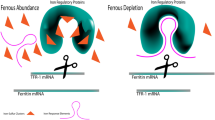
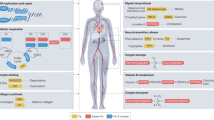
Abstract
Heart failure (HF) is a potentially debilitating condition, with a prognosis comparable to many forms of cancer. It is often complicated by anemia and iron deficiency (ID), which have been shown to even further harm patients’ functional status and hospitalization risk. Iron is a cellular micronutrient that is essential for oxygen uptake and transportation, as well as mitochondrial energy production. Iron is crucially involved in electrochemical stability, maintenance of structure, and contractility of cardiomyocytes. There is mounting evidence that ID indeed hampers the homeostasis of these properties. Animal model and stem cell research has verified these findings on the cellular level, while clinical trials that treat ID in HF patients have shown promising results in improving real patient outcomes, as electromechanically compromised cardiomyocytes translate to HF exacerbations and arrhythmias in patients. In this article, we review our current knowledge on the role of iron in cardiac muscle cells, the contribution of ID to anemia and HF pathophysiology and the capacity of IV iron therapy to ameliorate the patients’ arrhythmogenic profile, quality of life, and prognosis.

Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- EPO:
-
Erythropoietin
- FCM:
-
Ferric carboxymaltose
- HF:
-
Heart failure
- HFrEF:
-
Heart failure with reduced ejection fraction
- ID:
-
Iron deficiency
- IREs:
-
Iron-responsive elements
- IRP:
-
Iron regulatory proteins
- IV:
-
Intravenous
- LVEF:
-
Left ventricle ejection fraction
- mID:
-
Myocardial iron deficiency
- OXPHOS:
-
Oxidative phosphorylation
- Tfr1:
-
Transferrin receptor 1
- TSAT:
-
Transferrin saturation
References
Roger VL (2013) Epidemiology of heart failure. Circ Res 113:646–659. https://doi.org/10.1161/CIRCRESAHA.113.300268
Anand IS, Gupta P (2018) Anemia and iron deficiency in heart failure. Circulation 138:80–98. https://doi.org/10.1161/CIRCULATIONAHA.118.030099
Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI (2006) Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol 48:2485–2489. https://doi.org/10.1016/j.jacc.2006.08.034
Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJV, Anker SD, Ponikowski P (2010) Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 31:1872–1880. https://doi.org/10.1093/eurheartj/ehq158
Handelman GJ, Levin NW (2008) Iron and anemia in human biology: a review of mechanisms. Heart Fail Rev 13:393–404. https://doi.org/10.1007/s10741-008-9086-x
Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM (2006) Renal impairment and outcomes in heart failure. Systematic Review and Meta-Analysis. J Am Coll Cardiol 47:1987–1996. https://doi.org/10.1016/j.jacc.2005.11.084
Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, Mancini DM (2003) Hemodilution is common in patients with advanced heart failure. Circulation 107:226–229. https://doi.org/10.1161/01.CIR.0000052623.16194.80
Miller WL (2016) Fluid volume overload and congestion in heart failure. Circ Heart Fail 9:1–10. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002922
Grote Beverborg N, van Veldhuisen DJ, van der Meer P (2018) Anemia in heart failure. JACC Heart Fail 6:201–208. https://doi.org/10.1016/j.jchf.2017.08.023
Gossmann J, Thürmann P, Bachmann T, Weller S, Kachel HG, Schoeppe W, Scheuermann EH (1996) Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients. Kidney Int 50:973–978. https://doi.org/10.1038/ki.1996.398
Graafland AD, Doorenbos CJ, Saase JCLM (1992) Enalapril-induced anemia in two kidney transplant recipients. Transpl Int 5:51–53. https://doi.org/10.1007/BF00337190
Eckardt KU, Kurtz A, Bauer C (1989) Regulation of erythropoietin production is related to proximal tubular function. Am J Phys 256:F942–F947
von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD (2018) Iron deficiency in heart failure. JACC Heart Fail 7:36–46. https://doi.org/10.1016/j.jchf.2018.07.015
Zhang H, Zhabyeyev P, Wang S, Oudit GY (2018) Role of iron metabolism in heart failure: from iron deficiency to iron overload. Biochim Biophys Acta Mol Basis Dis 1865:1925–1937. https://doi.org/10.1016/j.bbadis.2018.08.030
Paul BT, Manz DH, Torti FM, Torti SV (2017) Mitochondria and iron: current questions. Expert Rev Hematol 10:65–79. https://doi.org/10.1080/17474086.2016.1268047
Hower V, Mendes P, Torti FM, Laubenbacher R, Akman S, Shulaev V, Torti SV (2009) A general map of iron metabolism and tissue-specific subnetworks. Mol BioSyst 5:422–443. https://doi.org/10.1039/b816714c
Lakhal-Littleton S, Wolna M, Chung YJ, Christian HC, Heather LC, Brescia M, Ball V, Diaz R, Santos A, Biggs D, Clarke K, Davies B, Robbins PA (2016) An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife 5:1–25. https://doi.org/10.7554/eLife.19804
Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flögel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T (2017) Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 38:362–372. https://doi.org/10.1093/eurheartj/ehw333
Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P (2018) Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 20:910–919. https://doi.org/10.1002/ejhf.1154
Klip IT, Comin-Colet J, Voors AA et al (2013) Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 165:575–582.e3. https://doi.org/10.1016/j.ahj.2013.01.017
Okonko DO, Mandal AKJ, Missouris CG, Poole-Wilson PA (2011) Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 58:1241–1251. https://doi.org/10.1016/j.jacc.2011.04.040
Cunha GJL, Rocha BML, Menezes Falcão L (2018) Iron deficiency in chronic and acute heart failure: a contemporary review on intertwined conditions. Eur J Intern Med 52:1–7. https://doi.org/10.1016/j.ejim.2018.04.013
Cappellini MD, Comin-Colet J, de Francisco A, Dignass A, Doehner W, Lam CS, Macdougall IC, Rogler G, Camaschella C, Kadir R, Kassebaum NJ, Spahn DR, Taher AT, Musallam KM, on behalf of the IRON CORE Group (2017) Iron deficiency across chronic inflammatory conditions: international expert opinion on definition, diagnosis, and management. Am J Hematol 92:1068–1078. https://doi.org/10.1002/ajh.24820
Arora NP, Ghali JK (2013) Iron deficiency anemia in heart failure. Heart Fail Rev 18:485–501. https://doi.org/10.1007/s10741-012-9342-y
Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P (2016) Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail 18:786–795. https://doi.org/10.1002/ejhf.473
Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P (2018) Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 20:125–133. https://doi.org/10.1002/ejhf.823
Andrews NC (2000) Iron homeostasis: insights from genetics and animal models. Nat Rev Genet 1:208–217. https://doi.org/10.1038/35042073
Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC (2015) Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep 13:533–545. https://doi.org/10.1016/j.celrep.2015.09.023
Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH (2006) Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med 84:349–364. https://doi.org/10.1007/s00109-005-0029-x
Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ (2006) Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci 103:13612–13617. https://doi.org/10.1073/pnas.0606424103
Kumfu S, Chattipakorn S, Chinda K, Fucharoen S, Chattipakorn N (2012) T-type calcium channel blockade improves survival and cardiovascular function in thalassemic mice. Eur J Haematol 88:535–548. https://doi.org/10.1111/j.1600-0609.2012.01779.x
Przybyszewska J, Zekanowska E (2014) The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol 9:208–213. https://doi.org/10.5114/pg.2014.45102
Lakhal-Littleton S, Wolna M, Carr CA, Miller JJJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D, Stillion R, Holdship P, Larner F, Tyler DJ, Clarke K, Davies B, Robbins PA (2015) Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc Natl Acad Sci 112:3164–3169. https://doi.org/10.1073/pnas.1422373112
Maeder MT, Khammy O, dos Remedios C, Kaye DM (2011) Myocardial and systemic iron depletion in heart failure. J Am Coll Cardiol 58:474–480. https://doi.org/10.1016/j.jacc.2011.01.059
Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, Pluhacek T, Spatenka J, Kovalcikova J, Drahota Z, Kautzner J, Pirk J, Houstek J (2017) Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail 19:522–530. https://doi.org/10.1002/ejhf.640
Sovari AA (2016) Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol Res Pract 2016:1–7. https://doi.org/10.1155/2016/9656078
Kumar V, Santhosh Kumar TR, Kartha CC (2018) Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail Rev 24:255–267. https://doi.org/10.1007/s10741-018-9756-2
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond atp production. Circ Res 113:709–724. https://doi.org/10.1161/CIRCRESAHA.113.300376
Kobak KA, Radwańska M, Dzięgała M, Kasztura M, Josiak K, Banasiak W, Ponikowski P, Jankowska EA (2018) Structural and functional abnormalities in iron-depleted heart. Heart Fail Rev 24:269–277. https://doi.org/10.1007/s10741-018-9738-4
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Beverborg NG, Klip IT, Meijers WC et al (2018) Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 11:1–11. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004519
Langer AL, Ginzburg YZ (2017) Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment of anemia of chronic inflammation. Hemodial Int 21:S37–S46. https://doi.org/10.1111/hdi.12543
Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, Steinbeck L, Kube J, Bekfani T, Scherbakov N, Valentova M, Sandek A, Doehner W, Springer J, Anker SD, von Haehling S (2016) The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co-morbidities aggravating heart failure. Int J Cardiol 205:6–12. https://doi.org/10.1016/j.ijcard.2015.11.178
Toblli JE, Lombraña A, Duarte P, Di Gennaro F (2007) Intravenous Iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 50:1657–1665. https://doi.org/10.1016/j.jacc.2007.07.029
Enjuanes C, Klip IT, Bruguera J et al (2014) Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 174:268–275. https://doi.org/10.1016/j.ijcard.2014.03.169
Lip GYH, Heinzel FR, Gaita F, Juanatey JRG, le Heuzey JY, Potpara T, Svendsen JH, Vos MA, Anker SD, Coats AJ, Haverkamp W, Manolis AS, Chung MK, Sanders P, Pieske B, Document Reviewers, Gorenek B, Lane D, Boriani G, Linde C, Hindricks G, Tsutsui H, Homma S, Brownstein S, Nielsen JC, Lainscak M, Crespo-Leiro M, Piepoli M, Seferovic P, Savelieva I (2016) European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 18:12–36. https://doi.org/10.1093/europace/euv191
Sciatti E, Lombardi C, Ravera A, Vizzardi E, Bonadei I, Carubelli V, Gorga E, Metra M (2016) Nutritional deficiency in patients with heart failure. Nutrients 8. https://doi.org/10.3390/nu8070442
McDonagh T, Macdougall IC (2015) Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail 17:248–262. https://doi.org/10.1002/ejhf.236
Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne-Nickens P, Butler J, Braunwald E, for the NHLBI Heart Failure Clinical Research Network (2017) Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency the IRONOUT HF randomized clinical trial. JAMA 317:1958–1966. https://doi.org/10.1001/jama.2017.5427
Hussain I, Bhoyroo J, Butcher A et al (2013) Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia 2013:1–10. https://doi.org/10.1155/2013/169107
Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P, FAIR-HF Trial Investigators (2009) Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 361:2436–2448. https://doi.org/10.1056/NEJMoa0908355
Ponikowski P, Van Veldhuisen DJ, Comin-Colet J et al (2015) Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 36:657–668. https://doi.org/10.1093/eurheartj/ehu385
van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen-Solal A, EFFECT-HF Investigators (2017) Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 136:1374–1383. https://doi.org/10.1161/CIRCULATIONAHA.117.027497
Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P (2008) Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. J Am Coll Cardiol 51:103–112. https://doi.org/10.1016/j.jacc.2007.09.036
Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N (2013) IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol 168:3439–3442. https://doi.org/10.1016/j.ijcard.2013.04.181
Mosterd A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93:1137–1146. https://doi.org/10.1136/hrt.2003.025270
Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp-Pedersen C, Andersson C (2017) Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation 135:1214–1223. https://doi.org/10.1161/CIRCULATIONAHA.116.025941
Rocha BML, Cunha GJL, Menezes Falcão LF (2018) The burden of iron deficiency in heart failure. Therapeutic Approach J Am Coll Cardiol 71:782–793. https://doi.org/10.1016/j.jacc.2017.12.027
Cohen-Solal A, Leclercq C, Deray G, Lasocki S, Zambrowski JJ, Mebazaa A, de Groote P, Damy T, Galinier M (2014) Iron deficiency: an emerging therapeutic target in heart failure. Heart 100:1414–1420. https://doi.org/10.1136/heartjnl-2014-305669
Yang KC, Kyle JW, Makielski JC, Dudley SC (2015) Mechanisms of sudden cardiac death: oxidants and metabolism. Circ Res 116:1937–1955. https://doi.org/10.1161/CIRCRESAHA.116.304691
van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ (2008) Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res 81:420–428. https://doi.org/10.1093/cvr/cvn282
Bertero E, Maack C (2018) Metabolic remodelling in heart failure. Nat Rev Cardiol 15:457–470. https://doi.org/10.1038/s41569-018-0044-6
Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJV, O'Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ (2013) Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 368:1210–1219. https://doi.org/10.1056/NEJMoa1214865
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakogiannis, C., Briasoulis, A., Mouselimis, D. et al. Iron deficiency as therapeutic target in heart failure: a translational approach. Heart Fail Rev 25, 173–182 (2020). https://doi.org/10.1007/s10741-019-09815-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09815-z