Abstract
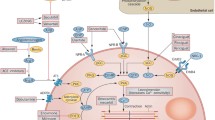
Heart failure (HF) represents a global public health and economic problem associated with unacceptable rates of death, hospitalization, and healthcare expenditure. Despite available therapy, HF carries a prognosis comparable to many forms of cancer with a 5-year survival rate of ~50 %. The current treatment paradigm for HF with reduced ejection fraction (EF) centers on blocking maladaptive neurohormonal activation and decreasing cardiac workload with therapies that concurrently lower blood pressure and heart rate. Continued development of hemodynamically active medications for stepwise addition to existing therapies carries the risk of limited tolerability and safety. Moreover, this treatment paradigm has thus far failed for HF with preserved EF. Accordingly, development of hemodynamically neutral HF therapies targeting primary cardiac pathologies must be considered. In this context, a partial adenosine A1 receptor (A1R) agonist holds promise as a potentially hemodynamically neutral therapy for HF that could simultaneous improve cardiomyocyte energetics, calcium homeostasis, cardiac structure and function, and long-term clinical outcomes when added to background therapies. In this review, we describe the physiology and pathophysiology of HF as it relates to adenosine agonism, examine the existing body of evidence and biologic rationale for modulation of adenosine A1R activity, and review the current state of drug development of a partial A1R agonist for the treatment of HF.




Similar content being viewed by others
References
Butler J, Fonarow GC, Gheorghiade M (2013) Need for increased awareness and evidence-based therapies for patients hospitalized for heart failure. JAMA 310:2035–2036
Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376:875–885
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR (2014) Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371:993–1004
Vaduganathan M, Greene SJ, Ambrosy AP, Gheorghiade M, Butler J (2013) The disconnect between phase II and phase III trials of drugs for heart failure. Nat Rev Cardiol 10:85–97
Vaduganathan M, Butler J, Pitt B, Gheorghiade M (2015) Contemporary drug development in heart failure: call for hemodynamically neutral therapies. Circ Heart Fail 8:826–831
Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP (2013) Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 309:1125–1135
Bayeva M, Sawicki KT, Butler J, Gheorghiade M, Ardehali H (2014) Molecular and cellular basis of viable dysfunctional myocardium. Circ Heart Fail 7:680–691
Bayeva M, Gheorghiade M, Ardehali H (2013) Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61:599–610
Schelbert EB, Fonarow GC, Bonow RO, Butler J, Gheorghiade M (2014) Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J Am Coll Cardiol 63:2188–2198
Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC Jr, Roessig L, Stasch JP, Solomon SD, Paulus WJ, Butler J (2013) The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc 2:e000536
Wilcox JE, Fonarow GC, Ardehali H, Bonow RO, Butler J, Sauer AJ, Epstein SE, Khan SS, Kim RJ, Sabbah HN, Diez J, Gheorghiade M (2015) “Targeting the Heart” in heart failure: myocardial recovery in heart failure with reduced ejection fraction. JACC Heart Fail 3:661–669
Sabbah HN, Gupta RC, Kohli S, Wang M, Rastogi S, Zhang K, Zimmermann K, Diedrichs N, Albrecht-Kupper BE (2013) Chronic therapy with a partial adenosine A1-receptor agonist improves left ventricular function and remodeling in dogs with advanced heart failure. Circ Heart Fail 6:563–571
Linden J (2005) Adenosine in tissue protection and tissue regeneration. Mol Pharmacol 67:1385–1387
Brodde OE, Michel MC (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51:651–690
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC (1996) Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 118:1461–1468
Akbar M, Okajima F, Tomura H, Shimegi S, Kondo Y (1994) A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase C and arachidonate release. Mol Pharmacol 45:1036–1042
Wang D, Belardinelli L (1994) Mechanism of the negative inotropic effect of adenosine in guinea pig atrial myocytes. Am J Physiol 267:H2420–2429
Yuan K, Cao C, Han JH, Kim SZ, Kim SH (2005) Adenosine-stimulated atrial natriuretic peptide release through A1 receptor subtype. Hypertension 46:1381–1387
Schutte F, Burgdorf C, Richardt G, Kurz T (2006) Adenosine A1 receptor-mediated inhibition of myocardial norepinephrine release involves neither phospholipase C nor protein kinase C but does involve adenylyl cyclase. Can J Physiol Pharmacol 84:573–577
Xiang F, Huang YS, Zhang DX, Chu ZG, Zhang JP, Zhang Q (2010) Adenosine A1 receptor activation reduces opening of mitochondrial permeability transition pores in hypoxic cardiomyocytes. Clin Exp Pharmacol Physiol 37:343–349
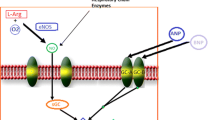
Martynyuk AE, Kane KA, Cobbe SM, Rankin AC (1996) Nitric oxide mediates the anti-adrenergic effect of adenosine on calcium current in isolated rabbit atrioventricular nodal cells. Pflugers Arch 431:452–457
Kirsch GE, Codina J, Birnbaumer L, Brown AM (1990) Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol 259:H820–826
Albrecht-Kupper BE, Leineweber K, Nell PG (2012) Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal 8:91–99
Vallon V, Muhlbauer B, Osswald H (2006) Adenosine and kidney function. Physiol Rev 86:901–940
Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC (2007) The effects of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 50:1551–1560
Dittrich HC, Gupta DK, Hack TC, Dowling T, Callahan J, Thomson S (2007) The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. J Card Fail 13:609–617
Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC (2010) Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 363:1419–1428
Teerlink JR, Iragui VJ, Mohr JP, Carson PE, Hauptman PJ, Lovett DH, Miller AB, Pina IL, Thomson S, Varosy PD, Zile MR, Cleland JG, Givertz MM, Metra M, Ponikowski P, Voors AA, Davison BA, Cotter G, Wolko D, Delucca P, Salerno CM, Mansoor GA, Dittrich H, O’Connor CM, Massie BM (2012) The safety of an adenosine A(1)-receptor antagonist, rolofylline, in patients with acute heart failure and renal impairment: findings from PROTECT. Drug Saf 35:233–244
Dunwiddie TV, Worth T (1982) Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther 220:70–76
Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L (2013) Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 15:1173–1184
Francis GS, Bartos JA, Adatya S (2014) Inotropes. J Am Coll Cardiol 63:2069–2078
Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN (2000) Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol 32:2361–2367
Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN (2007) Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol 42:150–158
Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A (1999) Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85:357–363
Siwik DA, Colucci WS (2004) Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev 9:43–51
Laskowski KR, Russell RR 3rd (2008) Uncoupling proteins in heart failure. Curr Heart Fail Rep 5:75–79
Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR 3rd (2011) Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer Chemother Pharmacol 67:1381–1388
Sabbah HN (2000) Apoptotic cell death in heart failure. Cardiovasc Res 45:704–712
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104:2923–2931
Sack MN, Kelly DP (1998) The energy substrate switch during development of heart failure: gene regulatory mechanisms (review). Int J Mol Med 1:17–24
Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J (2007) MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116:258–267
Staehr PM, Dhalla AK, Zack J, Wang X, Ho YL, Bingham J, Belardinelli L (2013) Reduction of free fatty acids, safety, and pharmacokinetics of oral GS-9667, an A(1) adenosine receptor partial agonist. J Clin Pharmacol 53:385–392
Mann DL, Barger PM, Burkhoff D (2012) Myocardial recovery and the failing heart: Myth, magic, or molecular target? J Am Coll Cardiol 60:2465–2472
Urmaliya VB, Pouton CW, Devine SM, Haynes JM, Warfe L, Scammells PJ, White PJ (2010) A novel highly selective adenosine A1 receptor agonist VCP28 reduces ischemia injury in a cardiac cell line and ischemia-reperfusion injury in isolated rat hearts at concentrations that do not affect heart rate. J Cardiovasc Pharmacol 56:282–292
Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB (1999) Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol 34:1711–1720
Shneyvays V, Mamedova LK, Leshem D, Korkus A, Shainberg A (2002) Insights into the cardioprotective function of adenosine A(1) and A(3) receptors. Exp Clin Cardiol 7:138–145
Kitakaze M, Hori M, Takashima S, Sato H, Inoue M, Kamada T (1993) Ischemic preconditioning increases adenosine release and 5′-nucleotidase activity during myocardial ischemia and reperfusion in dogs. Implications for myocardial salvage. Circulation 87:208–215
Tendera M, Gaszewska-Zurek E, Parma Z, Ponikowski P, Jankowska E, Kawecka-Jaszcz K, Czarnecka D, Krzeminska-Pakula M, Bednarkiewicz Z, Sosnowski M, Ochan Kilama M, Agrawal R (2012) The new oral adenosine A1 receptor agonist capadenoson in male patients with stable angina. Clin Res Cardiol 101:585–591
Bott-Flugel L, Bernshausen A, Schneider H, Luppa P, Zimmermann K, Albrecht-Kupper B, Kast R, Laugwitz KL, Ehmke H, Knorr A, Seyfarth M (2011) Selective attenuation of norepinephrine release and stress-induced heart rate increase by partial adenosine A1 agonism. PLoS ONE 6:e18048
Greene SJ, Gheorghiade M (2014) Matching mechanism of death with mechanism of action: considerations for drug development for hospitalized heart failure. J Am Coll Cardiol 64:1599–1601
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Greene reports no conflicts. Dr. Sabbah has received research Grants and/or is consultant to Bayer Healthcare AG, Stealth Peptides, Inc., Amgen Corp., Johnson & Johnson, Inc., Novartis Corp., Merck, and Takeda Pharmaceuticals. Dr. Butler reports research support from the National Institutes of Health, European Union, and Health Resources Service Administration and is a consultant to Amgen, Bayer, BG Medicine, Cardiocell, Celladon, Gambro, GE Healthcare, Medtronic, Novartis, Ono Pharma, Takeda, Trevena, and Zensun. Dr Voors received consultancy fees and/or research Grants from: Alere, Amgen, Bayer, Boehringer, Cardio3Biosciences, Celladon, GSK, Merck/MSD, Novartis, Servier, Singulex, Sphingotec, Trevena, Vifor, ZS Pharma, and is supported by a Grant from the European Commission: FP7-242209-BIOSTAT-CHF. Drs. Albrecht-Küpper and Dinh are employees of Bayer Healthcare. Dr. Düngen has received research grants and/or is consultant to Bayer Healthcare AG, Amgen Corp., Novartis, Trevena, Celladon, AstraZeneca and reports research support from German Ministry of Education and Research. Dr. Gheorghiade has been a consultant for Abbott Laboratories, AstraZeneca, Bayer Schering Pharma AG, Cardiocell LLC, Cardiorentis Ltd, GlaxoSmithKline, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Ono Pharmaceuticals USA, Otsuka Pharmaceuticals, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Stealth BioTherapeutics, Sticares InterACT, Takeda Pharmaceuticals North America, Inc and Trevena Therapeutics.
Rights and permissions
About this article
Cite this article
Greene, S.J., Sabbah, H.N., Butler, J. et al. Partial adenosine A1 receptor agonism: a potential new therapeutic strategy for heart failure. Heart Fail Rev 21, 95–102 (2016). https://doi.org/10.1007/s10741-015-9522-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9522-7