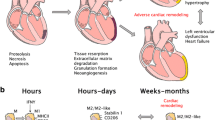
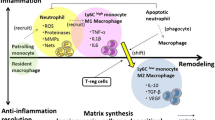
Abstract
Myocardial infarction (MI) is one of the cardiovascular diseases with high morbidity and mortality. MI causes large amounts of apoptotic and necrotic cells that need to be efficiently and instantly engulfed by macrophage to avoid second necrosis. Phagocytic macrophages can dampen or resolve inflammation to protect infarcted heart. Phagocytosis of macrophages is modulated by various factors including proteins, receptors, lncRNA and cytokines. A better understanding of mechanisms in phagocytosis will be beneficial to regulate macrophage phagocytosis capability towards a desired direction in cardioprotection after MI. In this review, we describe the phagocytosis effect of macrophages and summarize the latest reported signals regulating phagocytosis after MI, which will provide a new thinking about phagocytosis-dependent cardiac protection after MI.


Similar content being viewed by others
Abbreviations
- ABs:
-
Apoptotic bodies
- BMDM:
-
Bone marrow-derived macrophage
- BMP2:
-
Bone morphogenetic protein 2
- C1qa:
-
Complement pathway activators
- CCL2:
-
C-C motif chemokine ligand 2
- CCR2:
-
C-C chemokine receptor type 2
- CD36:
-
Cluster of differentiation 36
- CD47:
-
Cluster of differentiation 47
- CD86:
-
Cluster of differentiation 86
- CDC:
-
Cardiosphere-derived cells
- CX3CR1:
-
C-X3-C motif chemokine receptor 1
- CXCL4:
-
Chemokine (C-X-C motif) ligand 4
- DAMPs:
-
Danger-associated molecular patterns
- EDIL3:
-
EGF-like repeats and discoidin I-like domain 3
- ERK:
-
Extracellular signal-regulated kinase
- EVs:
-
Extracellular vesicles
- exos:
-
Exosomes
- FPR1:
-
Formyl peptide receptor 1
- FPR2:
-
Formyl peptide receptor 2
- GAS6:
-
Growth arrest-specific gene 6
- HLA-DR:
-
Human leukocyte antigen DR
- IL-4:
-
Interleukin-4
- IL-10:
-
Interleukin-10
- IL-1β:
-
Interleukin-1β
- IFN-γ:
-
Interferon gamma
- I/R:
-
Ischemia/reperfusion
- iRhom2:
-
Inactive rhomboid protein 2
- IRF4:
-
Interferon regulatory factor 4
- ITIM:
-
Immunoreceptor tyrosine-based inhibition motif
- KDM3A:
-
Lysine-specific demethylase 3A
- LC3-II:
-
Microtubule-associated protein light chain 3-II
- Lgmn:
-
Legumain
- LV:
-
Left ventricular
- MCP-1:
-
Monocyte chemoattractant protein-1
- M-CSF:
-
Macrophage colony-stimulating factor
- MerTK:
-
Myeloid-epithelial-reproductive tyrosine kinase
- MFG-E8:
-
Milk fat globule epidermal growth factor-like factor 8
- MHCII:
-
Major histocompatibility complex class II
- MI:
-
Myocardial infarction
- MMP9:
-
Matrix metalloproteinase-9
- Wnt1:
-
Wingless-type MMTV integration site family, member 1
- MSC:
-
Mesenchymal stem cell
- MVs:
-
Microvesicles
- MyS3KO:
-
Myeloid cell-specific Smad3 knockout mice
- NEAT1:
-
Nuclear enriched abundant transcript 1
- NET:
-
Neutrophil extracellular trap
- Nr4a1:
-
Nuclear receptor subfamily 4 group A member 1
- OPN:
-
Osteopontin
- PAR2:
-
Protease-activated receptor 2
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome 10
- PKCδ:
-
Protein kinase C δ
- PS:
-
Phosphatidyl serine
- PTX3:
-
Pentraxin-3
- RhoA:
-
Ras homolog gene family, member A
- ROS:
-
Reactive oxygen species
- RvD1:
-
Resolvin D1
- S100A9:
-
S100 calcium binding protein A9
- scRNA-seq :
-
Single-cell RNA-sequencing
- SIRPα:
-
Signal regulatory protein-α
- Smad3:
-
Sma-and Mad-related protein 3
- SPMs:
-
Specialized pro-resolving lipid mediators
- STAT3:
-
Signal transducer and activator of transcription 3
- STAT6:
-
Signal transducer and activator of transcription 6
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor α
- TRPV2:
-
Transient receptor potential vanilloid 2
- VEGFA:
-
Vascular endothelial growth factor A
- VEGFC:
-
Vascular endothelial growth factor C
References
Yan J, Horng T (2020) Lipid metabolism in regulation of macrophage functions. Trends Cell Biol 30:979–989. https://doi.org/10.1016/j.tcb.2020.09.006
Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140:871–882. https://doi.org/10.1016/j.cell.2010.02.029
Kain V, Halade GV (2015) Big eater macrophages dominate inflammation resolution following myocardial infarction. J Mol Cell Cardiol 87:225–227. https://doi.org/10.1016/j.yjmcc.2015.08.019
Horckmans M, Ring L, Duchene J et al (2017) Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 38:187–197. https://doi.org/10.1093/eurheartj/ehw002
Frangogiannis NG (2014) The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol 63:185–195. https://doi.org/10.1097/FJC.0000000000000003
Lambert JM, Lopez EF, Lindsey ML (2008) Macrophage roles following myocardial infarction. Int J Cardiol 130:147–158. https://doi.org/10.1016/j.ijcard.2008.04.059
Heidt T, Courties G, Dutta P et al (2014) Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115:284–295. https://doi.org/10.1161/CIRCRESAHA.115.303567
Pinto AR, Ilinykh A, Ivey MJ et al (2016) Revisiting cardiac cellular composition. Circ Res 118:400–409. https://doi.org/10.1161/CIRCRESAHA.115.307778
Ben-Mordechai T, Palevski D, Glucksam-Galnoy Y, Elron-Gross I, Margalit R, Leor J (2015) Targeting macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther 20:36–51. https://doi.org/10.1177/1074248414534916
Leuschner F, Nahrendorf M (2020) Novel functions of macrophages in the heart: insights into electrical conduction, stress, and diastolic dysfunction. Eur Heart J 41:989–994. https://doi.org/10.1093/eurheartj/ehz159
Epelman S, Lavine KJ, Beaudin AE et al (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40:91–104. https://doi.org/10.1016/j.immuni.2013.11.019
DeBerge M, Yeap XY, Dehn S et al (2017) MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res 121:930–940. https://doi.org/10.1161/CIRCRESAHA.117.311327
Bajpai G, Bredemeyer A, Li W et al (2019) Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 124:263–278. https://doi.org/10.1161/CIRCRESAHA.118.314028
Dewald O, Zymek P, Winkelmann K et al (2005) CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96:881–889. https://doi.org/10.1161/01.RES.0000163017.13772.3a
Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M (2004) Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol 165:439–447. https://doi.org/10.1016/S0002-9440(10)63309-3
Jung K, Kim P, Leuschner F et al (2013) Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res 112:891–899. https://doi.org/10.1161/CIRCRESAHA.111.300484
Walter W, Alonso-Herranz L, Trappetti V et al (2018) Deciphering the dynamic transcriptional and post-transcriptional networks of macrophages in the healthy heart and after myocardial injury. Cell Rep 23:622–636. https://doi.org/10.1016/j.celrep.2018.03.029
Swirski FK, Robbins CS, Nahrendorf M (2016) Development and function of arterial and cardiac macrophages. Trends Immunol 37:32–40. https://doi.org/10.1016/j.it.2015.11.004
Peet C, Ivetic A, Bromage DI, Shah AM (2020) Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res 116:1101–1112. https://doi.org/10.1093/cvr/cvz336
Garcia RA, Ito BR, Lupisella JA et al (2019) Preservation of post-infarction cardiac structure and function via long-term oral formyl peptide receptor agonist treatment. JACC Basic Transl Sci 4:905–920. https://doi.org/10.1016/j.jacbts.2019.07.005
Sun K, Li YY, Jin J (2021) A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther 6:79. https://doi.org/10.1038/s41392-020-00455-6
Nd DMJ, Tenkorang-Impraim MAA, Ma Y et al (2020) Exogenous IL-4 shuts off pro-inflammation in neutrophils while stimulating anti-inflammation in macrophages to induce neutrophil phagocytosis following myocardial infarction. J Mol Cell Cardiol 145:112–121. https://doi.org/10.1016/j.yjmcc.2020.06.006
Tourki B, Halade G (2017) Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J 31:4226–4239. https://doi.org/10.1096/fj.201700109R
Hochreiter-Hufford A, Ravichandran KS (2013) Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 5:a8748. https://doi.org/10.1101/cshperspect.a008748
Elliott MR, Ravichandran KS (2010) Clearance of apoptotic cells: implications in health and disease. J Cell Biol 189:1059–1070. https://doi.org/10.1083/jcb.201004096
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119:91–112. https://doi.org/10.1161/CIRCRESAHA.116.303577
Ishimoto Y, Ohashi K, Mizuno K, Nakano T (2000) Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem 127:411–417. https://doi.org/10.1093/oxfordjournals.jbchem.a022622
Seizer P, Schiemann S, Merz T et al (2010) CD36 and macrophage scavenger receptor a modulate foam cell formation via inhibition of lipid-laden platelet phagocytosis. Semin Thromb Hemost 36:157–162. https://doi.org/10.1055/s-0030-1251499
Rinne P, Guillamat-Prats R, Rami M et al (2018) Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler Thromb Vasc Biol 38:2562–2575. https://doi.org/10.1161/ATVBAHA.118.311185
Monaco C, Whitfield J, Jain SS, Spriet LL, Bonen A, Holloway GP (2015) Activation of AMPKalpha2 is not required for mitochondrial FAT/CD36 accumulation during exercise. PLoS ONE 10:e126122. https://doi.org/10.1371/journal.pone.0126122
Talle MA, Rao PE, Westberg E et al (1983) Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol 78:83–99. https://doi.org/10.1016/0008-8749(83)90262-9
Fadok VA, Warner ML, Bratton DL, Henson PM (1998) CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J Immunol 161:6250–6257
DeLeon-Pennell KY, Tian Y, Zhang B et al (2016) CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 9:14–25. https://doi.org/10.1161/CIRCGENETICS.115.001249
Silverstein RL, Febbraio M (2009) CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2:e3. https://doi.org/10.1126/scisignal.272re3
Lindsey ML, Jung M, Yabluchanskiy A et al (2019) Exogenous CXCL4 infusion inhibits macrophage phagocytosis by limiting CD36 signalling to enhance post-myocardial infarction cardiac dilation and mortality. Cardiovasc Res 115:395–408. https://doi.org/10.1093/cvr/cvy211
Glinton KE, Ma W, Lantz C et al (2022) Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J Clin Invest. https://doi.org/10.1172/JCI140685
Dehn S, Thorp EB (2018) Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 32:254–264. https://doi.org/10.1096/fj.201700450R
Driscoll WS, Vaisar T, Tang J, Wilson CL, Raines EW (2013) Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circ Res 113:52–61. https://doi.org/10.1161/CIRCRESAHA.112.300683
Wan E, Yeap XY, Dehn S et al (2013) Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113:1004–1012. https://doi.org/10.1161/CIRCRESAHA.113.301198
Lemke G, Rothlin CV (2008) Immunobiology of the TAM receptors. Nat Rev Immunol 8:327–336. https://doi.org/10.1038/nri2303
Lemke G (2019) How macrophages deal with death. Nat Rev Immunol 19:539–549. https://doi.org/10.1038/s41577-019-0167-y
Zizzo G, Hilliard BA, Monestier M, Cohen PL (2012) Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 189:3508–3520. https://doi.org/10.4049/jimmunol.1200662
Zhang S, Yeap XY, Grigoryeva L et al (2015) Cardiomyocytes induce macrophage receptor shedding to suppress phagocytosis. J Mol Cell Cardiol 87:171–179. https://doi.org/10.1016/j.yjmcc.2015.08.009
de Couto G, Jaghatspanyan E, DeBerge M et al (2019) Mechanism of enhanced MerTK-dependent macrophage efferocytosis by extracellular vesicles. Arterioscler Thromb Vasc Biol 39:2082–2096. https://doi.org/10.1161/ATVBAHA.119.313115
Hanayama R, Tanaka M, Miyasaka K et al (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304:1147–1150. https://doi.org/10.1126/science.1094359
Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC (2007) Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA 104:12005–12010. https://doi.org/10.1073/pnas.0704756104
Howangyin KY, Zlatanova I, Pinto C et al (2016) Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation 133:826–839. https://doi.org/10.1161/CIRCULATIONAHA.115.020857
Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I (2011) Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem 286:33335–33344. https://doi.org/10.1074/jbc.M111.263020
Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11:255–265. https://doi.org/10.1038/nrcardio.2014.28
Yu L, Yang G, Zhang X et al (2018) Megakaryocytic Leukemia 1 bridges epigenetic activation of NADPH oxidase in macrophages to cardiac ischemia-reperfusion injury. Circulation 138:2820–2836. https://doi.org/10.1161/CIRCULATIONAHA.118.035377
Sather S, Kenyon KD, Lefkowitz JB et al (2007) A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109:1026–1033. https://doi.org/10.1182/blood-2006-05-021634
Hanna A, Frangogiannis NG (2020) Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther 34:849–863. https://doi.org/10.1007/s10557-020-07071-0
White GE, Iqbal AJ, Greaves DR (2013) CC chemokine receptors and chronic inflammation–therapeutic opportunities and pharmacological challenges. Pharmacol Rev 65:47–89. https://doi.org/10.1124/pr.111.005074
Xia Y, Frangogiannis NG (2007) MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm Allergy Drug Targets 6:101–107. https://doi.org/10.2174/187152807780832265
Frangogiannis NG (2004) Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res 53:585–595. https://doi.org/10.1007/s00011-004-1298-5
Schack L, Stapulionis R, Christensen B et al (2009) Osteopontin enhances phagocytosis through a novel osteopontin receptor, the alphaXbeta2 integrin. J Immunol 182:6943–6950. https://doi.org/10.4049/jimmunol.0900065
Caberoy NB, Alvarado G, Bigcas JL, Li W (2012) Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol 227:401–407. https://doi.org/10.1002/jcp.22955
Shirakawa K, Endo J, Kataoka M et al (2018) IL (Interleukin)-10-STAT3-Galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation 138:2021–2035. https://doi.org/10.1161/CIRCULATIONAHA.118.035047
Shirakawa K, Endo J, Kataoka M et al (2020) MerTK expression and ERK activation are essential for the functional maturation of osteopontin-producing reparative macrophages after myocardial infarction. J Am Heart Assoc 9:e17071. https://doi.org/10.1161/JAHA.120.017071
Liu X, Chen J, Zhang B, Liu G, Zhao H, Hu Q (2019) KDM3A inhibition modulates macrophage polarization to aggravate post-MI injuries and accelerates adverse ventricular remodeling via an IRF4 signaling pathway. Cell Signal 64:109415. https://doi.org/10.1016/j.cellsig.2019.109415
Gast M, Rauch BH, Haghikia A et al (2019) Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res 115:1886–1906. https://doi.org/10.1093/cvr/cvz085
Chen B, Huang S, Su Y et al (2019) Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circ Res 125:55–70. https://doi.org/10.1161/CIRCRESAHA.119.315069
Humeres C, Venugopal H, Frangogiannis NG (2022) Smad-dependent pathways in the infarcted and failing heart. Curr Opin Pharmacol 64:102207. https://doi.org/10.1016/j.coph.2022.102207
Bujak M, Ren G, Kweon HJ et al (2007) Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116:2127–2138. https://doi.org/10.1161/CIRCULATIONAHA.107.704197
Dobaczewski M, Bujak M, Li N et al (2010) Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107:418–428. https://doi.org/10.1161/CIRCRESAHA.109.216101
Kong P, Shinde AV, Su Y et al (2018) Opposing actions of fibroblast and cardiomyocyte Smad3 Signaling in the infarcted myocardium. Circulation 137:707–724. https://doi.org/10.1161/CIRCULATIONAHA.117.029622
Huang S, Chen B, Su Y et al (2019) Distinct roles of myofibroblast-specific Smad2 and Smad3 signaling in repair and remodeling of the infarcted heart. J Mol Cell Cardiol 132:84–97. https://doi.org/10.1016/j.yjmcc.2019.05.006
Chen B, Li R, Hernandez SC et al (2022) Differential effects of Smad2 and Smad3 in regulation of macrophage phenotype and function in the infarcted myocardium. J Mol Cell Cardiol 171:1–15. https://doi.org/10.1016/j.yjmcc.2022.06.009
Humeres C, Shinde AV, Hanna A et al (2022) Smad7 effects on TGF-beta and ErbB2 restrain myofibroblast activation and protect from postinfarction heart failure. J Clin Invest. https://doi.org/10.1172/JCI146926
Giudice V, Wu Z, Kajigaya S et al (2019) Circulating S100A8 and S100A9 protein levels in plasma of patients with acquired aplastic anemia and myelodysplastic syndromes. Cytokine 113:462–465. https://doi.org/10.1016/j.cyto.2018.06.025
Yi W, Zhu R, Hou X, Wu F, Feng R (2022) Integrated analysis reveals S100a8/a9 regulates autophagy and apoptosis through the MAPK and PI3K-AKT signaling pathway in the early stage of myocardial infarction. Cells. https://doi.org/10.3390/cells11121911
Lin ZL, Liu YC, Gao YL et al (2022) S100A9 and SOCS3 as diagnostic biomarkers of acute myocardial infarction and their association with immune infiltration. Genes Genet Syst 97:67–79. https://doi.org/10.1266/ggs.21-00073
Foell D, Wittkowski H, Vogl T, Roth J (2007) S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 81:28–37. https://doi.org/10.1189/jlb.0306170
Marinkovic G, Koenis DS, de Camp L et al (2020) S100A9 links inflammation and repair in myocardial infarction. Circ Res 127:664–676. https://doi.org/10.1161/CIRCRESAHA.120.315865
Marinkovic G, Grauen Larsen H, Yndigegn T et al (2019) Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. Eur Heart J 40:2713–2723. https://doi.org/10.1093/eurheartj/ehz461
Dall E, Brandstetter H (2016) Structure and function of legumain in health and disease. Biochimie 122:126–150. https://doi.org/10.1016/j.biochi.2015.09.022
Jia D, Chen S, Bai P et al (2022) Cardiac resident macrophage-derived legumain improves cardiac repair by promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation 145:1542–1556. https://doi.org/10.1161/CIRCULATIONAHA.121.057549
Barnette DN, Cahill TJ, Gunadasa-Rohling M, Carr CA, Freeman M, Riley PR (2018) iRhom2-mediated proinflammatory signalling regulates heart repair following myocardial infarction. JCI Insight. https://doi.org/10.1172/jci.insight.98268
Garcia RA, Lupisella JA, Ito BR et al (2021) Selective FPR2 agonism promotes a proresolution macrophage phenotype and improves cardiac structure-function post myocardial infarction. JACC Basic Transl Sci 6:676–689. https://doi.org/10.1016/j.jacbts.2021.07.007
Entin-Meer M, Levy R, Goryainov P et al (2014) The transient receptor potential vanilloid 2 cation channel is abundant in macrophages accumulating at the peri-infarct zone and may enhance their migration capacity towards injured cardiomyocytes following myocardial infarction. PLoS ONE 9:e105055. https://doi.org/10.1371/journal.pone.0105055
Barclay AN, Van den Berg TK (2014) The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32:25–50. https://doi.org/10.1146/annurev-immunol-032713-120142
Tsai RK, Discher DE (2008) Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 180:989–1003. https://doi.org/10.1083/jcb.200708043
Zhang S, Yeap XY, DeBerge M et al (2017) Acute CD47 blockade during ischemic myocardial reperfusion enhances phagocytosis-associated cardiac repair. JACC Basic Transl Sci 2:386–397. https://doi.org/10.1016/j.jacbts.2017.03.013
Salio M, Chimenti S, De Angelis N et al (2008) Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117:1055–1064. https://doi.org/10.1161/CIRCULATIONAHA.107.749234
Maugeri N, Rovere-Querini P, Slavich M et al (2011) Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J Immunol 187:970–979. https://doi.org/10.4049/jimmunol.1100261
Guo T, Ke L, Qi B et al (2012) PTX3 is located at the membrane of late apoptotic macrophages and mediates the phagocytosis of macrophages. J Clin Immunol 32:330–339. https://doi.org/10.1007/s10875-011-9615-6
Jaillon S, Jeannin P, HamonY, et al (2009) Endogenous PTX3 translocates at the membrane of late apoptotic human neutrophils and is involved in their engulfment by macrophages. Cell Death Differ 16:465–474. https://doi.org/10.1038/cdd.2008.173
Shiraki A, Kotooka N, Komoda H, Hirase T, Oyama JI, Node K (2016) Pentraxin-3 regulates the inflammatory activity of macrophages. Biochem Biophys Rep 5:290–295. https://doi.org/10.1016/j.bbrep.2016.01.009
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. https://doi.org/10.3390/cells8070727
Lai RC, Arslan F, Lee MM et al (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4:214–222. https://doi.org/10.1016/j.scr.2009.12.003
Wysoczynski M, Khan A, Bolli R (2018) New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res 123:138–158. https://doi.org/10.1161/CIRCRESAHA.118.313251
Mentkowski KI, Mursleen A, Snitzer JD, Euscher LM, Lang JK (2020) CDC-derived extracellular vesicles reprogram inflammatory macrophages to an arginase 1-dependent proangiogenic phenotype. Am J Physiol Heart Circ Physiol 318:H1447–H1460. https://doi.org/10.1152/ajpheart.00155.2020
de Couto G, Gallet R, Cambier L et al (2017) Exosomal microRNA transfer into macrophages mediates cellular postconditioning. Circulation 136:200–214. https://doi.org/10.1161/CIRCULATIONAHA.116.024590
Patil M, Saheera S, Dubey PK et al (2021) Novel mechanisms of exosome-mediated phagocytosis of dead cells in injured heart. Circ Res 129:1006–1020. https://doi.org/10.1161/CIRCRESAHA.120.317900
Sun S, Wu Y, Maimaitijiang A, Huang Q, Chen Q (2022) Ferroptotic cardiomyocyte-derived exosomes promote cardiac macrophage M1 polarization during myocardial infarction. PeerJ 10:e13717. https://doi.org/10.7717/peerj.13717
Zheng C, Sui B, Zhang X et al (2021) Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles 10:e12109. https://doi.org/10.1002/jev2.12109
Thorp EB (2012) Contrasting inflammation resolution during atherosclerosis and post myocardial infarction at the level of monocyte/macrophage phagocytic clearance. Front Immunol 3:39. https://doi.org/10.3389/fimmu.2012.00039
Ma Y, Yabluchanskiy A, Iyer RP et al (2016) Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110:51–61. https://doi.org/10.1093/cvr/cvw024
Sreejit G, Abdel-Latif A, Athmanathan B et al (2020) Neutrophil-derived S100A8/A9 amplify Granulopoiesis after myocardial infarction. Circulation 41:1080–1094. https://doi.org/10.1161/CIRCULATIONAHA.119.043833
Daseke MJ II, Chalise U, Becirovic-Agic M et al (2021) Neutrophil signaling during myocardial infarction wound repair. Cell Signal 77:109816. https://doi.org/10.1016/j.cellsig.2020.109816
Sokol CL, Luster AD (2015) The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a016303
Daseke MJ, Valerio FM, Kalusche WJ, Ma Y, DeLeon-Pennell KY, Lindsey ML (2019) Neutrophil proteome shifts over the myocardial infarction time continuum. Basic Res Cardiol 114:37. https://doi.org/10.1007/s00395-019-0746-x
Ho HK, Jang JJ, Kaji S et al (2004) Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: its role in ischemia. Circulation 109:1314–1319. https://doi.org/10.1161/01.CIR.0000118465.36018.2D
Wei X, Zou S, Xie Z et al (2022) EDIL3 deficiency ameliorates adverse cardiac remodelling by neutrophil extracellular traps (NET)-mediated macrophage polarization. Cardiovasc Res 118:2179–2195. https://doi.org/10.1093/cvr/cvab269
Fredman G, Spite M (2017) Specialized pro-resolving mediators in cardiovascular diseases. Mol Aspects Med 58:65–71. https://doi.org/10.1016/j.mam.2017.02.003
Kain V, Ingle KA, Colas RA et al (2015) Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 84:24–35. https://doi.org/10.1016/j.yjmcc.2015.04.003
Chiang N, Serhan CN (2017) Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med 58:114–129. https://doi.org/10.1016/j.mam.2017.03.005
Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM (2006) Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281:38376–38384. https://doi.org/10.1074/jbc.M605146200
Dalli J, Serhan CN (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120:e60–e72. https://doi.org/10.1182/blood-2012-04-423525
Schmid M, Gemperle C, Rimann N, Hersberger M (2016) Resolvin D1 polarizes primary human macrophages toward a proresolution phenotype through GPR32. J Immunol 196:3429–3437. https://doi.org/10.4049/jimmunol.1501701
Cai B, Thorp EB, Doran AC et al (2016) MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci USA 113:6526–6531. https://doi.org/10.1073/pnas.1524292113
Vago JP, Amaral FA, van de Loo FAJ (2021) Resolving inflammation by TAM receptor activation. Pharmacol Ther 227:107893. https://doi.org/10.1016/j.pharmthera.2021.107893
Rymut N, Heinz J, Sadhu S et al (2020) Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. FASEB J 34:597–609. https://doi.org/10.1096/fj.201902126R
Park M, Shen YT, Gaussin V et al (2009) Apoptosis predominates in nonmyocytes in heart failure. Am J Physiol Heart Circ Physiol 297:H785–H791. https://doi.org/10.1152/ajpheart.00310.2009
Weinberger T, Rauber S, Schneider V et al (2021) Differential MHC-II expression and phagocytic functions of embryo-derived cardiac macrophages in the course of myocardial infarction in mice. Eur J Immunol 51:250–252. https://doi.org/10.1002/eji.202048560
Leblond AL, Klinkert K, Martin K et al (2015) Systemic and cardiac depletion of M2 macrophage through CSF-1R signaling inhibition alters cardiac function post myocardial infarction. PLoS ONE 10:e137515. https://doi.org/10.1371/journal.pone.0137515
Shiraishi M, Shintani Y, Shintani Y et al (2016) Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 126:2151–2166. https://doi.org/10.1172/JCI85782
Ma Y, Halade GV, Zhang J et al (2013) Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112:675–688. https://doi.org/10.1161/CIRCRESAHA.111.300502
Kubota A, Frangogiannis NG (2022) Macrophages in myocardial infarction. Am J Physiol Cell Physiol 323:C1304–C1324. https://doi.org/10.1152/ajpcell.00230.2022
DeBerge M, Zhang S, Glinton K et al (2017) Efferocytosis and outside-in signaling by cardiac phagocytes. Links to repair, cellular programming, and intercellular crosstalk in heart. Front Immunol 8:1428. https://doi.org/10.3389/fimmu.2017.01428
DeBerge M, Glinton K, Subramanian M et al (2021) Macrophage AXL receptor tyrosine kinase inflames the heart after reperfused myocardial infarction. J Clin Invest. https://doi.org/10.1172/JCI139576
Funding
This study was supported by the National Natural Science Foundation of China (82074053, 81930114, U22A20368), Scientific Program of Traditional Chinese Medicine Bureau of Guangdong Province (20231101), Key-Area Research and Development Program of Guangdong Province (No. 2020B1111100004), University Research Program of Guangdong Provincial Department Education (2021ZDZX1010), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab, 2020B1212030006).
Author information
Authors and Affiliations
Contributions
Jiahua Li and Qi Chen: writing—original draft preparation. Rong Zhang: manuscript revision. Zhongqiu Liu and Yuanyuan Cheng: manuscript design, supervision and revision.
Corresponding authors
Ethics declarations
Ethics approval
No human and/ or animal subjects were contained when summarizing this review article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Chen, Q., Zhang, R. et al. The phagocytic role of macrophage following myocardial infarction. Heart Fail Rev 28, 993–1007 (2023). https://doi.org/10.1007/s10741-023-10314-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10314-5