Abstract
Coronavirus disease 2019 (COVID-19) is due to severe acute respiratory syndrome coronavirus (SARS-CoV)-2 which binds and enters the host cells through the angiotensin-converting enzyme (ACE)2. While the potential for benefit with the use of renin-angiotensin-aldosterone system inhibitors (RAASi) and the risks from stopping them is more evident, potential harm by RAΑSi may also be caused by the increase in the activity of the ACE2 receptor, the inefficient counter regulatory axis in the lungs in which the proinflammatory prolyloligopeptidase (POP) is the main enzyme responsible for the conversion of deleterious angiotensin (ANG) II to protective ANG [1–7] and the proinflammatory properties of ACE2(+) cells infected with SARS-CoV-2. Acknowledging the proven RAΑSi benefit in patients with several diseases such as hypertension, heart failure, coronary disease, and diabetic kidney disease in the non-COVID-19 era, it is a reasonable strategy in this period of uncertainty to use these agents judiciously with careful consideration and to avoid the use of RAASi in select patients whenever possible, until definitive evidence becomes available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
“Prudence is the footprint of Wisdom.”
— Amos Bronson Alcott
Coronavirus disease 2019 (COVID-19) is due to severe acute respiratory syndrome coronavirus (SARS-CoV)-2 which binds and enters the host cells through the angiotensin-converting enzyme (ACE)2. Therapy with renin-angiotensin-aldosterone system (RAAS) inhibitors (ACE inhibitors [ACEi], angiotensin [ANG] II receptor blockers [ARB], and mineralocorticoid receptor antagonists [MRA]) may increase ACE2 [1]. The concern, therefore, has been raised that a potential increase in ACE2 by these agents may facilitate development and increase severity of COVID-19. In this brief review, we contend that a potential harmful effect of RAΑS inhibitors cannot be excluded in the COVID-19 era and thus, caution required when prescribing these agents.
Lung renin angiotensin system
The traditional view is that some of renin-angiotensin system intermediate products may be processed in alternative ways by ACE2, establishing a second axis through ACE2/ANG [1–7]/Mas receptor which counteracts the effects of the classical axis [2]. However, recent evidence suggests that prolyloligopeptidase (POP) is the main enzyme responsible for the conversion of deleterious ANG II to protective ANG [1–7] in the circulation and lungs, whereas ACE2 contributes to ANG [1–7] formation in the kidney [3]. In addition, POP is an important player in neutrophilic inflammation [4]. Regarding Mas, further studies need to clarify its relationship with ANG [1–7], which may depend on the specific cell types and their expression of other G protein-coupled receptors [5]. Thus, it is questionable whether an efficient ACE2/ANG [1–7]/Mas axis is operative in the lungs.
ACE2 and coronavirus entry
The major entry of SARS-CoV-2 is via the respiratory system where it readily affects differentiated cells that express more ACE2 [6]. The type II transmembrane serine proteases TMPRSS2 and ADAM17 (a disintegrin and metalloproteinase) promote SARS-CoV entry by ACE2 cleavage, promotes viral uptake, and SARS-S cleavage, resulting in activation of the viral S protein, which in turn fuses with cell membrane [7]. The attachment of the viruses to cell surface ACE2 protects them from immune surveillance mechanisms and provides the virus access to the host cell system, an environment not just to sustain and proliferate, but also to mutate and modify host evasion mechanisms. The precise relationship, however, between ACE2 levels and activity, viral infectivity, and severity of infection, is incompletely understood.
ACE2 and lung inflammatory injury
Prior to COVID-19 pandemic, it was suggested that ACE2 dysregulation is implicated in acute inflammatory lung injury (ILI) by inducing an imbalance in the RAS. It was proposed that in acute ILI: (i) a decrease in pulmonary ACE2 and an increase in ANG II levels occur; (ii) supplementation with ACE2 or inhibition of ANG II improves outcomes; and (iii) a lack or decrease of pulmonary ACE2 aggravates viral-induced ILI [8]. However, these findings are not applicable to COVID-19 because in pre COVID-19 studies, ACE2 was not involved in the pathogenesis of ILI [9,10,11,12]. Thus, the protective effect of ACE2 observed in non-SARS-CoV models of ILI could be deleterious in ILI related to COVID-19 where ACE2 serves as the receptor for viral entry [13]. The expression of ACE2, SARS-CoV spike (S) protein, and some proinflammatory cytokines (PICs) in autopsy tissues from patients who died of SARS was studied with immunohistochemistry (IHC) and in situ hybridization (ISH) assays [13]. SARS-CoV S protein and its RNA were only detected in ACE2(+) cells in the lungs and other organs, indicating that ACE2-expressing cells are the primary targets for SARS-CoV infection in vivo in humans. High levels of PICs were expressed in the SARS-CoV-infected ACE2(+) cells, but not in the uninfected cells suggesting that cells infected by SARS-CoV produce elevated levels of PICs which may cause immuno-mediated damage to the lungs resulting in ILI [13]. Similar were the findings in a recent study [14] which used gene set enrichment analysis (GSEA) and showed that the high expression of ACE2 is related to innate immune responses, adaptive immune responses, B cell regulation, and cytokine secretion, as well as an enhanced cytokine-induced inflammatory response. Based on these findings, it has been postulated that the immune system dysfunction involved in the high expression of ACE2 may be related to the development of the hyperinflammatory response in COVID-19 [14]. Consistent with these lung inflammatory changes, a persistent increase in inflammatory markers, such as c-reactive protein, d-dimer, ferritin, interleukin-6, is associated with major complications and increased mortality in COVID-19 [15]. Moreover, obesity, which is associated with chronic inflammation and high expression of ACE2, significantly increases the risk for severe COVID-19 even in younger age groups [16].
Effect of RAAS inhibitors on ACE
RAAS inhibitors (RAASi) with the possible exception of aliskiren (a direct inhibitor of renin), which is infrequently used, tend to increase tissue ACE2 activity [17,18,19]. However, professional scientific societies recommend the unrestricted prescription of RAASi during the COVID-19 pandemic. In a recent statement of the European Medicinal Agencies (EMA), it is emphasized (10 June 2020 EMA/284513/2020): “Recent observational studies of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs, also called sartans) have not shown an effect of these medicines on the risk of becoming infected with severe acute respiratory syndrome coronavirus 2 (the virus causing COVID-19) and do not indicate a negative impact on the outcome for patients with COVID-19 disease. EMA therefore reiterates its previous advice that patients should continue to use ACE inhibitors or ARBs as advised by their doctors”.
Table 1 provides a summary of all the references on which the EMA statement is based. It is obvious that (1) all studies, both positive and negative, are retrospective and observational (OSs); (2) some studies have a low sample size and other are preprints; (3) importantly, not all studies support the safety of RAASi; and (4) some studies supporting the safety of RAASi emphasize that caution is required in the interpretation of the findings. Thus, the recommendations of the EMA like those of the other scientific societies are based mostly on speculation and not on solid evidence.
Research in the COVID-19 era: the lack of randomized studies
In cases of epidemics when answers are urgently required, the quality of research is usually diminished when many teams of investigators are involved in the same field [20]. In this setting, even senior investigators may not follow the established rules and deviation of the rules may lead in unintentional errors and occasionally in catastrophic results. Further, members of scientific societies are under pressure and feel that they have an obligation to provide answers, even when such answers are not available.
Answering the question whether RAASi are safe in the COVID-19 context is crucial. The current prevailing view is that medical practices should be based on well-designed randomized controlled trials (RCTs). This is the best way to show a benefit or at least no harm in hard outcomes (mortality or morbidity), and current standards of care should be systematically subjected to such testing [21]. However, some questions about therapy cannot wait for RCTs to be conducted (e.g., RAASi safety in the COVID-19 context) and scientific societies feel that they have an obligation to make recommendations based on observational studies (OSs).
OSs, however, do not provide the final answer. Based on OSs, one cannot be certain that the recommendations are accurate and to assess if one drug is better or safer than another. Importantly, harm related to therapy cannot be excluded even after application of multiple adjustment techniques [22]. The conclusions of these studies, therefore, should not be taken as a surrogate for appropriate randomized data to guide the management with RAASi in the COVID-19 context [22].
Clinical implications
“ΩΦΕΛΕΕΙΝ Η ΜΗ ΒΛΑΠΤΕΙΝ” (if you cannot help at least do not harm)-Hippocrates
When it comes to one’s medical worldview, there is no neutral position. Every clinician makes the choice daily. For most of us, our decisions are capricious, uneven, and arbitrary based upon our personal experiences and beliefs [21]. For interventions we spurn, we proudly assert that “there is no evidence.” For others that we favor, we stress that “there are no negative studies” and rely on “anecdotal experience” and/or results from registries and not randomized trials. For other interventions with promising rationale and negative empirical trials, we argue that null data are flawed [23].
Over the past four decades, results from well-done RCTs have repeatedly contradicted practices supported by clinical observation [24]. A typical paradigm was hormone replacement therapy in post-menopausal women. In the Women’s Health Initiative (WHI, n = 151,870), the results of OSs and RCTs differed for the association of hormone therapy with outcome after adjusting for confounding factors and stratifying on factors that were hypothesized to modulate the effects of hormone therapy or that empirically modulated the effects of hormone therapy [25]. Likewise, beta-blockade therapy in patients with heart failure (HF) and preserved ejection fraction (HFpEF) reduced mortality in 15 OSs (n = 26,211), whereas it did not decrease mortality in two RCTs (n = 888) [26]. As a result, in the guidelines, beta-blockers are not recommended for the treatment of HFpEF. These are just few examples that OSs studies proved wrong. Thus, OSs cannot replace RCTs, even after application of multiple adjustment techniques.
In our current practice when it comes to prescribe a RAASi in the COVID-19 context, we base our decision on the following premises: (a) COVID-19 is a pandemic responsible for millions of infections and hundreds of thousands deaths worldwide; (b) COVID-19 is due to SARS-CoV-2 which binds and enters host cells through the ACE2 receptor; (c) the protective ACE2/ANG [1–7]/Mas axis may not be efficiently operative in the lungs; (d) RAASi may increase the tissue expression of ACE2; (e) lethal complications of COVID-19 are more common in diseases frequently treated with RAASi such as hypertension, other cardiovascular disease, and diabetes; (f) confounding cannot be corrected even after application of multiple adjustment techniques; (g) RAASi had proved lifesaving in the pre COVID-19 era; (h) RCTs testing the efficacy and safety of RAASi in the COVID-19 era are lacking; and (i) considering that RCTs are lacking we must follow the trail to the next best external evidence and work from there [27].
Based on the above, we believe that in the current period of uncertainty, the decision to prescribe RAASi should be individualized and based on evidence originating from physiology, pathophysiologic mechanisms, observational studies OSs, and clinical judgment. In this regard, the strategy that we follow is outlined below:
-
(A)
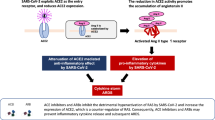
Patients in whom RAASi proved beneficial in the pre-COVID-19 era but are currently not indispensable (Fig. 1). Effective agents which potentially do not affect RAAS should be considered [28, 29]. Guideline-recommended alternatives depend on the underlying disease and include beta-blockers, which reduce plasma concentrations of ANG II (the ACE2 substrate) by reducing renin release from the kidneys as well as cleavage of ANG I to ANG II as well as calcium channel blockers, which are neutral concerning ANG II availability. Regarding diuretics, which are RAAS stimulators, torsemide is a highly effective diuretic agent that lowers blood pressure [30] and has favorable effects on neurohormones, electrolytes, cardiac remodeling, but has been predominantly used in patients with HF [31]. There is no doubt that switching from a RAASi to another antihypertensive therapy in stable ambulatory patients may occasionally be challenging. However, this challenge should be taken in the era of this lethal COVID-19 pandemic.
-
(B)
Patients in whom RAΑSi proved beneficial in the pre-COVID-19 era but are currently indispensable (Fig. 1). RAAS inhibitors should be prescribed and in patients already treated with these agents they should not be discontinued [28, 29]. Sodium-glucose cotransporter 2 inhibitors (SGLT2i), a new drug class approved for treatment of diabetes, have been shown to significantly reduce atherosclerotic events, hospitalization for HF, cardiovascular and total mortality, and progression of chronic kidney disease (CKD) even in patients without diabetes [32]. Increasing experimental and clinical data demonstrated a reduction in neurohormonal activity with these agents, including in key target organs such as the heart and the kidneys [32].
Rational and algorithm for the use of renin-angiotensin-aldosterone system (RAAS) inhibitors in the coronavirus disease 2019 (COVID-19) era. It should be noted that this strategy, like all the other recommendations on this issue, is not based on solid evidence due to the lack of the relevant randomized control trials. However, it is an individualized approach and, in this regard, better than the “one size fits all” approaches. (+): present; (−−/+): possibly absent; (−): absent; RAS, renin-angiotensin system; LVEF: left ventricular ejection fraction; ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; MRA: mineralocorticoid receptor antagonist; SGLT2, sodium glucose cotransporter 2; *, not yet in guidelines but effectiveness documented in randomized control trials
Undoubtedly, further experimental and clinical data are required to clarify the role of RAAS modulation in COVID-19. The important issue that the outcome of a viral infection may be related to therapy provided for another underlying disease should be taken into consideration not only for COVID-19 but for other future viral or non-viral infections as well. In the meantime, it is important to caution that the strategy that we follow is the result of obligatory decision making in a period that solid data are lacking and new ones emerge daily regarding mechanisms, clinical characteristics, treatment options, and outcomes for COVID-19. Steadfast use of ACEi/ARB based on current guidelines should be strongly encouraged when there are no alternatives. Deviation from these practices until solid evidence becomes available should be avoided.
Conclusion
Evidence regarding the efficacy and safety of RAΑSi in the COVID-19 context is based on OSs, which have provided useful information in the past but have also led to errors even after application of multiple adjustment techniques. While the potential for benefit with the use of RAASi and the risks from stopping them is more evident, potential harm by RAΑSi may also be caused by the increase in the activity of the ACE2 receptor which the SARS-CoV-2 binds and enters into the host, the doubtful protective effects of the ACE2/ANG [1–7]/Mas axis in the lungs, and the proinflammatory properties of ACE2(+) cells infected with SARS-CoV-2. Acknowledging the proven RAΑSi benefit in patients with several diseases such as hypertension, heart failure, coronary disease, and diabetic kidney disease in the non-COVID-19 era, it is a reasonable strategy in this period of uncertainty to use these agents judiciously with careful consideration to avoid the use of RAASi in select patients whenever possible, until definitive evidence becomes available. Shared decision making with patient and caregivers is important in this. Admittedly, this strategy, like all the other recommendations on this issue, is not based on solid evidence but is intended to be individualized and, in this regard, differs from the “one size fits all” approach. It is obvious that in a complex biological system where multiple factors interact, one cannot incorporate all these factors into the guidelines and/or algorithms. In this case, sound clinical judgment and common sense should be used by the clinician for the individual patient. There is no substitute for that.
Future perspective
Until now, studies evaluating the pharmacological properties of the different drugs focused on their pharmacokinetic and pharmacodynamic effects. The possibility that a viral infection may alter drug effects and consequently the final disease outcome was not taken into consideration. This is a new area of research in which pharmacologists, virologists, epidemiologists, and clinicians should be involved.
References
Sommerstein R, Kochen MM, Messerli FH, Grani C (2020) Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc 9(7):e016509. https://doi.org/10.1161/JAHA.120.016509
Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ (2018) The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 98(1):505–553. https://doi.org/10.1152/physrev.00023.2016
Serfozo P, Wysocki J, Gulua G, Schulze A, Ye M, Liu P, Jin J, Bader M, Myohanen T, Garcia-Horsman JA, Batlle D (2020) Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 75(1):173–182. https://doi.org/10.1161/HYPERTENSIONAHA.119.14071
Szul T, Bratcher PE, Fraser KB, Kong M, Tirouvanziam R, Ingersoll S, Sztul E, Rangarajan S, Blalock JE, Xu X, Gaggar A (2016) Toll-like receptor 4 engagement mediates prolyl endopeptidase release from airway epithelia via exosomes. Am J Respir Cell Mol Biol 54(3):359–369. https://doi.org/10.1165/rcmb.2015-0108OC
Bader M, Alenina N, Young D, Santos RAS, Touyz RM (2018) The meaning of Mas. Hypertension 72(5):1072–1075. https://doi.org/10.1161/HYPERTENSIONAHA.118.10918
Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr (2005) ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79(23):14614–14621. https://doi.org/10.1128/JVI.79.23.14614-14621.2005
Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pohlmann S (2014) TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88(2):1293–1307. https://doi.org/10.1128/JVI.02202-13
Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY (2020) Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res 126:1456–1474. https://doi.org/10.1161/CIRCRESAHA.120.317015
Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, Francis J, Katovich MJ, Daniell H, Raizada MK (2014) Oral delivery of angiotensin-converting enzyme 2 and angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64(6):1248–1259. https://doi.org/10.1161/HYPERTENSIONAHA.114.03871
Rigatto K, Casali KR, Shenoy V, Katovich MJ, Raizada MK (2013) Diminazene aceturate improves autonomic modulation in pulmonary hypertension. Eur J Pharmacol 713(1–3):89–93. https://doi.org/10.1016/j.ejphar.2013.04.017
Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, Thebaud B (2012) Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med (Berl) 90(6):637–647. https://doi.org/10.1007/s00109-012-0859-2
Treml B, Neu N, Kleinsasser A, Gritsch C, Finsterwalder T, Geiger R, Schuster M, Janzek E, Loibner H, Penninger J, Loeckinger A (2010) Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit Care Med 38(2):596–601. https://doi.org/10.1097/CCM.0b013e3181c03009
He L, Ding Y, Zhang Q, Che X, He Y, Shen H, Wang H, Li Z, Zhao L, Geng J, Deng Y, Yang L, Li J, Cai J, Qiu L, Wen K, Xu X, Jiang S (2006) Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 210(3):288–297. https://doi.org/10.1002/path.2067
Li G, He X, Zhang L, Ran Q, Wang J, Xiong A, Wu D, Chen F, Sun J, Chang C (2020) Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun:102463. https://doi.org/10.1016/j.jaut.2020.102463
Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, Metra M (2020) COVID 19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 22:957–966. https://doi.org/10.1002/ejhf.1871
Kassir R (2020) Risk of COVID-19 for patients with obesity. Obes Rev 21(6):e13034. https://doi.org/10.1111/obr.13034
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE (2005) Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111(20):2605–2610. https://doi.org/10.1161/CIRCULATIONAHA.104.510461
Karram T, Abbasi A, Keidar S, Golomb E, Hochberg I, Winaver J, Hoffman A, Abassi Z (2005) Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am J Physiol Heart Circ Physiol 289(4):H1351–H1358. https://doi.org/10.1152/ajpheart.01186.2004
Mourad JJ, Levy BI (2020) Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat Rev Cardiol 17(5):313. https://doi.org/10.1038/s41569-020-0368-x
Ioannidis JP (2005) Why most published research findings are false. PLoS Med 2(8):e124. https://doi.org/10.1371/journal.pmed.0020124
Prasad V (2013) Why randomized controlled trials are needed to accept new practices: 2 medical worldviews. Mayo Clin Proc 88(10):1046–1050. https://doi.org/10.1016/j.mayocp.2013.04.026
Shah R, Murthy VL, Koupenova M (2020) ACEing COVID-19: a role for angiotensin axis inhibition in SARS-CoV-2 infection? Circ Res 126:1682–1684. https://doi.org/10.1161/CIRCRESAHA.120.317174
Siontis GC, Tatsioni A, Katritsis DG, Ioannidis JP (2009) Persistent reservations against contradicted percutaneous coronary intervention indications: citation content analysis. Am Heart J 157(4):695–701. https://doi.org/10.1016/j.ahj.2008.11.023
Fanaroff AC, Califf RM, Harrington RA, Granger CB, McMurray JJV, Patel MR, Bhatt DL, Windecker S, Hernandez AF, Gibson CM, Alexander JH, Lopes RD (2020) Randomized trials versus common sense and clinical observation: JACC review topic of the week. J Am Coll Cardiol 76(5):580–589. https://doi.org/10.1016/j.jacc.2020.05.069
Hartz A, He T, Wallace R, Powers J (2013) Comparing hormone therapy effects in two RCTs and two large observational studies that used similar methods for comprehensive data collection and outcome assessment. BMJ Open 3(7):e002556. https://doi.org/10.1136/bmjopen-2013-002556
Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH (2015) Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev 20(2):193–201. https://doi.org/10.1007/s10741-014-9453-8
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn't. BMJ 312(7023):71–72. https://doi.org/10.1136/bmj.312.7023.71
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 69(12):1167. https://doi.org/10.1016/j.rec.2016.11.005
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD (2018) 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339
Roca-Cusachs A, Aracil-Vilar J, Calvo-Gomez C, Vaquer-Perez JV, Laporta-Crespo F, Rojas-Serrano MJ, Guglietta A, Gropper S, Torasemide PRiHCTIG (2008) Clinical effects of torasemide prolonged release in mild-to-moderate hypertension: a randomized noninferiority trial versus torasemide immediate release. Cardiovasc Ther 26(2):91–100. https://doi.org/10.1111/j.1527-3466.2008.00046.x
Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M (2006) Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart 92(10):1434–1440. https://doi.org/10.1136/hrt.2005.079764
Zelniker TA, Braunwald E (2020) Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol 75(4):422–434. https://doi.org/10.1016/j.jacc.2019.11.031
Author information
Authors and Affiliations
Contributions
FT, RCS, JB, and HB had the idea for the article.
FT, AX, and HB performed the literature search and data analysis.
FT, RCS, AX, JB, and HB drafted and/or critically revised the work.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
F.T. has received research support and honoraria from Amgen, Bayer, Boehringer Ingelheim, Elpen, Lilly, Menarini, Merck, Novartis, Sanofi, Servier, Vianex, and WinMedica.
R.C.S. has received research funding from Covia, Amgen, advisory board Medtronic, advisor and steering committee Cardiac Dimensions, steering committee Novartis.
A.X. has received honoraria from Novartis.
J.B. has received research support from the National Institutes of Health, Patient Centered Outcomes Research Institute and the European Union. He serves as a consultant for Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, LinaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, StealthPeptide, V-Wave Limited, Vifor, and ZS Pharma.
H.B. has no disclosures.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Triposkiadis, F., Starling, R.C., Xanthopoulos, A. et al. Renin-angiotensin-system inhibition in the context of corona virus disease-19: experimental evidence, observational studies, and clinical implications. Heart Fail Rev 26, 381–389 (2021). https://doi.org/10.1007/s10741-020-10022-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-10022-4