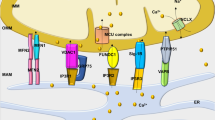
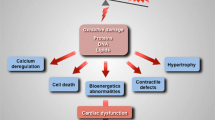
Abstract
Mitochondrial dysfunction is widely recognized as a major factor for the progression of cardiac failure. Mitochondrial uptake of metabolic substrates and their utilization for ATP synthesis, electron transport chain activity, reactive oxygen species levels, ion homeostasis, mitochondrial biogenesis, and dynamics as well as levels of reactive oxygen species in the mitochondria are key factors which regulate mitochondrial function in the normal heart. Alterations in these functions contribute to adverse outcomes in heart failure. Iron imbalance and oxidative stress are also major factors for the evolution of cardiac hypertrophy, heart failure, and aging-associated pathological changes in the heart. Mitochondrial ATP–binding cassette (ABC) transporters have a key role in regulating iron metabolism and maintenance of redox status in cells. Deficiency of mitochondrial ABC transporters is associated with an impaired mitochondrial electron transport chain complex activity, iron overload, and increased levels of reactive oxygen species, all of which can result in mitochondrial dysfunction. In this review, we discuss the role of mitochondrial ABC transporters in mitochondrial metabolism and metabolic switch, alterations in the functioning of ABC transporters in heart failure, and mitochondrial ABC transporters as possible targets for therapeutic intervention in cardiac failure.


Similar content being viewed by others
References
Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JGF, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M (2017) Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol 14(4):238–250. https://doi.org/10.1038/nrcardio.2016.203
O’Rourke B (2016) Metabolism: beyond the power of mitochondria. Nat Rev Cardiol 13:386–387. https://doi.org/10.1038/nrcardio.2016.95
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129. https://doi.org/10.1152/physrev.00006.2004
Barry WH (2004) Heart physiology from cell to circulation, 4th ed. Circulation 110:e313–e313. https://doi.org/10.1161/01.CIR.0000143724.99618.62
Andersson B, Blomström-Lundqvist C, Hedner T, Waagstein F (1991) Exercise hemodynamics and myocardial metabolism during long-term beta-adrenergic blockade in severe heart failure. J Am Coll Cardiol 18(4):1059–1066. https://doi.org/10.1016/0735-1097(91)90767-4
Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, Paolocci N, Tomaselli GF, Kass DA, van Eyk JE (2010) Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet 3:78–87. https://doi.org/10.1161/CIRCGENETICS.109.871236
Palmer JW, Tandler B, Hoppel CL (1985) Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys 236:691–702. https://doi.org/10.1016/0003-9861(85)90675-7
Lukyanenko V, Chikando A, Lederer WJ (2009) Mitochondria in cardiomyocyte Ca2+ signaling. Int J Biochem Cell Biol 41:1957–1971. https://doi.org/10.1016/j.biocel.2009.03.011
Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C (2005) Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Circ Physiol 289:H868–H872. https://doi.org/10.1152/ajpheart.00866.2004
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839. https://doi.org/10.1016/S0092-8674(00)81410-5
Kelly DP, Scarpulla RC (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368. https://doi.org/10.1101/gad.1177604.GENES
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79:208–217. https://doi.org/10.1093/cvr/cvn098
Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguère V (2007) Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab 5:345–356. https://doi.org/10.1016/j.cmet.2007.03.007
Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP (2004) Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator-1α promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94:525–533. https://doi.org/10.1161/01.RES.0000117088.36577.EB
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP (2000) Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106:847–856. https://doi.org/10.1172/JCI10268
Stojanovski D, Guiard B, Kozjak-Pavlovic V, Pfanner N, Meisinger C (2007) Alternative function for the mitochondrial SAM complex in biogenesis of α-helical TOM proteins. J Cell Biol 179:881–893. https://doi.org/10.1083/jcb.200706043
Wiedemann N, Pfanner N (2017) Mitochondrial machineries for protein import and assembly. Annu Rev Biochem 86:685–714. https://doi.org/10.1146/annurev-biochem-060815-014352
Hulbert AJ, Turner N, Hinde J, Else P, Guderley H (2006) How might you compare mitochondria from different tissues and different species? J Comp Physiol B Biochem Syst Environ Physiol 176:93–105. https://doi.org/10.1007/s00360-005-0025-z
Herbers E, Kekäläinen NJ, Hangas A et al (2018) Tissue specific differences in mitochondrial DNA maintenance and expression. Mitochondrion S1567-7249(17):30342–30342. https://doi.org/10.1016/j.mito.2018.01.004
Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S (1992) Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 24:1333–1347. https://doi.org/10.1016/0022-2828(92)93098-5
Sabbah HN (2016) Targeting mitochondrial dysfunction in the treatment of heart failure. Expert Rev Cardiovasc Ther 14:1305–1313. https://doi.org/10.1080/14779072.2016.1249466
Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT (2013) Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med 19:1111–1113. https://doi.org/10.1038/nm.3261
Wu F, Zhang J, Beard DA (2009) Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. Proc Natl Acad Sci U S A 106:7143–7148. https://doi.org/10.1073/pnas.0812768106
Aubert G, Vega RB, Kelly DP (2013) Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta 1833:840–847. https://doi.org/10.1016/j.bbamcr.2012.08.015
Ventura-Clapier R, Garnier A, Veksler V (2004) Energy metabolism in heart failure. J Physiol 555:1–13. https://doi.org/10.1113/jphysiol.2003.055095
Sarma S, Ardehali H, Gheorghiade M (2012) Enhancing the metabolic substrate: PPAR-alpha agonists in heart failure. Heart Fail Rev 17:35–43. https://doi.org/10.1007/s10741-010-9208-0
Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD (2011) Targeting fatty acid and carbohydrate oxidation — a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 1813:1333–1350. https://doi.org/10.1016/j.bbamcr.2011.01.015
Lodish H, Berk A, Zipursky SL et al (2000) Molecular cell biology, 4th edn. York, New
Chappell JB (1968) Systems used for the transport of substrates into mitochondria. Br Med Bull 24:150–157. https://doi.org/10.1093/oxfordjournals.bmb.a070618
Wohlrab H, Greaney J (1978) Mitochondrial phosphate transport and the N-ethylmaleimide binding proteins of the inner membrane. BBA-Bioenergetics 503:425–436. https://doi.org/10.1016/0005-2728(78)90142-1
Kaplan RS, Pedersen PL (1983) Characterization of phosphate efflux pathways in rat liver mitochondria. Biochem J 212:279–288. https://doi.org/10.1042/bj2120279
Gnoni GV, Priore P, Geelen MJH, Siculella L (2009) The mitochondrial citrate carrier: metabolic role and regulation of its activity and expression. IUBMB Life 61:987–994. https://doi.org/10.1002/iub.249
Hayes DJ, Taylor DJ, Bore PJ, Hilton-Jones D, Arnold DL, Squier MV, Gent AE, Radda GK (1987) An unusual metabolic myopathy: a malate-aspartate shuttle defect. J Neurol Sci 82:27–39. https://doi.org/10.1016/0022-510X(87)90004-9
Atlante A, Passarella S, Giannattasio S, Quagliariello E (1985) Fumarate permeation in rat liver mitochondria: fumarate/malate and fumarate/phosphate translocators. Biochem Biophys Res Commun 132:8–18. https://doi.org/10.1016/0006-291X(85)90981-7
De Bari L, Atlante A, Guaragnella N et al (2002) D-Lactate transport and metabolism in rat liver mitochondria. Biochem J 365:391–403. https://doi.org/10.1042/BJ20020139
Esparza A, Gerdtzen ZP, Olivera-Nappa A, Salgado JC, Núñez MT (2015) Iron-induced reactive oxygen species mediate transporter DMT1 endocytosis and iron uptake in intestinal epithelial cells. Am J Phys Cell Phys 309:C558–C567. https://doi.org/10.1152/ajpcell.00412.2014
Mouli S, Nanayakkara G, AlAlasmari A, Eldoumani H, Fu X, Berlin A, Lohani M, Nie B, Arnold RD, Kavazis A, Smith F, Beyers R, Denney T, Dhanasekaran M, Zhong J, Quindry J, Amin R (2015) The role of frataxin in doxorubicin mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol 309:H844–H859. https://doi.org/10.1152/ajpheart.00182.2015
Lakhal-Littleton S, Wolna M, Carr CA, Miller JJJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D, Stillion R, Holdship P, Larner F, Tyler DJ, Clarke K, Davies B, Robbins PA (2015) Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc Natl Acad Sci 112:3164–3169. https://doi.org/10.1073/pnas.1422373112
Sarkadi B, Homolya L, Szakács G, Váradi A (2006) Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86:1179–1236. https://doi.org/10.1152/physrev.00037.2005
Solbach TF, König J, Fromm MF, Zolk O (2006) ATP-binding cassette transporters in the heart. Trends Cardiovasc Med 16:7–15. https://doi.org/10.1016/j.tcm.2005.10.001
Zutz A, Gompf S, Schägger H, Tampé R (2009) Mitochondrial ABC proteins in health and disease. Biochim Biophys Acta Bioenerg 1787:681–690. https://doi.org/10.1016/j.bbabio.2009.02.009
Schaedler TA, Faust B, Shintre CA et al (2015) Structures and functions of mitochondrial ABC transporters. Biochem Soc Trans 43:943–951. https://doi.org/10.1042/BST20150118
Zhabyeyev P, Oudit GY (2017) Unravelling the molecular basis for cardiac iron metabolism and deficiency in heart failure. Eur Heart J 38:373–375. https://doi.org/10.1093/eurheartj/ehw386
Seguin A, Ward DM (2018) Mitochondrial ABC transporters and iron metabolism. J Clin Exp Pathol 8:338. https://doi.org/10.4172/2161-0681.1000338
Zhabyeyev P, Oudit GY (2016) Unravelling the molecular basis for cardiac iron metabolism and deficiency in heart failure. Eur Heart J 38:373–375. https://doi.org/10.1093/eurheartj/ehw386
Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flögel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T (2016) Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 38:362–372. https://doi.org/10.1093/eurheartj/ehw333
Kiss K, Kucsma N, Brozik A, Tusnady GE, Bergam P, van Niel G, Szakacs G (2015) Role of the N-terminal transmembrane domain in the endo-lysosomal targeting and function of the human ABCB6 protein. Biochem J 467:127–139. https://doi.org/10.1042/BJ20141085
Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, Tanaka M, Deybach JC, Puy H, le Gall M, Sureau C, Pham BN, le Pennec PY, Tani Y, Cartron JP, Arnaud L (2012) ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat Genet 44:170–173. https://doi.org/10.1038/ng.1069
Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV, Szakacs G, Akiyama SI, Gottesman MM (2007) Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry 46:9443–9452. https://doi.org/10.1021/bi700015m
Krishnamurthy PC, Du G, Fukuda Y et al (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443:586–589. https://doi.org/10.1038/nature05125
Mitsuhashi N, Miki T, Senbongi H, Yokoi N, Yano H, Miyazaki M, Nakajima N, Iwanaga T, Yokoyama Y, Shibata T, Seino S (2000) MTABC3, a novel mitochondrial ATP-binding cassette protein involved in iron homeostasis. J Biol Chem 275:17536–17540. https://doi.org/10.1074/jbc.275.23.17536
Matsumoto K, Hagiya Y, Endo Y, Nakajima M, Ishizuka M, Tanaka T, Ogura SI (2015) Effects of plasma membrane ABCB6 on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in vitro: tumor cell response to hypoxia. Photodiagn Photodyn Ther 12:45–51. https://doi.org/10.1016/j.pdpdt.2014.12.008
Fukuda Y, Cheong PL, Lynch J, Brighton C, Frase S, Kargas V, Rampersaud E, Wang Y, Sankaran VG, Yu B, Ney PA, Weiss MJ, Vogel P, Bond PJ, Ford RC, Trent RJ, Schuetz JD (2016) The severity of hereditary porphyria is modulated by the porphyrin exporter and Lan antigen ABCB6. Nat Commun 7:12353. https://doi.org/10.1038/ncomms12353
Paul VD, Lill R (2015) Biogenesis of cytosolic and nuclear iron–sulfur proteins and their role in genome stability. Biochim Biophys Acta Mol Cell Res 1853:1528–1539. https://doi.org/10.1016/j.bbamcr.2014.12.018
Li J, Cowan JA (2015) Glutathione-coordinated [2Fe–2S] cluster: a viable physiological substrate for mitochondrial ABCB7 transport. Chem Commun 51:2253–2255. https://doi.org/10.1039/C4CC09175B
Schaedler TA, Thornton JD, Kruse I, Schwarzländer M, Meyer AJ, van Veen HW, Balk J (2014) A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J Biol Chem 289:23264–23274. https://doi.org/10.1074/jbc.M114.553438
Taketani S, Kakimoto K, Ueta H et al (2003) Involvement of ABC7 in the biosynthesis of heme in erythroid cells: interaction of ABC7 with ferrochelatase. Blood 101:3274–3280. https://doi.org/10.1182/blood-2002-04-1212
Csere P, Lill R, Kispal G (1998) Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett 441:266–270. https://doi.org/10.1016/S0014-5793(98)01560-9
Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM (1999) Mutation of a putative mitochondrial Iron transporter gene (ABC7) in X-Linked Sideroblastic Anemia and Ataxia (XLSA/a). Hum Mol Genet 8:743–749. https://doi.org/10.1093/hmg/8.5.743
Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18:3981–3989. https://doi.org/10.1093/emboj/18.14.3981
Pondarré C, Antiochos BB, Campagna DR, Clarke SL, Greer EL, Deck KM, McDonald A, Han AP, Medlock A, Kutok JL, Anderson SA, Eisenstein RS, Fleming MD (2006) The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron–sulfur cluster biogenesis. Hum Mol Genet 15:953–964. https://doi.org/10.1093/hmg/ddl012
Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, Wu R, Khechaduri A, Jairaj Naik T, Ardehali H (2012) Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci 109:4152–4157. https://doi.org/10.1073/pnas.1119338109
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Prasad SVN, Mutharasan RK, Naik TJ, Ardehali H (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124:617–630. https://doi.org/10.1172/JCI72931
Hyde BB, Liesa M, Elorza AA, Qiu W, Haigh SE, Richey L, Mikkola HK, Schlaeger TM, Shirihai OS (2012) The mitochondrial transporter ABC-me (ABCB10), a downstream target of GATA-1, is essential for erythropoiesis in vivo. Cell Death Differ 19:1117–1126. https://doi.org/10.1038/cdd.2011.195
Liesa M, Luptak I, Qin F, Hyde BB, Sahin E, Siwik DA, Zhu Z, Pimentel DR, Xu XJ, Ruderman NB, Huffman KD, Doctrow SR, Richey L, Colucci WS, Shirihai OS (2011) Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation 124(68):806–813. https://doi.org/10.1161/CIRCULATIONAHA.110.003418
Chen W, Dailey HA, Paw BH (2010) Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 116:628–630. https://doi.org/10.1182/blood-2009-12-259614
Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J, Paw BH (2009) Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci 106:16263–16268. https://doi.org/10.1073/pnas.0904519106
Liesa M, Qiu W, Shirihai OS (2012) Mitochondrial ABC transporters function: the role of ABCB10 (ABC-me) as a novel player in cellular handling of reactive oxygen species. Biochim Biophys Acta Mol Cell Res 1823(10):1945–1957. https://doi.org/10.1016/j.bbamcr.2012.07.013
Couture L (2006) The ATP-binding cassette transporters and their implication in drug disposition: a special look at the heart. Pharmacol Rev 58:244–258. https://doi.org/10.1124/pr.58.2.7
J. Patterson A, Zhang L (2010) Hypoxia and fetal heart development. Curr Mol Med 10:653–666. https://doi.org/10.2174/156652410792630643
Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet SW, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Asaithamby A, Shah AM, Szweda LI, Sadek HA (2014) The oxygen rich postnatal environment induces cardiomyocyte cell cycle arrest through DNA damage response. Cell 157:565–579. https://doi.org/10.1016/j.cell.2014.03.032
Gibbs CL (1978) Cardiac energetics. Physiol Rev 58:174–254. https://doi.org/10.1152/physrev.1978.58.1.174
Kolwicz SC, Purohit S, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113:603–616. https://doi.org/10.1161/CIRCRESAHA.113.302095
Schaper J, Meiser E, Stammler G (1985) Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res 56:377–391. https://doi.org/10.1161/01.RES.56.3.377
Beer M, Seyfarth T, Sandstede J, Landschütz W, Lipke C, Köstler H, von Kienlin M, Harre K, Hahn D, Neubauer S (2002) Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274. https://doi.org/10.1016/S0735-1097(02)02160-5
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure. Circ Res 113:709–724. https://doi.org/10.1161/CIRCRESAHA.113.300376
Paolisso G, Gambardella A, Galzerano D, D'Amore A, Rubino P, Verza M, Teasuro P, Varricchio M, D’Onofrio F (1994) Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism 43:174–179. https://doi.org/10.1016/0026-0495(94)90241-0
Rimbaud S, Sanchez H, Garnier A, Fortin D, Bigard X, Veksler V, Ventura-Clapier R (2009) Stimulus specific changes of energy metabolism in hypertrophied heart. J Mol Cell Cardiol 46:952–959. https://doi.org/10.1016/j.yjmcc.2009.01.013
Camici P, Marraccini P, Marzilli M, Lorenzoni R, Buzzigoli G, Puntoni R, Boni C, Bellina CR, Klassen GA, L'Abbate A et al (1989) Coronary hemodynamics and myocardial metabolism during and after pacing stress in normal humans. Am J Phys 257:E309–E317. https://doi.org/10.1152/ajpendo.1989.257.3.E309
Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ (2002) Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40:271–277. https://doi.org/10.1016/S0735-1097(02)01967-8
Wallhaus TR, Taylor M, DeGrado TR et al (2001) Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 103:2441–2446. https://doi.org/10.1161/01.CIR.103.20.2441
Pellieux C, Aasum E, Larsen TS, Montessuit C, Papageorgiou I, Pedrazzini T, Lerch R (2006) Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol 41:459–466. https://doi.org/10.1016/j.yjmcc.2006.06.004
Pellieux C, Montessuit C, Papageorgiou I, Lerch R (2009) Angiotensin II downregulates the fatty acid oxidation pathway in adult rat cardiomyocytes via release of tumour necrosis factor-α. Cardiovasc Res 82:341–350. https://doi.org/10.1093/cvr/cvp004
Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD (1996) Creatine kinase system in failing and nonfailing human myocardium. Circulation 94:1894–1901. https://doi.org/10.1161/01.CIR.94.8.1894
Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G (1991) Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 122:795–801. https://doi.org/10.1016/0002-8703(91)90527-O
Wittels B, Spann JF (1968) Defective lipid metabolism in the failing heart. J Clin Invest 47:1787–1794. https://doi.org/10.1172/JCI105868
Panchal AR, Stanley WC, Kerner J, Sabbah HN (1998) Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail 4:121–126. https://doi.org/10.1016/S1071-9164(98)90252-4
Rouslin W, Broge CW (1993) Mechanisms of ATP conservation during ischemia in slow and fast heart rate hearts. Am J Physiol 264:C209–C216. https://doi.org/10.1152/ajpcell.1993.264.1.C209
Cannon MV, Sillje HH, Sijbesma JW, Vreeswijk-Baudoin I, Ciapaite J, van der Sluis B, van Deursen J, Silva GJ, de Windt LJ, Gustafsson JA, van der Harst P, van Gilst WH, de Boer RA (2015) Cardiac LXR protects against pathological cardiac hypertrophy and dysfunction by enhancing glucose uptake and utilization. EMBO Mol Med 7:1229–1243. https://doi.org/10.15252/emmm.201404669
O’Donnell JM, White LT, Lewandowski ED (1999) Mitochondrial transporter responsiveness and metabolic flux homeostasis in postischemic hearts. Am J Phys 277:H866–H873. https://doi.org/10.1152/ajpheart.1999.277.3.H866
Walther T, Tschöpe C, Sterner-Kock A et al (2007) Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation 115:333–344. https://doi.org/10.1161/CIRCULATIONAHA.106.643296
Sigauke E, Rakheja D, Kitson K, Bennett MJ (2003) Carnitine palmitoyltransferase II deficiency: a clinical, biochemical, and molecular review. Lab Investig 83:1543–1554. https://doi.org/10.1097/01.LAB.0000098428.51765.83
Djouadi F, Bonnefont J-P, Thuillier L, Droin V, Khadom N, Munnich A, Bastin J (2003) Correction of fatty acid oxidation in carnitine palmitoyl transferase 2–deficient cultured skin fibroblasts by bezafibrate. Pediatr Res 54:446–451. https://doi.org/10.1203/01.PDR.0000083001.91588.BB
Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D et al (2017) Exercise-induced changes in glucose metabolism promote physiologic cardiac growth. Circulation 136:2144–2157. https://doi.org/10.1161/CIRCULATIONAHA.117.028274
Rines AK, Ardehali H (2013) Transition metals and mitochondrial metabolism in the heart. J Mol Cell Cardiol 55:50–57. https://doi.org/10.1016/j.yjmcc.2012.05.014
Andrews NC (1999) Disorders of iron metabolism. N Engl J Med 341:1986–1995. https://doi.org/10.1056/NEJM199912233412607
Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P (2013) Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 34:816–829. https://doi.org/10.1093/eurheartj/ehs224
Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJV, Anker SD, Gheorghiade M, Ponikowski P (2013) Iron status in patients with chronic heart failure. Eur Heart J 34:827–834. https://doi.org/10.1093/eurheartj/ehs377
Sukumaran A, Chang J, Han M, Mintri S, Khaw BA, Kim J (2017) Iron overload exacerbates age-associated cardiac hypertrophy in a mouse model of hemochromatosis. Sci Rep 7(5756):5756. https://doi.org/10.1038/s41598-017-05810-2
Das SK, Wang W, Zhabyeyev P, Basu R, McLean B, Fan D, Parajuli N, DesAulniers J, Patel VB, Hajjar RJ, Dyck JRB, Kassiri Z, Oudit GY (2016) Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy. Sci Rep 5:18132. https://doi.org/10.1038/srep18132
Medeiros DM, Jiang Y, Klaahsen D, Lin D (2009) Mitochondrial and sarcoplasmic protein changes in hearts from copper-deficient rats: up-regulation of PGC-1α transcript and protein as a cause for mitochondrial biogenesis in copper deficiency. J Nutr Biochem 20:823–830. https://doi.org/10.1016/j.jnutbio.2008.08.001
Zeng H, Saari JT, Johnson WT (2007) Copper deficiency decreases complex IV but not complex I, II, III, or V in the mitochondrial respiratory chain in rat heart. J Nutr 137:14–18. https://doi.org/10.1093/jn/137.1.14
Johnson WT, Johnson LK (2009) Copper deficiency inhibits Ca2+-induced swelling in rat cardiac mitochondria. J Nutr Biochem 20:248–253. https://doi.org/10.1016/j.jnutbio.2008.02.009
Li Y, Wang L, Schuschke DA, Zhou Z, Saari JT, Kang YJ (2005) Marginal dietary copper restriction induces cardiomyopathy in rats. J Nutr 135:2130–2136. https://doi.org/10.1093/jn/135.9.2130
Jüllig M, Chen X, Hickey AJ, Crossman DJ, Xu A, Wang Y, Greenwood DR, Choong YS, Schönberger SJ, Middleditch MJ, Phillips ARJ, Cooper GJS (2007) Reversal of diabetes-evoked changes in mitochondrial protein expression of cardiac left ventricle by treatment with a copper(II)-selective chelator. Proteomics Clin Appl 1:387–399. https://doi.org/10.1002/prca.200600770
Miller KB, Caton JS, Finley JW (2006) Manganese depresses rat heart muscle respiration. BioFactors 28:33–46. https://doi.org/10.1002/biof.5520280104
Gunter TE, Gerstner B, Lester T, Wojtovich AP, Malecki J, Swarts SG, Brookes PS, Gavin CE, Gunter KK (2010) An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicol Appl Pharmacol 249:65–75. https://doi.org/10.1016/j.taap.2010.08.018
Van Remmen H, Williams MD, Guo Z et al (2001) Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Circ Physiol 281:H1422–H1432. https://doi.org/10.1152/ajpheart.2001.281.3.H1422
Shen X, Zheng S, Metreveli NS, Epstein PN (2006) Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 55:798–805. https://doi.org/10.2337/diabetes.55.03.06.db05-1039
Au C, Benedetto A, Aschner M (2008) Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology 29:569–576. https://doi.org/10.1016/j.neuro.2008.04.022
Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258. https://doi.org/10.1152/physrev.00015.2009
Paolisso G, De Riu S, Marrazzo G et al (1991) Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metabolism 40:972–977. https://doi.org/10.1016/0026-0495(91)90075-8
Di NP, Di GP, Gaeta MA et al (2007) Trimetazidine and reduction in mortality and hospitalization in patients with ischemic dilated cardiomyopathy: a post hoc analysis of the Villa Pini DʼAbruzzo Trimetazidine Trial. J Cardiovasc Pharmacol 50:585–589. https://doi.org/10.1097/FJC.0b013e31814fa9cb
Lopaschuk GD (2003) Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ Res 93:33e–37e. https://doi.org/10.1161/01.RES.0000086964.07404.A5
Fragasso G, Perseghin G, De Cobelli F et al (2006) Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J 27:942–948. https://doi.org/10.1093/eurheartj/ehi816
Mobini R, Fu M, Jansson P-A, Bergh CH, Scharin Täng M, Waagstein F, Andersson B (2006) Influence of central inhibition of sympathetic nervous activity on myocardial metabolism in chronic heart failure: acute effects of the imidazoline1-receptor agonist moxonidine. Clin Sci 110:329–336. https://doi.org/10.1042/CS20050037
Cohn JN, Pfeffer MA, Rouleau J, Sharpe N, Swedberg K, Straub M, Wiltse C, Wright TJ, for the MOXCON Investigators (2003) Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON). Eur J Heart Fail 5:659–667. https://doi.org/10.1016/S1388-9842(03)00163-6
Fragasso G (2016) Deranged cardiac metabolism and the pathogenesis of heart failure. Card Fail Rev 2:8–13. https://doi.org/10.15420/cfr.2016:5:2
Kumar V, Aneesh KA, Kshemada K et al (2017) Amalaki rasayana, a traditional Indian drug enhances cardiac mitochondrial and contractile functions and improves cardiac function in rats with hypertrophy. Sci Rep 7:8588. https://doi.org/10.1038/s41598-017-09225-x
Passarella S, Atlante A, Valenti D, de Bari L (2003) The role of mitochondrial transport in energy metabolism. Mitochondrion 2:319–343. https://doi.org/10.1016/S1567-7249(03)00008-4
Acknowledgments
We thank the Director, Rajiv Gandhi Centre for Biotechnology, for providing the facilities and funding the study. We thank Ms. Nimmy Francis for the help in the preparation of summary figure and Mr. Aneesh Kumar A for the contribution in the reference style of the manuscript.
Funding
We thank the Department of Science and Technology-Science and Engineering Research Board (DST-SERB), Government of India for funding the study. The study was supported by grants (CO/AB/006/2012) from DST-SERB, Government of India and Rajiv Gandhi Centre for Biotechnology (RGCB), Trivandrum, India.
Author information
Authors and Affiliations
Contributions
VK, TRSK, and CCK designed and directed the overall project. VK wrote and drafted the manuscript. CCK revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, V., Santhosh Kumar, T.R. & Kartha, C.C. Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail Rev 24, 255–267 (2019). https://doi.org/10.1007/s10741-018-9756-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9756-2