Abstract
Atrial fibrillation (AF) and atrial flutter (AFL) are associated with adverse outcomes in patients with heart failure and reduced ejection fraction (HFrEF). We investigated the effects of sodium-glucose cotransporter-2 inhibitors (SGLT2i) on the incidence of AF and/or AFL in HFrEF patients. PubMed and ClinicalTrials.gov were systematically searched until March 2022 for randomized controlled trials (RCTs) that enrolled patients with HFrEF. A total of six RCTs with 9467 patients were included (N = 4731 in the SGLT2i arms; N = 4736 in the placebo arms). Compared to placebo, SGLT2i treatment was associated with a significant reduction in the risk of AF [relative risk (RR) 0.62, 95% confidence interval CI 0.44–0.86; P = 0.005] and AF/AFL (RR 0.64, 95% CI 0.47–0.87; P = 0.004). Subgroup analysis showed that empagliflozin use resulted in a significant reduction in the risk of AF (RR 0.55, 95% CI 0.34–0.89; P = 0.01) and AF/AFL (RR 0.50, 95% CI 0.32–0.77; P = 0.002). By contrast, dapagliflozin use was not associated with a significant reduction in the risk of AF (RR 0.69, 95% CI 0.43–1.11; P = 0.12) or AF/AFL (RR 0.82, 95% CI 0.53–1.27; P = 0.38). Additionally, a “shorter” duration (< 1.5 years) of treatment with SGLT2i remained associated with a reduction in the risk of AF (< 1.5 years; RR 0.58, 95% CI 0.36–0.91; P = 0.02) and AF/AFL (< 1.5 years; RR 0.52, 95% CI 0.34–0.80; P = 0.003). In conclusion, SGLT2i therapy was associated with a significant reduction in the risk of AF and AF/AFL in patients with HFrEF. These results reinforce the value of using SGLT2i in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) and heart failure with reduced ejection fraction (HFrEF) frequently coexist, and each affects the course and treatment of the other [1]. In patients with HFrEF, AF, especially if new-onset or paroxysmal, has been associated with a significantly increased risk of adverse outcomes [2]. Additionally, atrial flutter (AFL), although much less well studied, is considered to have a similar clinical impact [3]. Furthermore, AF and AFL impose significant therapeutic challenges in the setting of HFrEF [4]. Therefore, prevention of these arrhythmias represents an important target in patients with HFrEF and treatments that reduce their burden may yield considerable clinical benefits.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce the risk of HF hospitalization and cardiovascular mortality in patients with HFrEF and represent an effective novel therapeutic modality for this condition [4,5,6,7,8]. Interestingly, recent clinical evidence is also indicative of a potential therapeutic effect of SGLT2i on AF and/or AFL. In particular, a post hoc analysis of the Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial demonstrated that dapagliflozin decreases the relative risk of AF/AFL in individuals with type 2 diabetes and high cardiovascular risk [9]. Furthermore, recent meta-analyses have shown that treatment with SGLT2i was associated with a lower incidence of AF and AF/AFL in individuals with type 2 diabetes [10], as well as in mixed populations comprised of patients with type 2 diabetes, chronic kidney disease, and HF [11,12,13,14,15]. However, to the best of our knowledge, no randomized controlled trials (RCTs) or meta-analyses have addressed the relationship between SGLT2i and these arrhythmias in patients with HFrEF.
Therefore, a systematic review and meta-analysis of RCTs that enrolled patients with HFrEF was performed, examining serious adverse event reports of AF and/or AFL according to randomized assignment to SGLT2i or placebo. Furthermore, subgroup analyses were conducted to compare the SGLT2i treatment effect between different drug types and length of follow-up.
Methods
This meta-analysis was conducted according to the Cochrane Handbook (Version 6.3) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy
PubMed and ClinicalTrials.gov were systematically searched for eligible studies from their inception to March 03, 2022. We screened published and unpublished RCTs using the following search strategy: (sodium-glucose cotransporter-2 inhibitors OR SGLT2 inhibitors OR SGLT-2 inhibitors OR SGLT 2 inhibitors OR empagliflozin OR dapagliflozin OR canagliflozin OR ertugliflozin OR tofogliflozin OR ipragliflozin OR luseogliflozin OR remogliflozin OR sergliflozin OR sotagliflozin) AND (heart failure OR heart failure with reduced ejection fraction OR HFrEF OR atrial fibrillation OR atrial flutter). No language restrictions were set. Reference lists of included studies were carefully checked to identify additional studies.
Inclusion and exclusion criteria
Eligible RCTs had to meet the following criteria: (i) enroll patients aged 18 years or older with a diagnosis of HFrEF [left ventricular ejection fraction (LVEF) ≤ 40%]; (ii) compare SGLT2i with placebo; and (iii) report at least one of the predefined outcomes of interest. RCTs focused on conditions other than HFrEF were excluded. Trial eligibility was assessed by two independent reviewers (D.S. and T.L.). Discrepancies between reviewers were resolved by consensus or, if necessary, by a third investigator (P.K.).
Outcomes of interest
Primary outcomes of interest included the incidence of AF, AFL, and the composite of AF/AFL (new-onset and recurrent events of relevant arrhythmias) reported as serious adverse events according to Medical Dictionary for Drug Regulatory Activities (MedDRA). Prespecified subgroup analyses by SGLT2i agent used and follow-up duration were conducted to compare the SGLT2i treatment effect between different drug types and length of follow-up. Subgroup analysis by dosage was not performed because the included studies examined only the doses of 10 mg empagliflozin and 10 mg dapagliflozin.
Data extraction and quality assessment
Two reviewers (D.S. and T.L.) independently performed data extraction using a standard form, including study characteristics, sample size, study design, follow-up duration, baseline characteristics of study population, interventions, comparisons, and outcomes of interest. Quality assessment of included RCTs was conducted using the Cochrane Risk of Bias Tool (performed by D.S. and T.L.). Discrepancies were resolved by consensus, or if necessary, by adjudication from a third investigator (P.K.).
Statistical analysis
The collected raw data were used to calculate relative risks (RRs) and 95% confidence intervals (CIs), which were then pooled in meta-analyses. The pooled RRs and corresponding 95% CIs were used to present the incidence of AF, AFL, and the composite of AF/AFL. Heterogeneity was assessed using the Cochran Q statistic and Higgins and Thompson I2 before the meta-analysis. For the Q test, a P value < 0.1 was considered statistically significant. Additionally, I2 > 50% indicated at least moderate heterogeneity. If heterogeneity was present, as suggested by P value < 0.1 or I2 > 50%, the random-effects (RE) model was applied; otherwise, the fixed effects (FE) model was applied. All analyses were conducted using Review Manager (RevMan, version 5.4, The Cochrane Collaboration, 2020).
Results
Characteristics of eligible studies
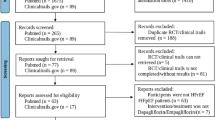
Among the 2164 records identified, six RCTs [5, 6, 16,17,18,19] including 9467 patients with HFrEF (N = 4731 in the SGLT2i arms; N = 4736 in the placebo arms) matched the predefined inclusion criteria and were included in the final meta-analysis. The PRISMA flow diagram is presented in Supplementary material online, Appendix Fig. S1. The baseline characteristics of the included studies are summarized in Table 1. Except for two unpublished trials (DETERMINE-reduced; NCT03877237 [17] and EMPERIAL-reduced; NCT03448419 [18]), the other four trials [5, 6, 16, 19] have been published. Six RCTs [5, 6, 16,17,18,19] reported AF, and two RCTs [5, 6] reported AFL outcomes. No RCTs reported AF/AFL as a composite event. The mean age of the participants was 61.3–69 years, the proportion of males ranged from 73.3% to 76.6%, and the median follow-up duration ranged from 12 weeks to 18.2 months. Among the included studies, three trials [6, 16, 18] examined the effects of empagliflozin and three trials [5, 17, 19] the effects of dapagliflozin. Notably, only the doses of 10 mg empagliflozin and 10 mg dapagliflozin were examined.
The results of the quality assessment are presented in Supplementary material online, Appendix Figs. S2 and S3. All the included RCTs were of high methodological quality. The participants included in the safety analysis set in some RCTs were fewer than those in the full analysis set, which yielded a 13-person difference between the baseline population (N = 9467) and the outcome evaluation population (N = 9454). Since the difference between the two sets was very small (0.13% of the total sample size), it was considered unlikely that it could have influenced the pooled results. Therefore, a low risk of bias was still considered.
Incidence of atrial fibrillation
AF was reported in six RCTs [5, 6, 16,17,18,19]. A total of 142 AF events were reported, of which 54 occurred in the SGLT2i group (N = 4725) and 88 in the placebo group (N = 4729). After pooling the six trials, treatment with SGLT2i was associated with a significant reduction in the risk of AF compared to placebo (RR, 0.62, 95% CI, 0.44–0.86; P = 0.005) (Fig. 1A). There was no significant heterogeneity across trials (P = 0.90; I2 = 0%). Visual inspection of the funnel plot suggested no apparent publication bias (Fig. 1B). In subgroup analysis by SGLT2i agent used, empagliflozin use resulted in a significant reduction in the risk of AF (RR, 0.55; 95% CI, 0.34–0.89; P = 0.01), whereas dapagliflozin use was not associated with a reduction in the risk of AF (RR, 0.69, 95% CI, 0.43–1.11; P = 0.12) (Fig. 2A and B). Given that there were five RCTs [6, 16,17,18,19] that had “shorter” duration of follow-up (< 1.5 years) and one RCT [5] that had “longer” duration of follow-up (> 1.5 years), subgroup analysis by follow-up duration was also conducted. When focusing the effects of follow-up duration on AF, “shorter” duration of follow-up (< 1.5 years; RR, 0.58; 95% CI, 0.36–0.91; P = 0.02) remained associated with a significant reduction in the risk of AF (Fig. 3A and B). In subgroup analysis by SGLT2i agent used, and by follow-up duration, the χ2 test for subgroup differences identified no significant subgroup effect (P = 0.50 and P = 0.67, respectively).
A Forest plot of subgroup analysis by SGLT2i agent used comparing the incidence of AF between SGLT2i and placebo. CI, confidence interval; AF, atrial fibrillation; SGLT2i, sodium-glucose cotransporter-2 inhibitors. B Funnel plot of subgroup analysis by SGLT2i agent used comparing the incidence of AF between SGLT2i and placebo. RR, relative risk; AF, atrial fibrillation; SE, standard error
A Forest plot of subgroup analysis by follow-up duration comparing the incidence of AF between SGLT2i and placebo. CI, confidence interval; AF, atrial fibrillation; SGLT2i, sodium-glucose cotransporter-2 inhibitors. B Funnel plot of subgroup analysis by follow-up duration comparing the incidence of AF between SGLT2i and placebo. RR, relative risk; AF, atrial fibrillation; SE, standard error
Incidence of atrial flutter
AFL was reported in two RCTs [5, 6]. A total of 25 AFL events were reported, of which 11 in the SGLT2i group (N = 4231) and 14 in the placebo group (N = 4231). Since substantial heterogeneity was identified (P = 0.02; I2 = 83%), the two trials were pooled using the random-effects model. Overall, there was no significant difference between the SGLT2i arm and the placebo arm in the incidence of AFL (RR, 0.85; 95% CI, 0.09–7.92; P = 0.88) (Supplementary material online, Appendix Fig. S4). Visual inspection of the funnel plot revealed no asymmetry (Supplementary material online, Appendix Fig. S5).
Incidence of the composite of atrial fibrillation and atrial flutter
When AF and AFL were combined as a composite endpoint, treatment with SGLT2i was associated with a significant reduction in the risk of AF/AFL compared to placebo (RR: 0.64, 95% CI: 0.47–0.87; P = 0.004) (Fig. 4A). There was no significant heterogeneity across trials (P = 0.62; I2 = 0%). Visual inspection of the funnel plot suggested no apparent publication bias (Fig. 4B). In subgroup analysis by SGLT2i agent used, empagliflozin use resulted in a significant reduction in the risk of AF/AFL (RR, 0.50; 95% CI, 0.32–0.77; P = 0.002), whereas dapagliflozin use was not associated with a significant reduction in the risk of AF/AFL (RR, 0.82; 95% CI, 0.53–1.27; P = 0.38) (Fig. 5 A and Supplementary material online, Appendix Fig. S6). Given that there were five RCTs [6, 16,17,18,19] that had “shorter” duration of follow-up (< 1.5 years) and one RCT [5] that had “longer” duration of follow-up (> 1.5 years), subgroup analysis by follow-up duration was also conducted. When focusing the effects of follow-up duration on the composite of AF/AFL, “shorter” duration of follow-up (< 1.5 years; RR, 0.52; 95% CI, 0.34–0.80; P = 0.003) was still associated with a significant reduction in the risk of AF/AFL (Fig. 5B and Supplementary material online, Appendix Fig. S7). In subgroup analysis by SGLT2i agent used, and by follow-up duration, the χ2 test for subgroup differences identified no significant subgroup effect (P = 0.11, and P = 0.16, respectively). Interestingly, there was substantial heterogeneity between the subgroups of empagliflozin and dapagliflozin (I2 = 61%) and moderate heterogeneity between the subgroups of shorter and longer duration of follow-up (I2 = 48.5%).
A Forest plot comparing the incidence of AF/AFL between SGLT2i and placebo. CI, confidence interval; AF, atrial fibrillation; AFL, atrial flutter; SGLT2i, sodium–glucose cotransporter-2 inhibitors. B Funnel plot of meta-analysis for the incidence of AF/AFL. RR, relative risk; AF, atrial fibrillation; AFL, atrial flutter; SE, standard error
A Forest plot of subgroup analysis by SGLT2i agent used comparing the incidence of AF/AFL between SGLT2i and placebo. CI, confidence interval; AF, atrial fibrillation; AFL, atrial flutter; SGLT2i, sodium-glucose cotransporter-2 inhibitors. B Forest plot of subgroup analysis by follow-up duration comparing the incidence of AF/AFL between SGLT2i and placebo. CI, confidence interval; AF, atrial fibrillation; AFL, atrial flutter; SGLT2i, sodium-glucose cotransporter-2 inhibitors
Discussion
The present meta-analysis demonstrated that SGLT2i treatment was associated with a significant reduction in the risk of AF and AF/AFL in patients with HFrEF. In subgroup analysis by SGLT2i agent used, the use of empagliflozin, but not dapagliflozin, resulted in a significant reduction in the risk of AF and AF/AFL. In subgroup analysis by follow-up duration, a “shorter” duration (< 1.5 years) of treatment with SGLT2i remained associated with a significant reduction in the risk of AF and AF/AFL. As far as AFL is concerned, no significant association was observed.
AF, especially if new-onset or paroxysmal, confers a worse prognosis in patients with HFrEF [2, 20]. In fact, new-onset AF has been associated with a significantly increased risk of HF hospitalization and all-cause mortality, as well as cardiovascular mortality and stroke [2]. Similarly, paroxysmal AF has been shown to increase the risks of HF hospitalization and stroke, as well as pump-failure death [2]. Furthermore, the presence of AF in patients with HFrEF may reduce or abolish the benefits of beta-blockers and cardiac resynchronization therapy (CRT) and renders ivabradine ineffective [4]. In the same context, the Catheter Ablation Versus Standard Conventional Treatment in Patients With Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) study indicated that maintenance of sinus rhythm in patients with HFrEF by catheter ablation of AF significantly reduces mortality [21]. Even though AFL is much less well studied, it is considered to have similar clinical significance and consequences to AF [3]. Therefore, prevention of AF and/or AFL is of particular importance in the setting of HFrEF.
The results of the present meta-analysis suggest that SGLT2i may have a favorable impact on the mechanistic pathways of AF and AF/AFL in patients with HFrEF. Although a significant association between SGLT2i and AFL was not observed, the number of AFL events was low, and this might have contributed to a wide confidence interval. Nonetheless, AFL is considered to have similar clinical significance and consequences to AF, and the combined analysis of AF/AFL showing a significant risk reduction might eliminate possible publication bias for AFL. In support of the results of this meta-analysis, SGLT2i have recently been shown to mitigate adverse cardiac remodeling and fibrosis [16, 22,23,24]. Furthermore, SGLT2i have been demonstrated to optimize loading conditions through their effect on natriuresis and diuresis [25]. Of note, SGLT2i have been shown to increase natriuresis without off-target electrolyte wasting, renal dysfunction, and neurohormonal activation [26]. In addition to the above, recent evidence suggests that SGLT2i might ameliorate HFrEF- and AF-related Na+ and Ca2+ handling abnormalities in the atrial cardiomyocyte level, which may prevent the triggering and maintenance of AF [27,28,29]. Even further, SGLT2i have been demonstrated to improve mitochondrial function and cardiac energy metabolism [30, 31]. Also, these agents ameliorate inflammation and oxidative stress through several mechanisms, including suppression of NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome and activation of 5′ AMP-activated protein kinase (AMPK) [30, 31]. Moreover, SGLT2i have been demonstrated to inhibit the sympathetic nervous system activity either directly or because of a reduction of renal afferent sympathetic activation [30, 32]. Nevertheless, SGLT2i have been shown to improve renal function which can indirectly improve cardiac function through a reduction in afferent sympathetic nervous system activation, attenuation of inflammation, and amelioration of oxidative stress [30]. Finally, SGLT2i have been shown to reduce epicardial fat mass, HbA1c, body weight, blood pressure, and uric acid levels, while they also increase erythropoietin levels and improve vascular function [30, 33].
Limitations
This meta-analysis has several potential limitations. Firstly, AF and AFL were not the prespecified outcomes of the included trials; therefore, there might be ascertainment bias. Secondly, only AF or AFL events that were reported as serious adverse events according to MedDRA were included. Thirdly, the trials included were underpowered to study arrhythmia outcomes. Fourthly, the included trials examined only empagliflozin and dapagliflozin. Fifthly, most of the weight of the statistical analysis depended only on two studies, namely, EMPEROR-Reduced and DAPA-HF. Sixthly, most of the weight of the empagliflozin and dapagliflozin subgroups depended on the EMPEROR-Reduced and DAPA-HF trials, respectively, and therefore the results of subgroup analysis by agent used may have been influenced by the different populations included in them. Consequently, whether the use of empagliflozin results in a significant reduction in the risk of AF and AF/AFL, and dapagliflozin does not, needs to be further investigated. Seventhly, there were five RCTs that had shorter duration of follow-up (< 1.5 years), and only one RCT that had relatively longer duration of follow-up (> 1.5 years), restricting the significance of the results of subgroup analysis by follow-up duration. Finally, the included trials examined only the doses of 10 mg empagliflozin and 10 mg dapagliflozin, rendering subgroup analysis by dosage unfeasible. In this context, further research with adequately powered RCTs to study arrhythmia outcomes is needed.
Conclusion
SGLT2i therapy was associated with a significant reduction in the risk of AF and AF/AFL in patients with HFrEF. These results reinforce the value of using SGLT2i in these patients. Further data with properly designed RCTs are needed to confirm the present findings, as well as to evaluate and elucidate the potential antiarrhythmic effects of SGLT2i in patients with HFrEF.
Data availability
Data are available from the corresponding author on reasonable request.
References
Verma A, Kalman JM, Callans DJ (2017) Treatment of patients with atrial fibrillation and heart failure with reduced ejection fraction. Circulation 135:1547–1563
Mogensen UM, Jhund PS, Abraham WT, Desai AS, Dickstein K, Packer M, PARADIGM-HF and ATMOSPHERE Investigators and Committees et al (2017) Type of atrial fibrillation and outcomes in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 70:2490–2500
Diamant MJ, Andrade JG, Virani SA, Jhund PS, Petrie MC, Hawkins NM (2021) Heart failure and atrial flutter: a systematic review of current knowledge and practices. ESC Heart Fail 8:4484–4496
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, ESC Scientific Document Group et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, DAPA-HF Trial Committees and Investigators et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381:1995–2008
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, EMPEROR-Reduced Trial Investigators et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383:1413–1424
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396:819–829
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, SOLOIST-WHF Trial Investigators et al (2021) Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384:117–128
Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA et al (2020) Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 Trial. Circulation 141:1227–1234
Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH (2020) SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol 19:130
Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C et al (2021) Protective effects of sodium-glucose transporter 2 inhibitors on atrial fibrillation and atrial flutter: a systematic review and meta-analysis of randomized placebo-controlled trials. Front Endocrinol (Lausanne) 12:619586
Zheng RJ, Wang Y, Tang JN, Duan JY, Yuan MY, Zhang JY (2021) Association of SGLT2 inhibitors with risk of atrial fibrillation and stroke in patients with and without type 2 diabetes: a systemic review and meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol 79:e145-152
Li HL, Lip GYH, Feng Q, Fei Y, Tse YK, Wu MZ et al (2021) Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol 20:100
Pandey AK, Okaj I, Kaur H, Belley-Cote EP, Wang J, Oraii A et al (2021) Sodium-glucose co-transporter inhibitors and atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 10:e022222
Okunrintemi V, Mishriky BM, Powell JR, Cummings DM (2021) Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes Metab 23:276–280
Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT et al (2021) Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 143:516–525
An International, Multicentre, Parallel-group, Randomised, Double-blind, Placebo-controlled, Phase III Study Evaluating the effect of Dapagliflozin on Exercise Capacity in Heart Failure Patients with Reduced Ejection Fraction (HFrEF) (DETERMINE-reduced). https://clinicaltrials.gov/ct2/show/NCT03877237
A phase III randomised, double-blind trial to evaluate the effect of 12 weeks treatment of once daily EMPagliflozin 10 mg compared with placebo on ExeRcise ability and heart failure symptoms, In patients with chronic HeArt FaiLure with reduced Ejection Fraction (HFrEF) (EMPERIAL-reduced). https://clinicaltrials.gov/ct2/show/NCT03448419
Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE et al (2019) Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation 140:1463–1476
Naka KK, Bazoukis G, Bechlioulis A, Korantzopoulos P, Michalis LK, Ntzani EE (2019) Association between atrial fibrillation and patient-important outcomes in heart failure patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes 5:96–104
Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L et al (2021) Atrial fibrillation burden and clinical outcomes in heart failure: the CASTLE-AF trial. JACC Clin Electrophysiol 7:594–603
Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbæk L et al (2021) Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire HF randomized clinical trial. JAMA Cardiol 6:836–840
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, EMPA-TROPISM (ATRU-4) Investigators et al (2021) Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 77:243–255
Zhang N, Wang Y, Tse G, Korantzopoulos P, Letsas KP, Zhang Q et al (2022) Effect of sodium-glucose cotransporter-2 inhibitors on cardiac remodelling: a systematic review and meta-analysis. Eur J Prev Cardiol 28:1961–1973
Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK et al (2020) Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 76:2740–2751
Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C et al (2020) Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 142:1028–1039
Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM et al (2018) Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail 5:642–648
Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R et al (2017) Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60:568–573
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A et al (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61:722–726
Lopaschuk GD, Verma S (2020) Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 5:632–644
Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW et al (2018) Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol 9:1575
Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, EMBODY trial investigators et al (2020) Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 19:148
Masson W, Lavalle-Cobo A, Nogueira JP (2021) Effect of SGLT2-inhibitors on epicardial adipose tissue: a meta-analysis. Cells 10:2150
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Dimitrios Sfairopoulos: acquisition, analysis, and interpretation of data. Drafted the manuscript. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Tong Liu: acquisition, analysis, and interpretation of data. Revised the manuscript critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Nan Zhang: acquisition, analysis, and interpretation of data. Drafted the manuscript. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Gary Tse: Analysis and interpretation of data. Drafted the manuscript. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. George Bazoukis: analysis and interpretation of data. Drafted the manuscript. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Konstantinos Letsas: analysis and interpretation of data. Revised the manuscript critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Christos Goudis: design of the work. Revised the manuscript critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Haralampos Milionis: conception and design of the work. Revised the manuscript critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Apostolos Vrettos: design of the work. Revised the manuscript critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Panagiotis Korantzopoulos: conception and design of the work. Revised the manuscript critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
What’s new
• SGLT2i therapy was associated with a significant reduction in the risk of AF and AF/AFL in patients with HFrEF.
• The use of empagliflozin, but not dapagliflozin, resulted in a significant reduction in the risk of AF and AF/AFL.
• A “shorter” duration (< 1.5 years) of treatment with SGLT2i remained associated with a significant reduction in the risk of AF and AF/AFL.
• These results reinforce the value of using SGLT2i in patients with HFrEF.
Supplementary Information
Below is the link to the electronic supplementary material.
10741_2022_10281_MOESM1_ESM.docx
Supplementary file1 (DOCX 39 KB) Supplementary material online, Appendix Figure S1: Prisma flow diagram of study selection process
10741_2022_10281_MOESM2_ESM.pdf
Supplementary file2 (PDF 238 KB) Supplementary material online, Appendix Figure S2: Results of the quality assessment (risk of bias graph)
10741_2022_10281_MOESM3_ESM.pdf
Supplementary file3 (PDF 263 KB) Supplementary material online, Appendix Figure S3: Results of the quality assessment (risk of bias summary)
10741_2022_10281_MOESM4_ESM.pdf
Supplementary file4 (PDF 257 KB) Supplementary material online, Appendix Figure S4: Forest plot comparing the incidence of AFL between SGLT2i and placebo. CI, confidence interval; AFL, atrial flutter; SGLT2i, sodium–glucose cotransporter-2 inhibitors
10741_2022_10281_MOESM5_ESM.pdf
Supplementary file5 (PDF 28 KB) Supplementary material online, Appendix Figure S5: Funnel plot of meta-analysis for the incidence of AFL. RR, relative risk; AFL, atrial flutter; SE, standard error
10741_2022_10281_MOESM6_ESM.pdf
Supplementary file6 (PDF 58 KB) Supplementary material online, Appendix Figure S6: Funnel plot of subgroup analysis by SGLT2i agent used comparing the incidence of AF/AFL between SGLT2i and placebo. RR, relative risk; AF, atrial fibrillation; AFL, atrial flutter; SE, standard error
10741_2022_10281_MOESM7_ESM.pdf
Supplementary file7 (PDF 86 KB) Supplementary material online, Appendix Figure S7: Funnel plot of subgroup analysis by follow-up duration comparing the incidence of AF/AFL between SGLT2i and placebo. RR, relative risk; AF, atrial fibrillation; AFL, atrial flutter; SE, standard error
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sfairopoulos, D., Liu, T., Zhang, N. et al. Association between sodium-glucose cotransporter-2 inhibitors and incident atrial fibrillation/atrial flutter in heart failure patients with reduced ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev 28, 925–936 (2023). https://doi.org/10.1007/s10741-022-10281-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10281-3