Abstract
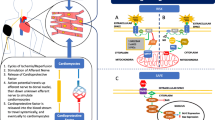
Ischemic preconditioning renders the heart resistant to infarction from ischemia/reperfusion. Over the past two decades a great deal has been learned about preconditioning’s mechanism. Adenosine, bradykinin, and opioids act in parallel to trigger the preconditioned state and do so by activating PKC. While adenosine couples directly to PKC through the phospholipases, bradykinin and opioids do so through a complex pathway that includes in order: phosphatidylinositol 3-kinase (PI3-kinase), Akt, nitric oxide synthase, guanylyl cyclase, PKG, opening of mitochondrial KATP channels, and activation of PKC by redox signaling. There are even differences between the opioid and bradykinin coupling as the former activates PI3-kinase through transactivation of the epidermal growth factor receptor while the latter has an unknown coupling mechanism. Protection stems from inhibition of formation of mitochondrial permeability transition pores early in reperfusion through activation of the survival kinases, Akt and ERK. These kinases are activated as a result of PKC somehow promoting signaling from adenosine A2 receptors early in reperfusion. The survival kinases are thought to inhibit pore formation by phosphorylating GSK-3β. The reperfused heart requires the support of the protective signals for only about an hour after which the ischemic injury is repaired and the signals are no longer needed.

Similar content being viewed by others
References
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Liu GS, Thornton J, Van Winkle DM, Stanley AWH, Olsson RA, Downey JM (1991) Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 84:350–356
Wall TM, Sheehy R, Hartman JC (1994) Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther 270:681–689
Schultz JEJ, Rose E, Yao Z, Gross GJ (1995) Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol 268:H2157–H2161
Goto M, Liu Y, Yang X-M, Ardell JL, Cohen MV, Downey JM (1995) Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res 77:611–621
Miki T, Cohen MV, Downey JM (1998) Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Mol Cell Biochem 186:3–12
Baines CP, Goto M, Downey JM (1997) Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29:207–216
Sakamoto J, Miura T, Goto M, Iimura O (1995) Limitation of myocardial infarct size by adenosine A1 receptor activation is abolished by protein kinase C inhibitors in the rabbit. Cardiovasc Res 29:682–688
Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB, Brew EC, Cairns CB et al (1993) Preconditioning against myocardial dysfunction after ischemia and reperfusion by an α1-adrenergic mechanism. Circ Res 73:656–670
Liu Y, Tsuchida A, Cohen MV, Downey JM (1995) Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol 27:883–892
Wang P, Gallagher KP, Downey JM, Cohen MV (1996) Pretreatment with endothelin-1 mimics ischemic preconditioning against infarction in isolated rabbit heart. J Mol Cell Cardiol 28:579–588
Pain T, Yang X-M, Critz SD, Yue Y, Nakano A, Liu GS et al (2000) Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 87:460–466
Van Winkle DM, Thornton JD, Downey DM, Downey JM (1991) The natural history of preconditioning: cardioprotection depends on duration of transient ischemia and time to subsequent ischemia. Coron Artery Dis 2:613–619
Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD et al (2002) Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol 97:365–373
Oldenburg O, Critz SD, Cohen MV, Downey JM (2003) Acetylcholine-induced production of reactive oxygen species in adult rabbit ventricular myocytes is dependent on phosphatidylinositol 3- and Src-kinase activation and mitochondrial KATP channel opening. J Mol Cell Cardiol 35:653–660
Oldenburg O, Qin Q, Sharma AR, Cohen MV, Downey JM, Benoit JN (2002) Acetylcholine leads to free radical production dependent on KATP channels, Gi proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovasc Res 55:544–552
Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C et al (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884–888
Krieg T, Cui L, Qin Q, Cohen MV, Downey JM (2004) Mitochondrial ROS generation following acetylcholine-induced EGF receptor transactivation requires metalloproteinase cleavage of proHB-EGF. J Mol Cell Cardiol 36:435–443
Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM (2002) ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol 283:H2322–H2330
Cohen MV, Philipp S, Krieg T, Cui L, Kuno A, Solodushko V et al (2007) Preconditioning-mimetics bradykinin and DADLE activate PI3-kinase through divergent pathways. J Mol Cell Cardiol 42:842–851
Qin Q, Downey JM, Cohen MV (2003) Acetylcholine but not adenosine triggers preconditioning through PI3-kinase and a tyrosine kinase. Am J Physiol 284:H727–H734
Cohen MV, Yang X-M, Liu GS, Heusch G, Downey JM (2001) Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ Res 89:273–278
Tong H, Chen W, Steenbergen C, Murphy E (2000) Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res 87:309–315
Mocanu MM, Bell RM, Yellon DM (2002) PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol 34:661–668
Andjelković M, Alessi DR, Meier R, Fernandez A, Lamb NJC, Frech M et al (1997) Role of translocation in the activation and function of protein kinase B. J Biol Chem 272:31515–31524
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB et al (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279:710–714
Krieg T, Qin Q, Philipp S, Alexeyev MF, Cohen MV, Downey JM (2004) Acetylcholine and bradykinin trigger preconditioning in the heart through a pathway that includes Akt and NOS. Am J Physiol 287:H2606–H2611
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605
Bolli R, Dawn B, Tang X-L, Qiu Y, Ping P, Xuan Y-T et al (1998) The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol 93:325–338
Lochner A, Marais E, Genade S, Moolman JA (2000) Nitric oxide: a trigger for classic preconditioning? Am J Physiol 279:H2752–H2765
Oldenburg O, Qin Q, Krieg T, Yang X-M, Philipp S, Critz SD et al (2004) Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol 286:H468–H476
Cohen MV, Yang X-M, Downey JM (2006) Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70:231–239
Nakano A, Liu GS, Heusch G, Downey JM, Cohen MV (2000) Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischemic preconditioning. J Mol Cell Cardiol 32:1159–1167
Qin Q, Yang X-M, Cui L, Critz SD, Cohen MV, Browner NC et al (2004) Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol 287:H712–H718
Yang X-M, Philipp S, Downey JM, Cohen MV (2006) Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol 101:311–318
Costa ADT, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV et al (2005) Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97:329–336
Forbes RA, Steenbergen C, Murphy E (2001) Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res 88:802–809
Tritto I, D’Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A et al (1997) Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res 80:743–748
Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U (2002) Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem 277:44327–44331
Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, García-Dorado D et al (2005) Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 97:583–586
Murry CE, Jennings RB, Reimer KA (1991) New insights into potential mechanisms of ischemic preconditioning. Circulation 84:442–445
Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol 288:H971–H976
Baines CP, Wang L, Cohen MV, Downey JM (1999) Myocardial protection by insulin is dependent on phosphatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol 94:188–198
Xu Z, Yang X-M, Cohen MV, Neumann T, Heusch G, Downey JM (2000) Limitation of infarct size in rabbit hearts by the novel adenosine receptor agonist AMP 579 administered at reperfusion. J Mol Cell Cardiol 32:2339–2347
Baxter GF, Mocanu MM, Brar BK, Latchman DS, Yellon DM (2001) Cardioprotective effects of transforming growth factor-β1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J Cardiovasc Pharmacol 38:930–939
Schulman D, Latchman DS, Yellon DM (2002) Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol 283:H1481–H1488
Liao Z, Brar BK, Cai Q, Stephanou A, O’Leary RM, Pennica D et al (2002) Cardiotrophin-1 (CT-1) can protect the adult heart from injury when added both prior to ischaemia and at reperfusion. Cardiovasc Res 53:902–910
Zhang SJ, Yang X-M, Liu GS, Cohen MV, Pemberton K, Downey JM (2003) CGX-1051, a peptide from Conus snail venom, attenuates infarction in rabbit hearts when administered at reperfusion. J Cardiovasc Pharmacol 42:764–771
Yang X-M, Krieg T, Cui L, Downey JM, Cohen MV (2004) NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol 36:411–421
Cai Z, Semenza GL (2004) Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation 109:2050–2053
Parsa CJ, Kim J, Riel RU, Pascal LS, Thompson RB, Petrofski JA et al (2004) Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J Biol Chem 279:20655–20662
Solenkova NV, Solodushko V, Cohen MV, Downey JM (2006) Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol 290:H441–H449
Philipp S, Yang X-M, Cui L, Davis AM, Downey JM, Cohen MV (2006) Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res 70:308–314
Vinten-Johansen J, Thourani VH, Ronson RS, Jordan JE, Zhao Z-Q, Nakamura M et al (1999) Broad-spectrum cardioprotection with adenosine. Ann Thorac Surg 68:1942–1948
Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR (2004) Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol 287:H841–H849
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res 61:372–385
Zhao Z-Q, Vinten-Johansen J (2002) Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res 55:438–455
Tong H, Imahashi K, Steenbergen C, Murphy E (2002) Phosphorylation of glycogen synthase kinase-3β during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res 90:377–379
Juhaszova M, Zorov DB, Kim S-H, Pepe S, Fu Q, Fishbein KW et al (2004) Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113:1535–1549
Xu Z, Downey JM, Cohen MV (2003) Timing and duration of administration are crucial for antiinfarct effect of AMP 579 infused at reperfusion in rabbit heart. Heart Dis 5:368–371
Bullard AJ, Govewalla P, Yellon DM (2005) Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol 100:397–403
Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K-i et al (2007) Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial KATP channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol 42:453–458
Asakura M, Jiyoong K, Minamino T, Shintani Y, Asanuma H, Kitakaze M (2004) Rationale and design of a large-scale trial using atrial natriuretic peptide (ANP) as an adjunct to percutaneous coronary intervention for ST-segment elevation acute myocardial infarction: Japan-Working groups of acute myocardial Infarction for the reduction of Necrotic Damage by ANP (J-WIND-ANP). Circ J 68:95–100
Minamino T, Jiyoong K, Asakura M, Shintani Y, Asanuma H, Kitakaze M (2004) Rationale and design of a large-scale trial using nicorandil as an adjunct to percutaneous coronary intervention for ST-segment elevation acute myocardial infarction: Japan-Working groups of acute myocardial Infarction for the reduction of Necrotic Damage by a K-ATP channel opener (J-WIND-KATP). Circ J 68:101–106
Ohno Y, Minatoguchi S, Uno Y, Kariya T, Arai M, Yamashita K et al (1997) Nicorandil reduces myocardial infarct size by opening the KATP channel in rabbits. Int J Cardiol 62:181–190
Imagawa J-i, Baxter GF, Yellon DM (1998) Myocardial protection afforded by nicorandil and ischaemic preconditioning in a rabbit infarct model in vivo. J Cardiovasc Pharmacol 31:74–79
Das B, Sarkar C, Karanth KS (2001) Effects of administration of nicorandil or bimakalim prior to and during ischemia or reperfusion on survival rate, ischemia/reperfusion-induced arrhythmias and infarct size in anesthetized rabbits. Naunyn Schmiedebergs Arch Pharmacol 364:383–396
Das B, Sarkar C (2003) Cardiomyocyte mitochondrial KATP channels participate in the antiarrhythmic and antiinfarct effects of KATP activators during ischemia and reperfusion in an intact anesthetized rabbit model. Pol J Pharmacol 55:771–786
Das B, Sarkar C (2005) Is the sarcolemmal or mitochondrial KATP channel activation important in the antiarrhythmic and cardioprotective effects during acute ischemia/reperfusion in the intact anesthetized rabbit model? Life Sci 77:1226–1248
D’Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A et al (2003) B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol 284:H1592–H1600
D’Souza SP, Davis M, Baxter GF (2004) Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther 101:113–129
Jonassen AK, Brar BK, Mjøs OD, Sack MN, Latchman DS, Yellon DM (2000) Insulin administered at reoxygenation exerts a cardioprotective effect in myocytes by a possible anti-apoptotic mechanism. J Mol Cell Cardiol 32:757–764
Jonassen AK, Aasum E, Riemersma RA, Mjøs OD, Larsen TS (2000) Glucose–insulin–potassium reduces infarct size when administered during reperfusion. Cardiovasc Drugs Ther 14:615–623
Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D et al (2005) Effect of glucose–insulin–potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 293:437–446
Vander Heide RS, Reimer KA (1996) Effect of adenosine therapy at reperfusion on myocardial infarct size in dogs. Cardiovasc Res 31:711–718
Xu Z, Downey JM, Cohen MV (2001) AMP 579 reduces contracture and limits infarction in rabbit heart by activating adenosine A2 receptors. J Cardiovasc Pharmacol 38:474–481
Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF et al (1999) Adenosine as an adjunct to thrombolytic therapy for actue myocardial infarction. Results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) Trial. J Am Coll Cardiol 34:1711–1720
Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW (2005) A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of actue myocardial infarction (AMISTAD-II). J Am Coll Cardiol 45:1775–1780
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Downey, J.M., Davis, A.M. & Cohen, M.V. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12, 181–188 (2007). https://doi.org/10.1007/s10741-007-9025-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-007-9025-2