it elevates injury risk. Yet, there is a large amount of evidence supporting the use and safety of 1RM testing in healthy populations [59], with emerging evidence of its reliability and safety for use with CHF patients [60]. However, 1RM testing is not necessary as intensity can be determined via the use of RPE scales [51, 54].
Conclusions
RT is beneficial and improves peak VO2 and partly restores normal muscle fibre profile and decreases inflammation. RT at higher percentages of 1RM such as ≥ 80% appears to provide optimal improvements in strength and function and in turn this leads to a reduced risk or impact of sarcopenia/cachexia via effect on appetite. The positive impact on activities of daily living is related to improved peak VO2 which in turn improves prognosis. RT appears to be safe as there has been only one serious event reported and rate pressure product (RPP) during RT is lower than for aerobic exercise. Only one event has been reported in > 8000 patient-hours to date.
Data Availability
The data was a pooled analysis of studies that are publicly available so anyone can extract this data of their own accord.
References
Smart NA, King N, Lambert JD, Pearson MJ, Campbell JL, Risom SS et al (2018) Exercise-based cardiac rehabilitation improves exercise capacity and health-related quality of life in people with atrial fibrillation: a systematic review and meta-analysis of randomised and non-randomised trials. Open Heart 5(2):e000880
Larsen AI, Lindal S, Aukrust P, Toft I, Aarsland T, Dickstein K (2002) Effect of exercise training on skeletal muscle fibre characteristics in men with chronic heart failure. Correlation between skeletal muscle alterations, cytokines and exercise capacity. Int J Cardiol 83(1):25–32
Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J et al (2003) Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42(5):861–868
Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM (2007) A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol 49(24):2329–2336
Koch M, Douard H, Broustet JP (1992) The benefit of graded physical exercise in chronic heart failure. Chest 101(5 Suppl):231S-S235
Jewiss D, Ostman C, Smart NA (2016) The effect of resistance training on clinical outcomes in heart failure: a systematic review and meta-analysis. Int J Cardiol 221:674–681
Sarullo FM, Fazio G, Brusca I, Fasullo S, Paterna S, Licata P et al (2010) Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J 4:127–134
Fisher S, Smart NA, Pearson MJ (2022) Resistance training in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 27(5):1665–1682
Levinger I, Bronks R, Cody DV, Linton I, Davie A (2005) The effect of resistance training on left ventricular function and structure of patients with chronic heart failure. Int J Cardiol 105(2):159–163
Maiorana A, O’Driscoll G, Cheetham C, Collis J, Goodman C, Rankin S et al (2000) Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. J Appl Physiol 88(5):1565–1570
Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD et al (2004) Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Card Fail 10(1):21–30
Sabelis LW, Senden PJ, Te Boekhorst BC, Hulzebos HJ, Van De Wiel A, Van Haeften TW et al (2004) Does physical training increase insulin sensitivity in chronic heart failure patients? Clin Sci (Lond) 106(5):459–466
Paluch AE, Boyer WR, Franklin BA, Laddu D, Lobelo F, Lee DC et al (2024) Resistance exercise training in individuals with and without cardiovascular disease: 2023 update: a scientific statement from the American Heart Association. Circulation 149(3):e217–e231
Smart NA, Steele M (2010) Systematic review of the effect of aerobic and resistance exercise training on systemic brain natriuretic peptide (BNP) and N-terminal BNP expression in heart failure patients. Int J Cardiol 140(3):260–265
Smart NA, Meyer T, Butterfield JA, Faddy SC, Passino C, Malfatto G et al (2012) Individual patient meta-analysis of exercise training effects on systemic brain natriuretic peptide expression in heart failure. Eur J Prev Cardiol 19(3):428–435
Ismail H, McFarlane JR, Dieberg G, Smart NA (2014) Exercise training program characteristics and magnitude of change in functional capacity of heart failure patients. Int J Cardiol 171(1):62–65
Maiorana AJ, Naylor LH, Exterkate A, Swart A, Thijssen DH, Lam K et al (2011) The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension 57(1):56–62
Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R et al (2011) Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 58(17):1780–1791
Lee CT, Chen LW, Chien MY (2017) Effects of exercise training on anabolic and catabolic markers in patients with chronic heart failure: a systematic review. Heart Fail Rev 22(6):723–730
Andreae C, Arestedt K, Evangelista L, Stromberg A (2019) The relationship between physical activity and appetite in patients with heart failure: a prospective observational study. Eur J Cardiovasc Nurs 18(5):410–417
Smart N, Steele M (2011) Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 16(7):626–632
StataCorp (2019) Stata statistical software: release 16. StataCorp LLC, College Station, TX
Wells G, Beaton D, Shea B, Boers M, Simon L, Strand V et al (2001) Minimal clinically important differences: review of methods. J Rheumatol 28(2):406–412
Smart NA, Waldron M, Ismail H, Giallauria F, Vigorito C, Cornelissen V et al (2015) Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 13(1):9–18
Cider A, Tygesson H, Hedberg M, Seligman L, Wennerblom B, Sunnerhagen KS (1997) Peripheral muscle training in patients with clinical signs of heart failure. Scand J Rehabil Med 29(2):121–127
Feiereisen P, Delagardelle C, Vaillant M, Lasar Y, Beissel J (2007) Is strength training the more efficient training modality in chronic heart failure? Med Sci Sports Exerc 39(11):1910–1917
Groennebaek T, Sieljacks P, Nielsen R, Pryds K, Jespersen NR, Wang J et al (2019) Effect of Blood Flow Restricted Resistance Exercise and Remote Ischemic Conditioning on Functional Capacity and Myocellular Adaptations in Patients With Heart Failure. Circ Heart Fail 12(12):e006427
Grosse T, Kreulich K, N.gele H, Reer R, Petersen B, Braumann K et al (2001) Peripheres Muskelkrafttraining bei schwerer Herzinsuffizienz. Deutsche Zeitschrift für Sportmedizin 52(1)
Jakovljevic DG, Donovan G, Nunan D, McDonagh S, Trenell MI, Grocott-Mason R et al (2010) The effect of aerobic versus resistance exercise training on peak cardiac power output and physical functional capacity in patients with chronic heart failure. Int J Cardiol 145(3):526–528
Lan NS, Lam K, Naylor LH, Green DJ, Minaee NS, Dias P et al (2020) The impact of distinct exercise training modalities on echocardiographic measurements in patients with heart failure with reduced ejection fraction. J Am Soc Echocardiogr 33(2):148–156
Munch GW, Rosenmeier JB, Petersen M, Rinnov AR, Iepsen UW, Pedersen BK et al (2018) Comparative effectiveness of low-volume time-efficient resistance training versus endurance training in patients with heart failure. J Cardiopulm Rehabil Prev 38(3):175–181
Palevo G, Keteyian SJ, Kang M, Caputo JL (2009) Resistance exercise training improves heart function and physical fitness in stable patients with heart failure. J Cardiopulm Rehabil Prev 29(5):294–298
Pu CT, Johnson MT, Forman DE, Hausdorff JM, Roubenoff R, Foldvari M et al (2001) Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol 90(6):2341–2350
Redwine LS, Wilson K, Pung MA, Chinh K, Rutledge T, Mills PJ et al (2019) A randomized study examining the effects of mild-to-moderate group exercises on cardiovascular, physical, and psychological well-being in patients with heart failure. J Cardiopulm Rehabil Prev 39(6):403–408
Sadek Z, Ahmaidi S, Youness M, Joumaa W, Awada C, Ramadan W (eds) (2018) Impact of resistance training in patients with chronic heart failure. The Seventh International Conference on Global Health Challenges, Athens, Greece
Tyni-Lenn R, Dencker K, Gordon A, Jansson E, Sylv.n C, (2001) Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail 3(1):47–52
Xu D, Wang B, Hou Y, Hui H, Meng S, Liu Y (2002) The effects of exercise training on plasma tumor necrosis factor-alpha, blood leucocyte and its components in congestive heart failure patients. Zhonghua Nei Ke Za Zhi 41(4):237–240
Ismail H, McFarlane J, Nojoumian AH, Dieberg G, Smart NA (2013) Clinical outcomes and cardiovascular responses to different exercise training volumes in heart failure patients: a systematic review and meta-analysis. JACC Heart Fail 1(6):514–522. https://doi.org/10.1016/j.jchf.2013.08.006. Epub 2013 Oct 23
Ciani O, Piepoli M, Smart N, Uddin J, Walker S, Warren FC et al (2018) Validation of exercise capacity as a surrogate endpoint in exercise-based rehabilitation for heart failure: a meta-analysis of randomized controlled trials. JACC Heart Fail 6(7):596–604
Smart NA, Larsen AI, Le Maitre J, Ferraz A, Marwick TH (2005) Does exercise training reduce inflammatory cytokines in patients with systolic heart failure? A multi-centre trial. Eur Heart J 26:180
Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO (2013) Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 1(2):93–102
Smart N, Marwick T (2004) Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 116(10):693–706
Myers J (2003) Cardiology patient pages. Exercise and cardiovascular health Circulation 107(1):e2-5
Norton K, Norton L, Sadgrove D (2010) Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 13(5):496–502
Levinger I, Bronks R, Cody DV, Linton I, Davie A (2005) Resistance training for chronic heart failure patients on beta blocker medications. Int J Cardiol 102(3):493–499
Wood G, Murrell A, van der Touw T, Smart N (2019) HIIT is not superior to MICT in altering blood lipids: a systematic review and meta-analysis. BMJ Open Sport Exerc Med 5(1):e000647
Chan E, Giallauria F, Vigorito C, Smart NA (2016) Exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. Monaldi Arch Chest Dis 86(1–2):759
Brubaker PH, Nicklas BJ, Houston DK, Hundley WG, Chen H, Molina AJA et al (2023) A randomized, controlled trial of resistance training added to caloric restriction plus aerobic exercise training in obese heart failure with preserved ejection fraction. Circ Heart Fail 16(2):e010161
Coletti C, Acosta GF, Keslacy S, Coletti D (2022) Exercise-mediated reinnervation of skeletal muscle in elderly people: an update. Eur J Transl Myol 32(1):10416. https://doi.org/10.4081/ejtm.2022.10416
Meyer K (2006) Resistance exercise in chronic heart failure—landmark studies and implications for practice. Clin Invest Med 29(3):166–169
Chen YC, Chen W-C, Liu C-W, Huang W-Y, Lu I, Lin CW et al (2023) Is moderate resistance training adequate for older adults with sarcopenia? A systematic review and network meta-analysis of RCTs. Eur Rev Aging Phys Act 20(1):22
Sasaki K, Fukumoto Y (2022) Sarcopenia as a comorbidity of cardiovascular disease. J Cardiol 79(5):596–604
Grgic J, Schoenfeld BJ, Orazem J, Sabol F (2022) Effects of resistance training performed to repetition failure or non-failure on muscular strength and hypertrophy: a systematic review and meta-analysis. J Sport Health Sci 11(2):202–211
Robertson RJ, Goss FL, Rutkowski J, Lenz B, Dixon C, Timmer J, Frazee K, Dube J, Andreacci J (2003) Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc 35(2):333–341
Naclerio F, Rodríguez-Romo G, Barriopedro-Moro MI, Jiménez A, Alvar BA, Triplett NT (2011) Control of resistance training intensity by the omni perceived exertion scale. JSCR 25(7):1879–1888
Gearhart RFJ, Lagally KM, Riechman SE, Andrews RD, Robertson RJ (2009) Strength tracking using the OMNI resistance exercise scale in older men and women. JSCR 23(3):1011–1015
Zourdos MC, Klemp A, Dolan C, Quiles JM, Schau KA, Jo E et al (2016) Novel resistance training–specific rating of perceived exertion scale measuring repetitions in reserve. J Strength Cond Res 30(1):267–75. https://doi.org/10.1519/JSC.0000000000001049
Mansfield SK, Peiffer JJ, Hughes LJ, Scott BR (2020) Estimating repetitions in reserve for resistance exercise: an analysis of factors which impact on prediction accuracy. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000003779
Grgic J, Lazinica B, Schoenfeld BJ, Pedisic Z (2020) Test–retest reliability of the one-repetition maximum (1RM) strength assessment: a systematic review. Sports Medicine - Open 6(1):31
Ellis R, Holland AE, Dodd K, Shields N (2019) Reliability of one-repetition maximum performance in people with chronic heart failure. Disabil Rehabil 41(14):1706–1710
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions There was no funding received for this work.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission:
1. Made substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data used in the work
2. Drafted the work or revised it critically for important intellectual content
3. Approved the version to be published
4. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented as an oral abstract at the Scientific Sessions of the American Heart Association Nov 10–13, 2023 in Philadelphia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morris, B.A., Sinaei, R. & Smart, N.A. Resistance is not futile: a systematic review of the benefits, mechanisms and safety of resistance training in people with heart failure. Heart Fail Rev 29, 827–839 (2024). https://doi.org/10.1007/s10741-024-10402-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-024-10402-0