Abstract
Heart failure (HF) is a significant public health problem worldwide. It has long been noted that premenopausal women, compared to postmenopausal women and men, have lower rates for developing this disease, as well as subsequent morbidity and mortality. This difference has been attributed to estrogen playing a cardioprotective role in these women, though exactly how it does so remains unclear. In this review, we examine the presence of estrogen receptors within the cardiovascular system, as well as the role they play behind the cardioprotective effect attributed to estrogen. Furthermore, we highlight the underlying mechanisms behind their alleviation of HF, as well as possible treatment approaches, such as hormone replacement therapy and exercise regimens, to manipulate these mechanisms in treating and preventing HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease continues to be a major cause of mortality worldwide [1]. One of its most significant manifestations is heart failure (HF), stemming from structural and/or functional cardiac defects [2]. However, it has long been noted that premenopausal women have lower cardiovascular disease risk, compared to aged-matched males, which may be owed, at least in part, to the presence of sex hormones like estrogen [3]. This is further supported by observations of HF being more highly prevalent among postmenopausal women [4]. As a result, sex hormones have been considered to possibly play key roles in protecting against HF development [5], and substantial research has been conducted on the role of estrogen in cardiovascular disease etiology [6].
Estrogens have been found to exert a variety of beneficial effects on the cardiovascular system, such as antioxidative [5] and anti-inflammatory [7] activities, as well as being able to prevent atherogenesis [8], thrombosis [8], and cell proliferation [2]; it exerts cardiovascular protective effect mainly by inhibiting the proliferation of vascular smooth muscle cells [9]. All of these findings thus point to estrogen potentially serving as a cardioprotective agent, via its ability to alter cardiovascular physiology and function in health and disease. In this review, we examine the literature regarding the molecular bases of estrogen and its receptor in counteracting against HF and how their dysregulation results in disease development. This research, in turn, provides a foundation for developing therapies to treat HF.
The role of 17-β-estradiol (E2) and its receptors in the cardiovascular system
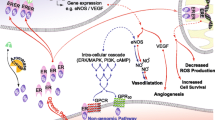
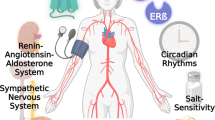
The most common form of circulating estrogen is 17-β-estradiol (E2), whose effects on the cardiovascular system are mediated through nuclear and membrane estrogen receptors (ERs), such as ERα, ERβ, and G-protein-coupled ER (GPR30/GPER) [10]. One process mediated by E2 binding on ERs is nitric oxide (NO) production in cerebral and peripheral endothelial cells, through endothelial NO synthase (eNOS) [10]. NO is a key vasodilator and cardio-protective factor, being able to maintain blood vessel dilation, regulate blood pressure, and activate a variety of vaso-protective and anti-atherosclerotic processes in vascular endothelial cells [11]. By contrast, NO and cyclic guanosine phosphate (cGMP) pathways are downregulated in postmenopausal women, owing to the activation of the renin–angiotensin–aldosterone system (RAAS) in response to lowered estrogen levels. These women have been characterized as possessing more rigid arteries and cardiac muscles, rendering them less sensitive to aberrant blood flow loads [12].
ERα
Among HF individuals with preserved ejection fraction (HFpEF), right ventricular (RV) dysfunction is a major predictor of mortality [13]. E2 has been documented to play a protective role in maintaining RV function and counteracting against maladaptive remodeling, via ERα. Investigations of the underlying mechanisms revealed that E2-ERα binding activated bone morphogenetic protein receptor 2 (BMPR2) signaling, which has been identified as a potent effector of cardiac contractile force [14, 15]. Previously, BMPR2, along with the peptide hormone Apelin, have been noted to be required for cardiac development and pulmonary vascular homeostasis [16]. Newer evidence, though, has indicated that the E2-ERα-BMPR2 pathway plays a role in maintaining RV function, serving as the molecular basis for the cardioprotective effect of E2 there [17]. This is further supported by ERα slowing down RV remodeling among female rats with pulmonary hypertension [7], as well as by Cheng et al., who induced RV hypertrophy, via pulmonary artery banding (PAB), among male rats, as well as both wild-type (WT) and ERα loss-of-function mutant females [18]. They discovered that mutant females, compared to WT and males, had diastolic dysfunction and higher collagen type I to III ratios, indicating that ERα signaling in females defended against collagen buildup and diastolic dysfunction during the RV pressure overload response. Therefore, the protective effect of E2-ERα against diastolic dysfunction is sex-specific [18].
ERβ
ERβ has also been identified as playing cardioprotective roles in recent years [19,20,21]. For instance, ovariectomized (OVX) and ERβ-deleted mice showed aberrant vascular function, hypertension, higher death rates, and worsened HF [22]. Furthermore, ERβ has been observed to exert anti-hypertrophic and anti-fibrotic actions among both OVX and non-OVX mice [21, 23, 24]. ERβ-deleted mice, compared to WT, also have been found to exhibit significant increases in inflammatory pathway activation, when the heart is subject to transverse aortic constriction (TAC)-induced hypertrophy [25]. By contrast, the presence of ERβ was able to attenuate cardiac remodeling and apoptosis in the TAC mouse model, according to Fliegner et al. [26]. Additionally, ERβ was determined to be essential for controlling the proteome response to pressure overload, which might be a key factor in delaying the beginning of HF [27]. Following these findings, several unbiased studies also showed that ERβ activation was responsible for some of those anti-apoptotic effects attributed to E2 and sex difference. In accordance with these findings, Cao et al. [28] showed that E2 treatment in vivo reduced cardiac rupture likelihood among myocardial infarcted (MI) male mice, which was accompanied by decreased matrix metalloproteinase-9 (MMP-9) activation and increased anti-apoptotic Bcl-2, compared to un-infarcted controls. E2 treatment was also found by Pedram et al. to prevent angiotensin II (Ang II)-induced cardiac hypertrophy, a precursor to diastolic stiffness, among female mice. Additionally, Ang II-induced myosin heavy chain synthesis, ERK activation, calcineurin activity, and interstitial fibrosis were all inhibited. Such prevention was only present among WT or ERα-null mice, not for ERβ-null, reinforcing that E2 operated through ERβ to exert its cardioprotective effects [29]. All these findings thus suggest that ERβ could serve as a potential therapeutic target against HF, which is further supported by studies investigating ERα and ERβ agonists, in which ERβ was found to be the main receptor for E2 being able to rescue cardiac functioning in HF [30].
Aside from its cardioprotective role, ERβ activation is also a prerequisite for estrogen-dependent upregulation of both eNOS and inducible NOS (iNOS) in rat neonatal cardiomyocytes [31]. eNOS, as well as neuronal NOS (nNOS) activities, have significant impacts on diastolic function, in which nNOS inhibition enhanced diastolic function in OVX rats, according to Jessup et al. [32] Tetrahydrobiopterin, a NOS cofactor, is hypothesized to be activated by E2 in order to control NOS production. Therefore, E2 deficiency in OVX rats leads to less activated tetrahydrobiopterin being available, which may have caused nNOS uncoupling, resulting in them shifting from catalyzing NO formation to generating superoxides [32]. This increase in nNOS-produced superoxides and decrease in proper NOS metabolites may result in diastolic stiffness, impaired cardiac remodeling, and eventually HF.
GPR30
G-protein-couped receptors (GPCRs) are the main membrane receptor class involved in mediating the effects of cardiac disorders [33]. One of those GPCRs is GPR30/GPER, which has been found to exert positive impacts on female HF patients [34], via its engagement in non-genomic estrogen signal transduction in nervous, reproductive, skeletal, immune, and cardiovascular systems, as well as in metabolism [35]. For instance, G1, a GPR30 agonist, was able to lower Ang II-induced hypertrophy among neonatal cardiomyocytes, via stimulating the upregulation of the PI3K-Akt-mTOR signaling pathway and inhibiting autophagy [36, 37]. GPR30, like ERβ, also appears to play a cardioprotective role against oxidative stress, as evidenced by the finding that a mitochondria-targeted antioxidant was able to reduce cardiac oxidative stress, which was otherwise elevated, among female animals possessing cardiomyocyte-specific mutant GPR30 [38]. This is further supported by the fact that G1 lowered cardiac atrial (ANP) and brain natriuretic peptides (BNP), as well as myosin heavy chain (MHC) levels, in Ang II-induced cardiac hypertrophy rats. With respect to Ang II stimulation, reactive oxygen species (ROS) are produced when it binds to the Ang II type 1 receptor (AT1R) [39, 40], which eventually leads to HF by increasing oxidative stress, hypertrophy, and apoptosis [41]. On the other hand, G1 administration suppressed cardiac fibrosis, apoptosis, and oxidative stress, demonstrating that GPR30 activation was able to counteract against cardiac remodeling [42], which was further proven by Da Silva et al. [43], who discovered that G1 administration was necessary for improving diastolic function among spontaneously hypertensive OVX rats. Chronic G1 treatment was also found to enhance aortic ring reactivity to acetylcholine by lowering cardiac angiotensin-converting enzyme activity, AT1R protein expression, and Ang II immunoreactivity[44].
To further examine the role of GPR30 in cardiomyocytes, a cardiomyocyte-specific KO animal model was developed by Wang et al. [43]. There, they found that GPR30 KO had diastolic dysfunction, as well as other cardiovascular disease-associated traits, thereby proving that GPR30 may be necessary for maintaining overall cardiac function. An isoproterenol-induced HF model was also examined, in which the progression of left ventricular (LV) cardiomyocyte dysfunction, with significantly decreased dL/dtmax, dR/dtmax, and [Ca2+]i, as well as β-adrenergic receptor (AR) desensitization and maladaptive remodeling in terms of their shapes, was paralleled by LV chamber abnormalities. All of these changes, however, were reversed towards normal levels after G1 treatment, which could be due to the normalization of basal and β-AR-stimulated Ca2+ handling, leading to the reversal of cardiomyocyte relaxation and force generation abnormalities stemming from HF. Therefore, G1 could serve as a potential therapeutic approach to counteract against HF, via restoring normal [Ca2+] regulation [34]. This is further supported by reports of HF, or GPR30 deficiencies, both leading to reduced LV SERCA2a expression and activity [45,46,47], along with increased sarcolemmal Na+-Ca2+ exchange, sarcoplasmic reticulum Ca2+ leakage, and faulty Ca2+ removal. On the other hand, GPR30 increases SERCA2a expression and activity to bolster myocardial Ca2+ mobilization [47, 48], via reversing HF-induced alterations in cardiac β1- and β2-AR expression and activity. Overall, data suggests that elevated oxidative stress leads to cellular damage, defective [Ca2+]i control, and remodeling during HF, whereas GPR30 activation was able to prevent cardiomyocyte apoptosis and unfavorable LV remodeling [45, 47]. Therefore, GPR30 could possibly counteract against HFpEF, by restoring cardiac β-AR responsiveness, as well as counteracting against LV and cardiomyocyte contractile abnormalities [34] (see Table 1).
Pharmacological therapy
The beneficial effects of estrogen/ER activation on cardiac functioning in HF have resulted in the development of numerous treatment approaches. One possible approach is to upregulate myocardial cGMP signaling, as examined in the Vericiguat Global trial in Subjects with Heart Failure and Reduced Ejection Fraction (VICTORIA), where it was found that the soluble guanylate cyclase (sGC) stimulant, vericiguat, exerted cardioprotective effects [49]. As lowering estrogen levels have been associated with lowered cGMP, it is thus reasonable to speculate that similar approaches to the VICTORIA trial could be used for activating estrogen/ER signaling in HF. Indeed, the myocardial cGMP-PKG signaling pathway has been found to be deactivated in HFpEF, which has been found to occur among females independently of obesity and diabetes. Furthermore, among female mice, cGMP-PKG activation among cardiomyocytes by the phosphodiesterase type 5 (PDE5) inhibitor sildenafil was able to alleviate HF [50, 51], which, however, requires estrogen signaling to activate eNOS-dependent cGMP and PKGIα [51]. More specifically, it involves a nonnuclear signaling mechanism, triggered by ER binding to striatin; this is blocked, though, in ER mutants [52].
However, discrepancies in the outcomes of clinical studies comparing hormone replacement therapies (HRT) for treating HF have been noted [53]. This may be due to cellular and organ functioning among postmenopausal women with cardiovascular disease being affected by the complex hormonal environment. Exogenous substances, such as the progesterone endocrine disruptor medroxyprogesterone acetate, as well as conjugated estrogen from horse urine, failed to exhibit the same positive effects as endogenous hormones [54]. Furthermore, they were associated with significant negative side effects, such as increased blood clotting and inflammation, compared to transdermal estradiol [55]. Therefore, the timing for the initiation of hormone therapy, as well as the agents used, are likely factors behind its failure to achieve beneficial cardiac effects [56].
Despite the failures in hormonal therapy, though, estrogen has been found to improve cardiovascular-associated indices, such as exercise endurance and arterial NO-dependent dilation, as well as slowing down subclinical atherosclerosis development. It is also crucial, in terms of diastolic function, for lowering isovolumetric relaxation time and raising the E/A ratio. Therefore, we believe that the timing and route of estrogen administration may be essential for obtaining its beneficial effects. This was supported by evidence showing transdermal administration of estrogen balanced out its benefits and side effects, transdermal delivery prevents the cardiovascular thromboembolic damage associated with oral oestrogen, while oral administration was associated with some negative cardiovascular effects [57]. The use of oral estrogens (diethylstilbestrol) increases thromboembolic cardiovascular disease [58]. The “timing hypothesis” postulates that the recipient’s age and hormonal environment affect the effect of E2 injection on the vasculature [59, 60]. Age has been proven to be a significant factor in determining the vascular effects of E2 in women, with positive effects being seen in younger (60 years) postmenopausal women but not in older (> 60 years) postmenopausal women [61]. Hodis et al. [62] discovered that the effects of estradiol (with or without progesterone) on the development of atherosclerosis varied depending on the timing of therapy initiation, with benefit noted when it was started in women who were less than 6 years past menopause but not in those who were 10 or more years past menopause. Therefore, the finding that estrogen administration during early menopause has more beneficial cardiovascular effects [62, 63], compared to other time points, suggests that future estrogen therapy approaches may be most successful if applied at younger ages. In fact, Gersh et al. suggested that if HRT was required, it should be started right away after the cessation of ovarian hormone production, and administered as transdermal estradiol, in conjunction with cyclic dosing of human-identical progesterone [64].Therefore, utilizing the proper timing, administration method, and formulation for estrogen replacement therapy may maximize its benefits and minimize its side effects. At present, the existing clinical data on the timing and pathway of estrogen administration are insufficient, which may provide a possible new approach for clinical research on estrogen receptor activation, which still needs to be further studied.
Exercise therapy
It is worth noting that reaching the recommended 150 min/week of physical activity is more difficult for female patients than for men because they face multiple barriers, such as time constraints caused by family or work responsibilities and a feeling that corporate responsibility is either tedious or inappropriate for young people. Therefore, to address increased cardiovascular disease risk among postmenopausal women, which may not be addressed by standard CR, alternative, cutting-edge exercise therapies must be investigated [65]. In fact, female congestive HF patients are able to dramatically increase their fitness level with CR, and actually exhibit greater improvements in tests like the 6-min walk test (6MWT), compared to male patients. Additionally, similar findings imply that exercise capacity, in the form of 6-min walk distance (6MWD), self-reported exercise levels, and mood (CDS scores) are able to simultaneously improve over time in female HF patients [66]. Aerobic, as well as resistance exercise, has also been found to lower pro-inflammatory biomarkers among obese postmenopausal women, who had lower levels of IL-2, IL-4, IL-6, and TNF-α [67]. This may be due to it counteracting the effects of increased adiposity and altered lipid profiles, stemming from hormonal changes, among postmenopausal women [68] (see Table 2).
Conclusion
In conclusion, the stimulation of estrogen receptors, particularly through the administration of a specifically arranged HRT regimen, or via cardiac rehabilitation exercises, could serve as a possible treatment approach for improving cardiac functioning post-HF, especially among postmenopausal females. However, these treatment regimens should be tailored for the needs of each patient.
Data availability
The data that support the findings of this study are available from the corresponding author, Dr. Jingjin Liu, upon reasonable request.
Abbreviations
- HF:
-
Heart failure
- HFrEF:
-
Heart failure with decreased ejection fraction
- GPR30/GPER:
-
G-protein-coupled ER
References
Virani SS et al (2021) Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 143(8):e254–e743
Bozkurt B et al (2021) Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail
Ueda K et al (2021) Sex differences and regulatory actions of estrogen in cardiovascular system. Front Physiol 12:738218
Zhao D et al (2018) Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 71(22):2555–2566
Murphy E (2011) Estrogen signaling and cardiovascular disease. Circ Res 109(6):687–696
Gerber Y et al (2015) A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 175(6):996–1004
Frump AL et al (2015) Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 308(9):L873–90
Iorga A et al (2017) The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 8(1):33
Pellegrini M et al (2014) Endocrine disruptors differently influence estrogen receptor β and androgen receptor in male and female rat VSMC. J Cell Physiol 229(8):1061–1068
Nevzati E et al (2015) Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir Suppl 120:141–145
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Euro Heart J 33(7):829–837
Shuaishuai D et al (2023) Sex differences and related estrogenic effects in heart failure with preserved ejection fraction. Heart Fail Rev 28(4):937–948
Chatterjee NA, Steiner J, Lewis GD (2014) It is time to look at heart failure with preserved ejection fraction from the right side. Circulation 130(25):2272–2277
Brash L et al (2018) Short-term hemodynamic effects of apelin in patients with pulmonary arterial hypertension. JACC Basic Transl Sci 3(2):176–186
Dai T, Ramirez-Correa G, Gao WD (2006) Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol 553(1–3):222–228
Szokodi I et al (2002) Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res 91(5):434–440
Frump AL et al (2021) 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Investig 131(6)
Cheng T-C et al (2020) Estrogen receptor-α prevents right ventricular diastolic dysfunction and fibrosis in female rats. Am J Physiol Hear Circ Physiol 319(6):H1459-H1473
Umar S et al (2011) Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184(6):715–723
Nikolic I et al (2007) Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol 42(4):769–780
Pedram A et al (2010) Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol (Baltimore, Md.) 24(11):2152–2165
Pelzer T et al (2005) Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation 111(12):1492–1498
Zhu Y et al (2002) Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science (New York, N.Y.) 295(5554):505–508.
Skavdahl M et al (2005) Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288(2):H469–476
Kararigas G et al (2011) Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol Genomics 43(8):438–446
Fliegner D et al (2010) Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298(6):R1597–1606
Kararigas G et al (2014) Comparative proteomic analysis reveals sex and estrogen receptor β effects in the pressure overloaded heart. J Proteome Res 13(12):5829–5836
Cao J et al (2011) Estrogen induces cardioprotection in male C57BL/6J mice after acute myocardial infarction via decreased activity of matrix metalloproteinase-9 and increased Akt-Bcl-2 anti-apoptotic signaling. Int J Mol Med 28(2):231–237
Pedram A et al (2008) Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology 149(7):3361–3369
Iorga A et al (2018) Estrogen rescues heart failure through estrogen receptor Beta activation. Biol Sex Differ 9(1):48
Nuedling S et al (2001) Activation of estrogen receptor beta is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Lett 502(3):103–108
Jessup JA et al (2011) Neuronal nitric oxide synthase inhibition improves diastolic function and reduces oxidative stress in ovariectomized mRen2.Lewis rats. Menopause (New York, N.Y.) 18(6):698–708
Xu Y et al (2003) Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res 57(2):388–394
Zhang X et al (2021) Chronic GPR30 agonist therapy causes restoration of normal cardiac functional performance in a male mouse model of progressive heart failure: insights into cellular mechanisms. Life Sci 285:119955
Olde B, Leeb-Lundberg LMF (2009) GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab 20(8):409–416
Morselli E et al (2017) The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol 13(6):352–364
Pei H et al (2019) G Protein-coupled estrogen receptor 1 inhibits angiotensin II-induced cardiomyocyte hypertrophy via the regulation of PI3K-Akt-mTOR signalling and autophagy. Int J Biol Sci 15(1):81–92
Wang H et al (2018) G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Translational research : the journal of laboratory and clinical medicine 199:39–51
Maulik SK, Kumar S (2012) Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods 22(5):359–366
Zhang G-X et al (2007) Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res 76(2):204–212
Togliatto G, Lombardo G, Brizzi MF (2017) The future challenge of reactive oxygen species (ROS) in hypertension: from bench to bed side. Int J Mol Sci 18(9)
Wang Y et al (2022) G-protein coupled receptor 30 attenuates myocardial hypertrophy by reducing oxidative stress and apoptosis in Ang II-treated mice. Peptides 157:170878
Wang H et al (2017) Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: a sex-specific gene profiling analysis. Biochimica et biophysica Acta Mol Basis Dis 1863(8):1870–1882
Silva JSD et al (2019) G-protein-coupled estrogen receptor agonist G1 improves diastolic function and attenuates cardiac renin-angiotensin system activation in estrogen-deficient hypertensive rats. J Cardiovasc Pharmacol 74(5):443–452
Aryan L et al (2020) The role of estrogen receptors in cardiovascular disease. Int J Mol Sci 21(12)
Mishra S, Kass DA (2021) Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 18(6):400–423
Groban L et al (2019) Female heart health: is GPER the missing link? Front Endocrinol 10:919
Alencar AK et al (2017) Effect of Age, Estrogen status, and late-life GPER activation on cardiac structure and function in the Fischer344×Brown Norway female rat. J Gerontol Series A Biol Sci Med Sci 72(2):152–162
Armstrong PW et al (2020) Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 382(20):1883–1893
Fisher PW et al (2005) Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111(13):1601–1610
Sasaki H et al (2014) PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Investig 124(6):2464–2471
Fukuma N et al (2020) Estrogen receptor-α non-nuclear signaling confers cardioprotection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic to translational science 5(3):282–295
Rossouw JE et al (2007) Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297(13):1465–1477
Miller VM, Mankad R (2018) Sex steroids and incident cardiovascular disease in post-menopausal women: new perspective on an old controversy. J Am Coll Cardiol 71(22):2567–2569
Gersh FL, Lavie CJ (2020) Menopause and hormone replacement therapy in the 21st century. Heart (British Cardiac Society) 106(7):479–481
Silva JSD et al (2021) Estrogen receptors: therapeutic perspectives for the treatment of cardiac dysfunction after myocardial infarction. Int J Mol Sci 22(2)
Langley RE et al (2021) Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised Prostate Adenocarcinoma Transcutaneous Hormone (PATCH) trial programme. Lancet (London, England) 397(10274):581–591
Byar DP (1973) Proceedings: The Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer 32(5):1126–1130
Phillips LS, Langer RD (2005) Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertil Steril 83(3):558–566
Turgeon JL et al (2004) Hormone therapy: physiological complexity belies therapeutic simplicity. Science (New York, N.Y.) 304(5675):1269–1273
Sherwood A et al (2007) Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol 27(8):1782–1787
Hodis HN et al (2016) Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 374(13):1221–1231
Keaney JF, Solomon CG (2016) Postmenopausal hormone therapy and atherosclerosis–time is of the essence. N Engl J Med 374(13):1279–1280
Gersh FL, O’Keefe JH, Lavie CJ (2021) Postmenopausal hormone therapy for cardiovascular health: the evolving data. Heart (British Cardiac Society) 107(14):1115–1122
Muzzarelli S et al (2010) Predictors of early readmission or death in elderly patients with heart failure. Am Heart J 160(2):308–314
Wise FM, Patrick JM (2012) Cardiac rehabilitation outcomes in women with chronic heart failure: mood, fitness, and exercise safety. J Cardiopulm Rehabil Prev 32(2):78–84
El-Kader SMA, Al-Jiffri OH (2019) Impact of aerobic versus resisted exercise training on systemic inflammation biomarkers and quality of life among obese post-menopausal women. Afr Health Sci 19(4):2881–2891
Biteli P et al (2021) Dyslipidemia influences the effect of physical exercise on inflammatory markers on obese women in post-menopause: a randomized clinical trial. Exp Gerontol 150:111355
Funding
This study is supported by Natural Science Foundation of Shenzhen Science and Technology Innovation Commission (JCYJ20190806153207263), National Natural Science Foundation (Project #82200315), Guangdong Basic and Applied Basic Research Foundation (2021A1515111145), Sanming Project of Medicine in Shenzhen (No. SZSM201412012), and Major scientific research project of Shenzhen People’s Hospital (SYWGSJCYJ202301).
Author information
Authors and Affiliations
Contributions
QCY, LJJ, and LHD contributed to manuscript conceptualization and design. QCY and LJJ contributed to first draft writing. All authors contributed to manuscript writing and critical review. All authors have read the final version of the manuscript and agreed to its submission.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, C., Liu, J. & Liu, H. Targeting estrogen receptor signaling for treating heart failure. Heart Fail Rev 29, 125–131 (2024). https://doi.org/10.1007/s10741-023-10356-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10356-9