Abstract
Studies over recent years have redeveloped our understanding of uremic cardiomyopathy, defined as left ventricular hypertrophy, congestive heart failure, and associated cardiac hypertrophy plus other abnormalities that result from chronic kidney disease and are often the cause of death in affected patients. Definitions of uremic cardiomyopathy have conflicted and overlapped over the decades, complicating the body of published evidence, and making comparison difficult. New and continuing research into potential risk factors, including uremic toxins, anemia, hypervolemia, oxidative stress, inflammation, and insulin resistance, indicates the increasing interest in illuminating the pathways that lead to UC and thereby identifying potential targets for intervention. Indeed, our developing understanding of the mechanisms of UC has opened new frontiers in research, promising novel approaches to diagnosis, prognosis, treatment, and management. This educational review highlights advances in the field of uremic cardiomyopathy and how they may become applicable in practice by clinicians. Pathways to optimal treatment with current modalities (with hemodialysis and angiotensin-converting enzyme inhibitors) will be described, along with proposed steps to be taken in research to allow evidence-based integration of developing investigational therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is the leading cause of death in patients with chronic kidney disease (CKD), ahead of even end-stage renal disease (ESRD) [1]. While the contribution of coronary artery disease to this burden of mortality is well established [2], research beginning in the 1970s and 1980s identified left ventricular hypertrophy (LVH) and congestive heart failure (CHF) as more significant causes of death in this patient group [3]. These conditions, along with associated cardiac hypertrophy and abnormalities resulting from CKD such as left ventricular dilatation and systolic and diastolic dysfunction, are often described under the term uremic cardiomyopathy (UC), although definitions have conflicted and overlapped over the decades, complicating the body of published evidence and making comparison difficult.
These conflicting definitions have made it challenging to precisely define the size of this patient population worldwide, but reports from the USA estimate that 2 million people in that country alone will require dialysis for ESRD by 2030 [4] and that LVH is found in some 70% of patients with ESRD [5, 6].
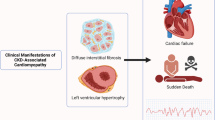
Among those patients with ESRD, cardiovascular disease is the cause of approximately half of deaths [6], and an analysis of over 1 million patients revealed an independent graded association between reduced glomerular filtration rate (GFR) and risk of cardiovascular events and death [7]. However, evidence indicates that this level of cardiovascular death is largely secondary to LVH and CHF rather than to atherosclerosis [4, 5]. The primary manifestation and hallmark of UC is LVH [8], often accompanied by increased ventricular thickness, arterial stiffening, coronary atherosclerosis, and coronary artery calcification. Clinical symptoms, while similar to those resulting from critical coronary artery disease, arise in patients with UC because of the reduced coronary reserve that results from adaptive (and eventually maladaptive) LVH [9].
Established risk factors associated with the development of UC include hypertension, hyperlipidemia, and diabetes, which are also well-known risk factors for atherosclerotic disease [10]. UC also appears to be associated with certain other and related risk factors for cardiovascular disease (e.g., increased age, obesity/sedentary lifestyle, tobacco use) that suggest a future global increase in UC incidence, and likely in CKD incidence overall, will accompany the aging of populations and Westernization of lifestyles.
However, other more specific potential risk factors for UC have been identified, including uremic toxins, anemia, hypervolemia, oxidative stress, inflammation, insulin resistance, and CKD-mineral and bone disorder (MBD) [9]. Continuing research in these areas indicates the increasing interest in illuminating the pathways that lead to UC and thereby identifying potential targets for intervention. Indeed, our developing understanding of the mechanisms of UC has opened new frontiers in research, promising novel approaches to diagnosis, prognosis, treatment, and management.
The topics covered in this review are aimed at providing an overall understanding of UC (Fig. 1), particularly advances in the field of UC that are applicable in practice for clinicians. Pathways to optimal treatment with current modalities (including hemodialysis and angiotensin-converting enzyme (ACE) inhibitors) will also be described, alongside the integration of developing investigational therapies.
Current knowledge
Clinical background
UC was first described by Bailey et al. in 1967 [11]. From that time on, it was described as characterized by LVH, diffuse interstitial fibrosis (DIF), focal scarring, and systolic and diastolic dysfunction. In the intervening decades, UC was considered a severe cardiomyopathy associated with ESRD and characterized by severe functional abnormalities that could be reversed by renal transplantation. This theoretical framework, however, has been increasingly replaced by an understanding of UC as a clinical phenotype of cardiac disease that accompanies CKD, with a multitude of contributing factors and best characterized as diastolic dysfunction seen in conjunction with LVH and fibrosis [12].
Pathogenesis
The definition of a typical time duration for the development of UC after the onset of CKD is difficult because of the lack of a firm definition or threshold for UC. However, the disease process itself becomes evident in the early stages of CKD, at relatively preserved estimated glomerular filtration rates [13].
As the field progresses, researchers should take care to identify any potential differential or atypical patient subsets in whom UC may develop differently. In children and adolescents with CKD, for example, there is typically no clinical background of pre-existing cardiac disease, although the risk of cardiovascular death is similar to that in adults with CKD [14]. The implication is that the high cardiac death rate is not fully explained by traditional cardiac risk factors, with much of the increase attributable to uremia-related risk factors. This assumption is given credence by the lower mortality observed in young patients after kidney transplant versus with those receiving continued dialysis [14]. Regardless, the development of CVD in children with CKD is clearly influenced by multiple factors, meaning that prevention efforts must focus on the identification of modifiable risk factors [15]. The picture is further complicated by the prominence of congenital (non-modifiable) anomalies of the kidney and urinary tract, which are recognized as the leading cause of ESRD in children and present a different pathophysiology from adult-onset ESRD [16]. To date in this subset of patients with CKD, there has been no high-quality evaluation of the associations between childhood CVD and later cardiac mortality, and interventional studies controlling modifiable CV risk have not been reported; no standard guidelines for screening or treatment in this population are yet available. Clearly, CVD in the setting of children with CKD remains poorly understood, with notable gaps in the research remaining to be filled.
Regardless of patient type or stage of development, the pathogenesis of UC is clearly multifactorial, involving multiple pathways including circulating uremic toxins, mineral metabolism, insulin resistance, and eventually hemodynamic overload. These pathways contribute to the increased left ventricular (LV) mass common in CKD and attributable to both myocyte hypertrophy and an expansion of the interstitial space caused by DIF [12].
A deficiency in carnitine, an important factor in the oxidation of fatty acids, is postulated to play a role in insulin resistance and the development of UC, on the basis of observations in patients undergoing dialysis [17]. Insulin resistance is an independent risk factor for CVD in patients with CKD, believed to be related to interruption of the intracellular insulin pathway that occurs as a result of increased angiotensin II, inflammation, metabolic acidosis, and uremic toxins [18].
The process of cardiac metabolic remodeling in patients with CKD is characterized by various forms of cardiac metabolic maladaptation including altered mitochondrial function, myocardial substrate utilization, metabolic transporter function and expression, and impaired insulin response and phosphoinositide-3 kinase-AKT signaling, leading to impaired cardiac function [19]. These maladaptations are mediated via the interplay between contributions of volume overload and pressure overload: LV pressure overload mediates hypertrophy by increasing LV wall thickness with minimal change in chamber size, whereas LV volume overload results in increased chamber size but normal LV wall thickness [20].
Uremic toxins, by definition uremic retention solutes that impede normal organ function, represent another pathway leading to UC and include P-cresyl sulfate (pCS), β2-microglobulin, indoxyl sulfate, and homocysteine [21]. Many of these retained compounds are not effectively removed or eliminated by current methods of dialysis.
Additionally, certain monocyte/macrophage subsets have been shown to be positively or negatively associated with cardiovascular calcification, an important predictor of CVD, in patients with CKD [22]. The influence of uremic toxins (as well as the treatment a patient may be undergoing for CKD) may affect the phenotype and function of these monocyte/macrophage subsets, resulting in osteogenic transformation and mineralization of the vascular cells.
One of the most promising areas of understanding UC is the recent progress in understanding the role of low serum levels of Klotho and elevated serum levels of FGF-23, both regulators of phosphate metabolism, in the development of LVH [23]. In patients with CKD, deficiencies in renal excretory capacity typically lead to massive increases in FGF-23 to compensate and maintain phosphate levels. These levels have been identified as being associated with CVD and mortality in a dose-dependent manner [24]. Klotho, a single-pass transmembrane protein expressed in the kidney, is a co-receptor for FGF-23 that mediates the effects of FGF-23 in phosphate regulation; some factors that are elevated in kidney dysfunction (angiotensin II and inflammatory cytokines) downregulate Klotho expression in the kidney. There remains a lack of consensus over whether Klotho and FGF-23 act on an axis or whether Klotho is protective independently of FGF-23 levels [25], but this area of research represents a potential therapeutic target for the treatment of UC, as described below.
Importantly, none of the established treatments for CKD directly targets the metabolism of the uremic heart, implying a potential area for the development of new treatments.
Screening and diagnosis
Because of the heterogeneous nature and involvement of multiple systems in UC, current clinical methods for its diagnosis involve multiple modalities.
Electrocardiogram (ECG) is the most widely available, least invasive, and least expensive method for assessing LV dysfunction and identifying volume and pressure abnormalities in patients with CKD. ECG changes that are characteristic or indicative of UC include the presence of Q waves, ST segment changes, prolonged QRS intervals, and tachycardia [26]. Associations between QT interval, spatial QRS-T angle, signal-averaged ECG, heart rate variability, and T-wave alternans and mortality have also been reported in patients undergoing dialysis [27], although the causal direction of these associations remains unclear. This modality is also of value in differentiating UC from other forms of cardiomyopathy.
As summarized in Table 1 [33], cardiac imaging in patients with CKD can involve multiple modalities, including echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac positron emission tomography (PET).
Transthoracic echocardiography (TTE) is a readily available, inexpensive, and noninvasive modality offering detailed observation of cardiac structures and potential abnormalities, particularly LVH. TTE is a well-established method to assess LV mass, commonly used to provide prognostic information or as an endpoint in studies of CVD risk. However, these methods involve indirect calculation of LV mass rather than direct observation of the actual measurements, and a large meta-analysis showed no clear association between changes in LV mass and CV mortality in patients with CKD [34], limiting the utility of such measures as a surrogate endpoint and highlighting the need for further imaging for assessment.
Cardiac computed tomography (CT) scanning can be used in UC, for example, to define areas of myocardial fibrosis [35]. This is of particular interest because the extent of fibrosis has been shown to be a strong predictor of CV death [31].
Cardiac MRI (cMRI) can be used to provide information on multiple cardiac structural and functional parameters including coronary arterial flow, perfusion, myocardial scarring, and interstitial fibrosis [36]. cMRI is of particular use in the diagnosis of UC because of its reproducibility and significantly greater quantitative information allowing for smaller sample sizes versus TTE. As a result, cMRI has become the standard for cardiac imaging where available and practical.
Additionally, there has been some research into the use of PET in the setting of CKD to evaluate myocardial perfusion and identify infarcted areas, as well as to assess coronary flow reserve [37] and areas of viability and inflammation [32].
Each of these imaging methods has some limitations in diagnosing or predicting UC, particularly in differentiating UC from other cardiomyopathies. However, these methods continue to evolve and become more practical and specific.
New and potential methods for the diagnosis of UC
Continuing advances in imaging have led to promising techniques for improved diagnosis of UC. For example, the fibrotic process can now be observed on cardiac MRI by T1 mapping, a technique that quantifies the relaxation time of protons on inversion recovery prepared images (longitudinal relaxation (T1) times) by using analytical expression of image-based signal intensities [5]. Fibrotic areas show greater accumulation of gadolinium, appearing as an area of high-intensity signal with a shorter T1 time compared with adjacent normal tissue. A comparison of myocardial T1 and T2 times before and after kidney transplantation suggests that T1 times are indeed a stable measure of DIF [38].
These cardiac MRI T1 and T2 (transverse relaxation time) mapping techniques can be used to characterize myocardial involvement in CKD, with higher scores related to worsening clinical status and myocardial damage [39] (Fig. 2). Importantly, some native T1 and T2 mapping techniques may be performed without the need for gadolinium, the repeated use of which may represent a patient safety issue. These findings obtained via techniques could therefore potentially be used as surrogate endpoints to gauge the efficacy of interventions.
a, b Cardiac MRI images in a patient with normal heart function. This image also shows feature tracking using cmr42 (Circle Cardiovascular Imaging Inc., Calgary, Canada) in short-axis cine images at the end-diastole (a) and end-systole (b). The yellow and cyan curves delineate the endocardial and epicardial contours, respectively. The yellow dots represent the right ventricle myocardial voxel points, and the short yellow line on the images shows the tracking of the ventricle myocardial voxel points. Adapted from Hu et al. [56]. CC-BY 4.0. c, d Cardiac MRI images in a patient with stage 4 chronic kidney disease, short-axis, mid-slice, region of interest conservatively drawn in the mid-septum. Native T1 is increased at 1067 ms (c). The T2 value is also slightly increased at 50 ms (d). The left ventricle showed eccentric hypertrophy and dilation; no late gadolinium enhancement was detected. The examination was performed with a 1.5-T scanner (Siemens Aera); in-center cut-offs for normality are 995 ms (native T1) and 49 ms (T2). White arrows indicate pericardial effusion. Adapted from Arcari et al. [30]. CC-BY-NC 4.0.
In other developments in cardiac MRI, the feature-tracking analysis technique involves a post-processing algorithm that uses a block-matching approach to assess the movement of specific anatomical features and identify longitudinal strain throughout the course of the diastole and systole [40]. A newer imaging technique analogous to cMRI feature tracking is speckle-tracking echocardiography (STE), which tracks the movement of specific echoes within the myocardium during systole to identify deformations and abnormal systolic myocardial LV function in patients with CKD and normal LV ejection fraction (LVEF) [41] and to evaluate LV global longitudinal strain.
New and more useful quantification measures are being developed along with the abovementioned novel imaging techniques. For example, global longitudinal strain (measured via conventional 2-dimensional echocardiography) has been reported to be a more sensitive predictor of myocardial dysfunction in ESRD compared with LVEF [42].
One obvious step to further advance screening and diagnosis procedures in the clinic would be to identify and quantify risk factors for UC and to apply that information to develop a predictive scoring system. This will be challenging because, compared with other cardiomyopathies, there are probably few findings that are specific to UC. To date, unfortunately, not enough is known about the use of existing or emerging diagnostic biomarkers or other means of detecting asymptomatic patients who may be at high risk for UC. The establishment of specific imaging methods should also be a priority. Echocardiography and MRI are the most promising noninvasive modalities.
In addition to developing the means to diagnose UC, in general practice, it will be important to detect ischemic changes early, based on their association with CKD, using ECG, echocardiography, chest X-ray, or coronary artery CT. This means that cardiac function should be screened and followed up from the early stages of CKD. In age-related decline in cardiac function, diastolic dysfunction is generally accepted to appear prior to systolic dysfunction. Echocardiography is the preferred modality for this identification, but as mentioned above, ischemic changes may also be detected by ECG. For patients who are already showing typical symptoms of cardiomyopathy, it may be too late to implement interventions that could change the clinical course.
Significant clinical experience has shown that UC is closely associated with CKD and that cardiac function declines over time in patients with CKD. The most common cause of this decline is ischemic heart disease. Therefore, if coronary artery stenosis is observed, revascularization procedures such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) may be performed, with resulting improvement in cardiac function. However, myocardial injury in UC is likely to extend beyond myocardial ischemia to endothelial damage in the microvasculature and cellular-level damage, as well as to the development of myocardial fibrosis. There is sufficient overlap between ischemic cardiomyopathy and UC to make it clinically difficult to distinguish between these two conditions, although it is important to treat UC when it cannot be explained as ischemic cardiomyopathy. To date, however, there is no specific treatment for UC, and the utility of current treatments may be insufficient at this point. Clarification in this area will be an important future issue for both diagnosis and treatment.
In terms of advances that can easily and immediately be adapted to the clinic, it is important to note that in no area related to UC does there already exist a sufficient evidence base to adopt new procedures based on current knowledge. For this reason, filling the knowledge gaps will be of critical importance.
Disease management
This section will discuss the application of new findings in the therapeutic management of UC, the monitoring of disease progression, and the overall efficacy of available treatment. Current available care options, the limitations thereof, and the potential of new therapeutics are presented in Table 2.
Current options for pharmacotherapy for patients with UC include many therapies used for heart failure in general. In particular, these include ACE inhibitors and angiotensin receptor blockers, which have been shown to reduce the progression of renal and vascular damage in patients with CKD [43]; carvedilol, which improves LV end-diastolic pressure, LV end-systolic volume, and LVF and appears to reduce symptoms and hospital admissions in patients with dilated cardiomyopathy undergoing dialysis [44]; and spironolactone to suppress the production of aldosterone, which stimulates ventricular hypertrophy [45]. The use of statins in the UC population has shown mixed results, appearing to vary depending on the specific patient group under study.
Regardless of pharmacotherapy, the eventual treatment for UC in most cases is renal replacement therapy, which can be accomplished by hemodialysis, peritoneal dialysis, or renal transplantation.
Hemodialysis has been shown in many studies to potentiate reverse cardiac remodeling and to reverse some of the clinical sequelae of UC [46]. This is perhaps the most important treatment for UC in our current armamentarium.
Peritoneal dialysis is a home therapy that may represent a preferable treatment option for some patients versus hemodialysis, as it does not require patients to travel 3–5 times a week to a dialysis center and may even be carried out while the patient is sleeping. Costs may also be lower than those for dialysis, depending on the treatment setting. However, there may be greater risk of treatment discontinuation with peritoneal dialysis because it is self-administered, and some studies suggest that if not adequately monitored, patients undergoing treatment via this modality appear to have worse CV outcomes, including increased LV mass index, in the long term (beyond 2 years of dialysis treatment) [47].
Finally, renal transplantation confers a significant survival advantage versus other treatment modalities, as well as long-term improvement in LVEF and reduction in LVH [48]. These advantages must be balanced with difficulties in finding donors (and risks posed by potential wait times), the cost of the procedure, and the risk of perioperative adverse events. Palliative/supportive treatment options for UC patients to date do not differ from those for heart failure patients in general; this treatment should be per accepted guidelines unless further evidence emerges that is specific to this patient group [49, 50].
Beyond these existing treatments, new and experimental therapies offer the potential for superior outcomes. Among these is a potential role for salvianolic acid B (Sal B), the active component in Salvia miltiorrhiza Bunge (red sage), which is widely used as an herbal medication in traditional Chinese medicine. A recent study using a rat model of UC (which also used speckle-tracking echocardiography) reported efficacy in reducing cardiac hypertrophy, edema, inflammation, and fibrosis [51].
The role of recombinant human erythropoietin (EPO) administration in the treatment of anemia in CKD has been explored with controversial results. Initial studies in anemic patients undergoing dialysis showed improved quality of life and reduced need for transfusions; the use of erythropoiesis-stimulating agents (ESAs) subsequently became widespread. However, some recent studies have indicated an increased risk of stroke without a survival benefit [52]. It appears that while EPO may be necessary for some anemic patients with CKD, clinicians should be aware of the potential for adverse events.
The role of vitamin D supplementation in this population is also being explored, with some positive findings in terms of LV fiber shortening [53]. Intravenous iron supplementation has also shown utility in patients with CKD undergoing dialysis who have low ferritin concentrations, resulting in fewer adverse CV events and lower risk of death [54].
Future directions
Further research is clearly needed to address the gaps in the literature regarding the etiology, diagnosis, and management of UC. This section describes the shortcomings in the current understanding of UC and offers suggestions for the types of prospective or retrospective research that could be carried out to address these gaps. There is an overall lack of research in this population in general; for example, many trials of therapies aimed at reducing CV risk have systematically excluded patients with CKD. As a result, there is a relative paucity of specific data, with much research focusing either on all patients with CKD or all those with heart failure.
A promising area for research is that related to the cardiac effects resulting from the interplay of FGF23 and Klotho [55]. If this relationship can be further illuminated, the balance of these factors presents an appealing therapeutic target.
The contributions of inflammation and systemic oxidant stress should be further explored, especially as oxidative stress appears to play some role in the molecular pathways postulated to be associated with the development of UC via myocyte hypertrophy and DIF causing expansion of interstitial spaces [12].
Besides these specific factors, research should be aimed at improving detection and treatment and at addressing the limitations of current methods, as noted above. There is a gap between the understanding of the underlying pathomechanisms and the development of actual treatments (including pharmacotherapy). In other words, we can diagnose the disease, but there are virtually no specific treatment options for UC. The potential and emerging modalities identified in Table 2 present opportunities that, with further research, could bring effective treatments to fruition. There also exists a gap when it comes to diagnosis: Early diagnosis is difficult because UC frequently overlaps with ischemic cardiomyopathy, hypertensive cardiomyopathy, and diabetic cardiomyopathy as a result of their common risk factors. Given the burden of this disease and its poor outcomes, future research should focus on differentiating UC from other types of cardiomyopathies in terms of identifying specific risk factors, quantifying risk, and optimizing treatment. Researchers are urged to publish their findings regardless of the success of diagnostic and treatment approaches.
Conclusions
The research to date has been essential in forming the modern understanding of the etiology, pathogenesis, diagnosis, and treatment of UC. However, this improved understanding has not yet led to applicable modifications to practice for the treating clinician, apart from substantial improvements in imaging technology. Innovative research must take place to fill these gaps in current research. Differential diagnosis, risk quantification and stratification, and specific treatment options for UC are important areas where research must transform our current understanding into applicable tools for the clinician. Clinicians, institutions, and organizations must work toward furthering the science and acting on it to improve patient outcomes in this disease that has such a significant global burden.
Data availability
Not applicable.
References
Saritas T, Floege J (2020) Cardiovascular disease in patients with chronic kidney disease. Herz 45(2):122–128. https://doi.org/10.1007/s00059-019-04884-0
Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR (2008) Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol 28(2):354–360. https://doi.org/10.1159/000111829
Rostand SG, Gretes JC, Kirk KA, Rutsky EA, Andreoli TE (1979) Ischemic heart disease in patients with uremia undergoing maintenance hemodialysis. Kidney Int 16(5):600–611. https://doi.org/10.1038/ki.1979.170
Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M (2013) Uremic cardiomyopathy: an underdiagnosed disease. Congest Heart Failure 19(4):E40–E50. https://doi.org/10.1111/chf.12030
Radhakrishnan A, Pickup LC, Price AM et al (2019) Coronary microvascular dysfunction: a key step in the development of uraemic cardiomyopathy? Heart 105(17):1302–1309. https://doi.org/10.1136/heartjnl-2019-315138
Kundhal K, Lok CE (2005) Clinical epidemiology of cardiovascular disease in chronic kidney disease. Nephron Clin Pract 101(2):c47–c52. https://doi.org/10.1159/000086221
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305. https://doi.org/10.1056/NEJMoa041031.Erratum.In:NEnglJMed.2008;18(4):4
Mark PB, Johnston N, Groenning BA et al (2006) Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 69(10):1839–1845. https://doi.org/10.1038/sj.ki.5000249
Parfrey PS, Foley RN (1999) The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol 10(7):1606–1615. https://doi.org/10.1681/asn.v1071606
Garikapati K, Goh D, Khanna S, Echampati K (2021) Uraemic cardiomyopathy: a review of current literature. Clin Med Insights Cardiol 15:1179546821998347. https://doi.org/10.1177/1179546821998347
Bailey GL, Hampers CL, Merrill JP (1967) Reversible cardiomyopathy in uremia. ASAIO J 13(1):263–270
Wang X, Shapiro JI (2019) Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol 15(3):159–175. https://doi.org/10.1038/s41581-018-0101-8
Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP (2014) Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 7(7):703–714. https://doi.org/10.1016/j.jcmg.2013.09.025
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23(4):578–585. https://doi.org/10.1681/ASN.2011111115
Kouri AM, Rheault MN (2021) Cardiovascular disease in children with chronic kidney disease. Curr Opin Nephrol Hypertens 30(2):231–236. https://doi.org/10.1097/MNH.0000000000000684
Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV (2015) Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11(12):720–731. https://doi.org/10.1038/nrneph.2015.140
Bellinghieri G, Santoro D, Calvani M, Mallamace A, Savica V (2003) Carnitine and hemodialysis. Am J Kidney Dis 41(3 Suppl 1):S116–S122. https://doi.org/10.1053/ajkd.2003.50099
Thomas SS, Zhang L, Mitch WE (2015) Molecular mechanisms of insulin resistance in chronic kidney disease. Kidney Int 88(6):1233–1239. https://doi.org/10.1038/ki.2015.305
Patel N, Yaqoob MM, Aksentijevic D (2022) Cardiac metabolic remodelling in chronic kidney disease. Nat Rev Nephrol 18(8):524–537. https://doi.org/10.1038/s41581-022-00576-x
Brønnum H, Kalluri R (2012) Chapter 29 – Cardiac fibrosis: cellular and molecular determinants. Muscle 1:389–404. https://doi.org/10.1016/B978-0-12-381510-1.00029-6
Lisowska-Myjak B (2014) Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract 128(3–4):303–311. https://doi.org/10.1159/000369817
Hénaut L, Candellier A, Boudot C et al (2019) New insights into the roles of monocytes/macrophages in cardiovascular calcification associated with chronic kidney disease. Toxins (Basel) 11(9):529. https://doi.org/10.3390/toxins11090529
Grabner A, Faul C (2016) The role of fibroblast growth factor 23 and klotho in uremic cardiomyopathy. Curr Opin Nephrol Hypertens 25(4):314–324. https://doi.org/10.1097/MNH.0000000000000231
Gutierrez OM, Mannstadt M, Isakova T et al (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359:584–592. https://doi.org/10.1056/NEJMoa0706130
Xie J, Yoon J, An SW, Kuro-o M, Huang CL (2015) Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26(5):1150–1160. https://doi.org/10.1681/ASN.2014040325
Shafi S, Saleem M, Anjum R, Abdullah W, Shafi T (2017) ECG abnormalities in patients with chronic kidney disease. J Ayub Med Coll Abbottabad 29(1):61–64
Waks JW, Tereshchenko LG, Parekh RS (2016) Electrocardiographic predictors of mortality and sudden cardiac death in patients with end stage renal disease on hemodialysis. J Electrocardiol 49(6):848–854. https://doi.org/10.1016/j.jelectrocard.2016.07.020
Arrigo M, Cippà PE, Mebazaa A (2018) Cardiorenal interactions revisited: how to improve heart failure outcomes in patients with chronic kidney disease. Curr Heart Fail Rep 15:307–314. https://doi.org/10.1007/s11897-018-0406-8
Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE (2000) Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol 11:912–916. https://doi.org/10.1681/ASN.V115912
Arcari L, Hinojar R, Engel J et al (2020) Native T1 and T2 provide distinctive signatures in hypertrophic cardiac conditions–comparison of uremic, hypertensive and hypertrophic cardiomyopathy. Int J Cardiol 306:102–108. https://doi.org/10.1016/j.ijcard.2020.03.002
McIntyre CW, John SG, Jefferies HJ (2008) Advances in the cardiovascular assessment of patients with chronic kidney disease. Oxford University Press. https://doi.org/10.1093/ndtplus/sfn146
Lau JMC, Raptis DA, Laforest R et al (2018) Cardiac positron emission tomography-magnetic resonance imaging: current status and future directions. J Thorac Imaging 33(3):139–146. https://doi.org/10.1097/RTI.0000000000000327
Kott J, Reichek N, Butler J, Arbeit L, Mallipattu SK (2020) Cardiac imaging in dialysis patients. Kidney Med 2(5):629–638. https://doi.org/10.1016/j.xkme.2020.05.010
Badve SV, Palmer SC, Strippoli GFM et al (2016) The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis 68(4):554–563. https://doi.org/10.1053/j.ajkd.2016.03.418
Shiozaki AA, Santos TS, Artega E, Rochitte CE (2007) Myocardial delayed enhancement by computed tomography in hypertrophic cardiomyopathy. Circulation 115(17):e430–e431. https://doi.org/10.1161/CIRCULATIONAHA.106.674911
Chiu DY, Green D, Abidin N, Sinha S, Kalra PA (2015) Cardiac imaging in patients with chronic kidney disease. Nat Rev Nephrol 11(4):207–220. https://doi.org/10.1038/nrneph.2014.243
Shah NR, Charytan DM, Murthy VL et al (2016) Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 27(6):1823–1829. https://doi.org/10.1681/ASN.2015030301
Hayer MK, Radhakrishnan A, Price AM et al (2019) Early effects of kidney transplantation on the heart: a cardiac magnetic resonance multi-parametric study. Int J Cardiol 293:272–277. https://doi.org/10.1016/j.ijcard.2019.06.007
Arcari L, Camastra G, Ciolina F, Danti M, Cacciotti L (2022) T1 and T2 mapping in uremic cardiomyopathy: an update. Card Fail Rev 8:e02. https://doi.org/10.15420/cfr.2021.19
Muser D, Castro SA, Santangeli P, Nucifora G (2018) Clinical applications of feature-tracking cardiac magnetic resonance imaging. World J Cardiol 10(11):210–221. https://doi.org/10.4330/wjc.v10.i11.210
Kramann R, Erpenbeck J, Schneider RK et al (2014) Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J Am Soc Nephrol 25(10):2351–2365. https://doi.org/10.1681/ASN.2013070734
Carrasco-Ruiz MF, Ruiz-Rivera A, Soriano-Ursúa MA et al (2022) Global longitudinal strain is superior to ejection fraction for detecting myocardial dysfunction in end-stage renal disease with hyperparathyroidism. World J Cardiol 14(4):239–249. https://doi.org/10.4330/wjc.v14.i4.239
Brown NJ (2008) Aldosterone and vascular inflammation. Hypertension 51(2):161–167. https://doi.org/10.1161/HYPERTENSIONAHA.107.095489
Cice G, Ferrara L, Di Benedetto A et al (2001) Dilated cardiomyopathy in dialysis patients–beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol 37(2):407–411. https://doi.org/10.1016/S0735-1097(00)01158-X
Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN (2009) Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol 54(6):505–512. https://doi.org/10.1016/j.jacc.2009.03.066
Adhyapak SM, Iyengar SS (2011) Characteristics of a subset of patients with reversible systolic dysfunction in chronic kidney disease. Congest Heart Fail 17(3):120–126. https://doi.org/10.1111/j.1751-7133.2011.00214.x
Lai S, Molfino A, Russo GE et al (2015) Cardiac, inflammatory and metabolic parameters: hemodialysis versus peritoneal dialysis. Cardiorenal Med 5(1):20–30. https://doi.org/10.1159/000369588
Wali RK, Wang GS, Gottlieb SS et al (2005) Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol 45(7):1051–1060. https://doi.org/10.1016/j.jacc.2004.11.061
Yancy CW, Jessup M, Bozkurt B et al (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62:e147-239. https://doi.org/10.1016/j.jacc.2013.05.019
Jaarsma T, Beattie JM, Ryder M et al (2009) Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 11:433–443. https://doi.org/10.1093/eurjhf/hfp041
Ma D, Mandour AS, Elfadadny A et al (2022) Changes in cardiac function during the development of uremic cardiomyopathy and the effect of salvianolic acid B administration in a rat model. Front Vet Sci 9:905759. https://doi.org/10.3389/fvets.2022.905759
Pfeffer MA, Burdmann EA, Chen CY, for TREAT Investigators, et al (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361(21):2019–2032. https://doi.org/10.1056/NEJMoa0907845
McGonigle R, Fowler M, Timmis A, Weston M, Parsons V (1984) Uremic cardiomyopathy: potential role of vitamin D and parathyroid hormone. Nephron 36(2):94–100. https://doi.org/10.1159/000183125
Macdougall IC, White C, Anker SD, for PIVOTAL Investigators and Committees, et al (2019) Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 380(5):447–458. https://doi.org/10.1056/NEJMoa1810742
Bao JF, Hu PP, She QY, Li A (2020) A land of controversy: fibroblast growth factor-23 and uremic cardiac hypertrophy. J Am Soc Nephrol 31(7):1423–1434. https://doi.org/10.1681/ASN.2020010081
Hu BY, Wang J, Yang ZG et al (2019) Cardiac magnetic resonance feature tracking for quantifying right ventricular deformation in type 2 diabetes mellitus patients. Sci Rep 9(1):11148. https://doi.org/10.1038/s41598-019-46755-y
Acknowledgements
The authors would like to thank Ryota Morimoto, MD, PhD; Toru Kondo, MD, PhD; Shingo Kazama, MD, PhD; Yuki Kimura, MD, PhD; Ryota Ito, MD; and Yuichiro Koyama, MD, for their useful discussion and helpful comments on the manuscript. We also thank John Daniel of Edanz (https://jp.edanz.com/ac) for providing medical writing support, which was funded in part by a research grant from the Nagoya University Medical Association in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
The authors received support from a research grant from the Nagoya University Medical Association for the submitted work.
Author information
Authors and Affiliations
Contributions
Hiroaki Hiraiwa contributed to the conceptualization, investigation, and visualization of this review. Daisuke Kasugai, Takahiro Okumura, and Toyoaki Murohara contributed to the investigation and visualization. All authors contributed to the writing and review of, and read and approved, the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interest
Hiroaki Hiraiwa received lecture fees from Kowa Co. Ltd., Bayer Pharmaceutical Co. Ltd., and Daiichi-Sankyo Co. Ltd. Takahiro Okumura received research grants from Ono Pharmaceutical Co. Ltd., Bayer Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., and Amgen Astellas BioPharma K.K., Pfizer Japan Inc., Alnylam Pharmaceuticals, Inc., Alexion Pharmaceuticals, Inc. Takahiro Okumura received lecture fees from Ono Pharmaceutical Co. Ltd., Otsuka Pharma Ltd., Novartis Pharma K.K., and AstraZeneca K.K. Toyoaki Murohara received an unrestricted research grant from the Department of Cardiology, Nagoya University Graduate School of Medicine, from Astellas Pharma Inc., Daiichi-Sankyo Co. Ltd., Dainippon Sumitomo Pharma Co. Ltd., Kowa Co. Ltd., MSD K.K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Otsuka Pharma Ltd., Pfizer Japan Inc., Sanofi-Aventis K.K., Takeda Pharmaceutical Co. Ltd., and Teijin Pharma Ltd. All other authors declare that they have no relationships with the industry relevant to the contents of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hiraiwa, H., Kasugai, D., Okumura, T. et al. Implications of uremic cardiomyopathy for the practicing clinician: an educational review. Heart Fail Rev 28, 1129–1139 (2023). https://doi.org/10.1007/s10741-023-10318-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10318-1