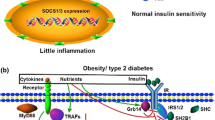
Abstract
Heart diseases are major causes of mortality. Cardiac hypertrophy, myocardial infarction (MI), viral cardiomyopathy, ischemic and reperfusion (I/R) heart injury finally lead to heart failure and death. Insulin and IGF1 signal pathways play key roles in normal cardiomyocyte growth and physiological cardiac hypertrophy while inflammatory signal pathway is associated with pathological cardiac hypertrophy, MI, viral cardiomyopathy, I/R heart injury, and heart failure. Adapter proteins are the major family proteins, which transduce signals from insulin, IGF1, or cytokine receptors to the downstream pathways and have been shown to regulate variety of heart diseases. Here, we summarized the recent advances in understanding the physiological and pathological roles of adapter proteins in heart failure.

Similar content being viewed by others
References
Heidenreich PA et al (2011) Forecasting the future of cardiovascular disease in the United States a policy statement from the American Heart Association. Circulation 123(8):933–944
McMullen JR et al (2004) The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J Biol Chem 279(6):4782–4793
Bugger H et al (2012) Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J Mol Cell Cardiol 52(5):1019–1026
Qi Y et al (2013) Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes 62(11):3887–3900
McMullen JR (2008) Role of insulin-like growth factor 1 and phosphoinositide 3-kinase in a setting of heart disease. Clin Exp Pharmacol Physiol 35(3):349–354
Li Q et al (1997) Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Investig 100(8):1991–1999
McMullen JR et al (2007) Protective effects of exercise and phosphoinositide 3-kinase(p110α) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 104(2):612–617
Riehle C et al (2014) Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol 34(18):3450–3460
Vinciguerra M et al (2012) mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell 11(1):139–149
Arslan F, de Kleijn DP, Pasterkamp G (2011) Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 8(5):292–300
Blauwet LA, Cooper LT (2010) Myocarditis. Prog Cardiovasc Dis 52(4):274–288
Mezzaroma E et al (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA 108(49):19725–19730
Yang Y et al (2016) The emerging role of toll-like receptor 4 in myocardial inflammation. Cell Death Dis 7(5):e2234
Avlas O et al (2010) Toll-like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid Redox Signal 15(7):1895–1909
Fallach R et al (2010) Cardiomyocyte toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol 48(6):1236–1244
Leo A et al (2002) Adapters in lymphocyte signaling. J Clin Invest 109(3):301–309
Pawson T, Scott JD (1997) Signaling through scaffold, anchoring, and adaptor proteins. Science 278(5346):2075–2080
Bradley JR, Pober JS (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20(44):6482–6491
Yoshimura A, Naka T, Kubo M (2007) SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7(6):454–465
Chen Z (2016) Adapter proteins regulate insulin resistance and lipid metabolism in obesity. Sci Bull. doi:10.1007/s11434-016-1058-2
Tao L et al (2015) Exercise for the heart: signaling pathways. Oncotarget 6(25):20773–20784
Kolwicz SC, Purohit S, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of the cardiomyocte. Circ Res. doi:10.1161/CIRCRESAHA.113.302095
Dikow R et al (2009) Effect of insulin and glucose infusion on myocardial infarction size in uraemic rats. Basic Res Cardiol 104(5):571–579
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414(6865):799–806
Du Y, Wei T (2014) Inputs and outputs of insulin receptor. Protein Cell 5(3):203–213
Ravichandran KS (2001) Signaling via Shc family adapter proteins. Oncogene 20(44):6322–6330
Obreztchikova M et al (2006) Distinct signaling functions for Shc isoforms in the heart. J Biol Chem 281(29):20197–20204
Cesselli D et al (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89(3):279–286
Giorgio M et al (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122(2):221–233
Pani G (2010) P66SHC and ageing: ROS and TOR? Aging (Albany NY) 2(8):514–518
Camici GG et al (2007) Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA 104(12):5217–5222
Graiani G et al (2005) Genetic deletion of the p66Shc adaptor protein protects from angiotensin II—induced myocardial damage. Hypertension 46(2):433–440
Diogo CV et al (2013) Cardiac mitochondrial dysfunction during hyperglycemia—the role of oxidative stress and p66Shc signaling. Int J Biochem Cell Biol 45(1):114–122
Di Lisa F et al (2009) Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66Shc and monoamine oxidase. Basic Res Cardiol 104(2):131–139
Baysa A et al (2015) The p66ShcA adaptor protein regulates healing after myocardial infarction. Basic Res Cardiol 110(2):13
Akhmedov A et al (2015) Genetic deletion of the adaptor protein p66(Shc) increases susceptibility to short-term ischaemic myocardial injury via intracellular salvage pathways. Eur Heart J 36(8):516-U79
Dance M et al (2006) The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor receptor-2 to the activation of phosphoinositide 3-kinase. J Biol Chem 281(32):23285–23295
Sun Y et al (2004) Role of Gab1 in UV-induced c-Jun NH2-terminal kinase activation and cell apoptosis. Mol Cell Biol 24(4):1531–1539
Holgado-Madruga M, Wong AJ (2003) Gab1 is an integrator of cell death versus cell survival signals in oxidative stress. Mol Cell Biol 23(13):4471–4484
Itoh M et al (2000) Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol 20(10):3695–3704
Sachs M et al (2000) Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol 150(6):1375–1384
Nakaoka Y et al (2007) Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling. J Clin Investig 117(7):1771–1781
Zhao J et al (2016) Cardiac Gab1 deletion leads to dilated cardiomyopathy associated with mitochondrial damage and cardiomyocyte apoptosis. Cell Death Differ 23(4):695–706
Sun L et al (2014) Grb2-associated binder 1 is essential for cardioprotection against ischemia/reperfusion injury. Basic Res Cardiol 109(4):1–15
Rui L (2014) SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J Diabetes 5(4):511–526
Morris DL et al (2009) SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes 58(9):2039–2047
Duan C et al (2004) Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol 24(17):7435–7443
Ren D et al (2005) Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2(2):95–104
Ren D et al (2007) Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest 117(2):397–406
Chen Z et al (2014) SH2B1 in beta-cells regulates glucose metabolism by promoting beta-cell survival and islet expansion. Diabetes 63(2):585–595
Chen Z et al (2014) SH2B1 in beta-cells promotes insulin expression and glucose metabolism in mice. Mol Endocrinol 28(5):696–705
Wu G et al (2015) SH2B1 is critical for the regulation of cardiac remodelling in response to pressure overload. Cardiovasc Res 107(2):203–215
Gery S, Koeffler HP (2013) Role of the adaptor protein LNK in normal and malignant hematopoiesis. Oncogene 32(26):3111–3118
Devallière J, Charreau B (2011) The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol 82(10):1391–1402
Gudbjartsson DF et al (2009) Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 41(3):342–347
Flister MJ et al (2015) SH2B3 is a genetic determinant of cardiac inflammation and fibrosis. Circ Cardiovasc Genet 8(2):294
Mitsuuchi Y et al (1999) Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18(35):4891–4898
Fang X et al (2010) An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab 299(5):E721–E729
Dadson K et al (2016) Cellular, structural and functional cardiac remodelling following pressure overload and unloading. Int J Cardiol 216:32–42
Cao T et al (2014) AdipoR1/APPL1 potentiates the protective effects of globular adiponectin on angiotensin II-induced cardiac hypertrophy and fibrosis in neonatal rat atrial myocytes and fibroblasts. PLoS ONE 9(8):e103793
Park M et al (2013) APPL1 transgenic mice are protected from high-fat diet-induced cardiac dysfunction. Am J Physiol Endocrinol Metab 305(7):E795–E804
Yasukawa H et al (2012) SOCS3: a novel therapeutic target for cardioprotection. JAK-STAT 1(4):234–240
Cittadini A et al (2012) SOCS1 gene transfer accelerates the transition to heart failure through the inhibition of the gp130/JAK/STAT pathway. Cardiovasc Res 96(3):381–390
Yasukawa H et al (2001) Suppressor of cytokine signaling-3 is a biomechanical stress–inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J Clin Investig 108(10):1459–1467
Yajima T et al (2011) Absence of SOCS3 in the cardiomyocyte increases mortality in a gp130 dependent manner accompanied by contractile dysfunction and ventricular arrhythmias. Circulation 124(24):2690–2701
Oba T et al (2012) Cardiac-specific deletion of SOCS-3 prevents development of left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 59(9):838–852
Knowlton KU (2008) CVB infection and mechanisms of viral cardiomyopathy. Curr Top Microbiol Immunol 323:315–335
Yasukawa H et al (2003) The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J Clin Invest 111(4):469–478
Yajima T et al (2006) Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation 114(22):2364–2373
Tsygankov AY et al (2001) Beyond the RING: CBL proteins as multivalent adapters. Oncogene 20(44):6382–6402
Rafiq K et al (2014) c-Cbl inhibition improves cardiac function and survival in response to myocardial ischemia. Circulation 129(20):2031–2043
Rafiq K et al (2012) c-Cbl ubiquitin ligase regulates focal adhesion protein turnover and myofibril degeneration induced by neutrophil protease cathepsin G. J Biol Chem 287(8):5327–5339
Valaperti A et al (2014) The adapter protein c-Cbl-associated protein (CAP) protects from acute CVB3-mediated myocarditis through stabilization of type I interferon production and reduced cytotoxicity. Basic Res Cardiol 109(3):1–14
Chung JY et al (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci 115(Pt 4):679–688
Chen Z et al (2012) Hepatic TRAF2 regulates glucose metabolism through enhancing glucagon responses. Diabetes 61(3):566–573
Chen Z et al (2015) Hepatocyte TRAF3 promotes insulin resistance and type 2 diabetes in mice with obesity. Mol Metab 4(12):951–960
Chen Z et al (2015) Myeloid cell TRAF3 promotes metabolic inflammation, insulin resistance, and hepatic steatosis in obesity. Am J Physiol Endocrinol Metab 308(6):E460–E469
Huang Y et al (2014) Cardiac-specific Traf2 overexpression enhances cardiac hypertrophy through activating AKT/GSK3β signaling. Gene 536(2):225–231
Divakaran VG et al (2013) Tumor necrosis factor receptor associated factor 2 signaling provokes adverse cardiac remodeling in the adult mammalian heart. Circ Heart Fail 6(3):535–543
Tzeng HP et al (2014) Dysferlin mediates the cytoprotective effects of TRAF2 following myocardial ischemia reperfusion injury. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 3(1):e000662
Jiang X et al (2015) Tumor necrosis factor receptor-associated factor 3 is a positive regulator of pathological cardiac hypertrophy. Hypertension 66(2):356–367
Deguine J, Barton GM (2014) MyD88: a central player in innate immune signaling. F100Prime Rep 6:97
Ha T et al (2005) Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res 68(2):224–234
Vilahur G et al (2011) Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 50(3):522–533
Riad A et al (2008) Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol 180(10):6954–6961
Oyama J et al (2004) Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109(6):784–789
Singh MV et al (2012) MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol 52(5):1135–1144
Ha T et al (2006) Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 290(3):H985–H994
Blyszczuk P et al (2009) Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res 105(9):912–920
Zhu Y et al (2011) The TIR/BB-loop mimetic AS-1 prevents cardiac hypertrophy by inhibiting IL-1R-mediated MyD88-dependent signaling. Basic Res Cardiol 106(5):787–799
Singh MV et al (2015) Dual activation of TRIF and MyD88 adaptor proteins by angiotensin II evokes opposing effects on pressure, cardiac hypertrophy, and inflammatory gene expression. Hypertension 66(3):647–656
Riad A et al (2011) TRIF is a critical survival factor in viral cardiomyopathy. J Immunol 186(4):2561–2570
Chen C et al (2014) Role of extracellular RNA and TLR3-trif signaling in myocardial ischemia-reperfusion injury. J Am Heart Assoc Cardiovasc Cerebrovascu Dis 3(1):e000683
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801
Acknowledgments
This study was supported by Changbai Mountain Scholars Program of The People’s Government of Jilin Province 2013046 (to Z. C.), the Jilin Science and Technology Development Program 20160101204JC (to Z. C.), the Jilin Talent Development Foundation 111860000 (to Z. C.), the Fok Ying Tong Education Foundation 151022 (to Z. C.), the National Natural Science Foundation of China Grant 31500957 (to Z. C.), and the startup funds from Northeast Normal University 120401204 (to Z. C.). We apologize to colleagues whose relevant work could not be cited here due to space limitation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
L.T., L.J., Y.L., C.S. and Z.C. declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tao, L., Jia, L., Li, Y. et al. Recent advances of adapter proteins in the regulation of heart diseases. Heart Fail Rev 22, 99–107 (2017). https://doi.org/10.1007/s10741-016-9582-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9582-3