Abstract
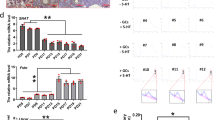
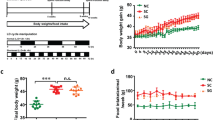
mTOR (mammalian target of Rapamycin) is an important signaling pathway involved in several crucial ovarian functions including folliculogenesis and oocyte maturation. The circadian rhythm regulates multiple physiological processes and PER2 is one of the core circadian rhythm components. mTOR is regulated by the circadian clock and in turn, the rhythmic mTOR activities strengthen the clock function. Our current study aims to investigate a possible interconnection between the circadian clock and the mTORC1 signaling pathway in folliculogenesis and oocyte maturation. Here we demonstrate that the circadian system regulates mTORC1 signaling via Per2 dependent mechanism in the mouse ovary. To investigate the effect of constant light on ovarian and oocyte morphology, animals were housed 12:12 h L:D group in standard lightening conditions and the 12:12 h L:L group in constant light for one week. Food intake and body weight changes were measured. Ovarian morphology, follicle counting, and oocyte aging were evaluated. Afterward, western blot for mTOR, p-mTOR, p70S6K, p-p70S6K, PER2, and Caspase-3 protein levels was performed. The study demonstrated that circadian rhythm disruption caused an alteration in their food intake and decrease in primordial follicle numbers and an increase in the number of atretic follicles. It caused an increase in oxidative stress and a decrease in ZP3 expression in oocytes. Decreased protein levels of mTOR, p-mTOR, p70S6K, and PER2 were shown. The results showed that the circadian clock regulates mTORC1 through PER2 dependent mechanism and that decreased mTORC1 activity can contribute to premature aging of mouse ovary. In conclusion, these results suggest that the circadian clock may control ovarian aging by regulating mTOR signaling pathway through Per2 expression.








Similar content being viewed by others
Data availability (data transparency)
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability (software application or custom code)
None.
Abbreviations
- Bmal1:
-
Brain and muscle ARNT-like protein 1
- Clock:
-
Circadian locomotor output cycles kaput
- Cry1:
-
Cryptochrome circadian regulator 1
- Cry2:
-
Cryptochrome circadian regulator 2
- L:D:
-
Light-dark cycle
- L:L:
-
Light-light cycle
- mTORC1:
-
The mammalian target of rapamycin complex 1
- NTY:
-
Nitrotyrosine
- p-mTOR:
-
Phosphorylated mammalian target of rapamycin
- Per1:
-
Period Circadian Clock 1
- Per2:
-
Period Circadian Clock 2
- Per3:
-
Period Circadian Clock 3
- P70 S6K:
-
Ribosomal protein S6 Kinase
- p-P70 S6K:
-
Phosphorylated ribosomal protein S6 Kinase
- ROS:
-
Reactive Oxygen Species
- TSC1:
-
Tuberous Sclerosis Complex 1
- ZP3:
-
Zona pellucida sperm binding protein 3
References
Arble DM et al (2009) Circadian timing of food intake contributes to weight gain. Obes (Silver Spring) 17(11):2100–2102
Brainard J et al (2015) Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology 122(5):1170–1175
Cao R (2018) mTOR Signaling, Translational Control, and the circadian clock. Front Genet 9:367
Castillo MR et al (2004) Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol 287(3):R551–555
Cissé YM et al (2017) Effects of Dim Light at Night on Food Intake and Body Mass in developing mice. Front Neurosci 11:294
Fahrenkrug J et al (2006) Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 147(8):3769–3776
Fonken LK, Nelson RJ (2014) The effects of light at night on circadian clocks and metabolism. Endocr Rev 35(4):648–670
Fonken LK et al (2010) Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci 107(43):18664–18669
Fonken LK et al (2013) Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms 28(4):262–271
Ford EA et al (2020) Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 159(1):R15–r29
Guo J et al (2018) Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc Natl Acad Sci 115(23):E5326–E5333
Hepler C et al (2022) Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 378(6617):276–284
Herta AC et al (2018) In vitro follicle culture in the context of IVF. Reproduction 156(1):F59–f73
Holmes MM, Mistlberger RE (2000) Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol Behav 68(5):655–666
Jain MV et al (2013) Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med 17(1):12–29
Jiao ZX et al (2012) Age-associated alteration of oocyte-specific gene expression in polar bodies: potential markers of oocyte competence. Fertil Steril 98(2):480–486
Jiao X et al (2018) Molecular Genetics of premature ovarian insufficiency. Trends Endocrinol Metab 29(11):795–807
Kennaway DJ (2005) The role of circadian rhythmicity in reproduction. Hum Reprod Update 11(1):91–101
Lananna BV, Musiek ES (2020) The wrinkling of time: aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol Dis 139:104832
Liu J et al (2018) The role of mTOR in ovarian Neoplasms, polycystic ovary syndrome, and ovarian aging. 31(6):891–898
Meng L et al (2018) Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biol Reprod 99(4):853–863
Miao YL et al (2009) Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 15(5):573–585
Miller BH, Takahashi JS (2013) Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 4:195
Mills J, Kuohung W (2019) Impact of circadian rhythms on female reproduction and infertility treatment success. Curr Opin Endocrinol Diabetes Obes 26(6):317–321
Myers M et al (2004) Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127(5):569–580
Palaniappan M, Menon KM (2012) Luteinizing hormone/human chorionic gonadotropin-mediated activation of mTORC1 signaling is required for androgen synthesis by theca-interstitial cells. Mol Endocrinol 26(10):1732–1742
Percie du Sert N et al (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. 18(7). e3000410
Pickel L, Sung HK (2020) Feeding Rhythms and the circadian regulation of metabolism. Front Nutr 7:39
Prasad S et al (2016) Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 23:36
Ramanathan C et al (2018) mTOR signaling regulates central and peripheral circadian clock function. 14(5):e1007369
Reinke H, Asher G (2019) Crosstalk between metabolism and circadian clocks. 20(4):227–241
Richards J, Gumz ML (2013) Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol 304(12):R1053–1064
Sacks D et al (2018) Multisociety Consensus Quality Improvement revised Consensus Statement for Endovascular Therapy of Acute ischemic stroke. Int J Stroke 13(6):612–632
Sellix MT, Menaker M (2010) Circadian clocks in the ovary. Trends Endocrinol Metab 21(10):628–636
Shaw E et al (2019) Temporal pattern of eating in night shift workers. Chronobiol Int 36(12):1613–1625
Stangherlin A, Reddy AB (2013) Regulation of circadian clocks by redox homeostasis. J Biol Chem 288(37):26505–26511
Sudo M et al (2003) Constant light housing attenuates circadian rhythms of mPer2 mRNA and mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience 121(2):493–499
Turek FW et al (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308(5724):1043–1045
Vollenhoven B, Hunt S (2018) Ovarian ageing and the impact on female fertility. F1000Res, p 7
Wang J et al (2016) Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal 28(8):1099–1104
Wang Z et al (2020) The circadian rhythm and core gene Period2 regulate the chemotherapy effect and multidrug resistance of ovarian cancer through the PI3K signaling pathway.Biosci Rep, 40(11)
Wu R et al (2019) The circadian protein Period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 complex. Cell Metab 29(3):653–667e656
Yaba A, Demir N (2012) The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS). J Ovarian Res 5(1):38
Yaba A et al (2008) A putative mitotic checkpoint dependent on mTOR function controls cell proliferation and survival in ovarian granulosa cells. Reprod Sci 15(2):128–138
Yaba A et al (2015) Expression of CCM2 and CCM3 during mouse gonadogenesis. J Assist Reprod Genet 32(10):1497–1507
Yang S et al (2009) The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150(5):2153–2160
Yu J et al (2011) mTOR controls ovarian follicle growth by regulating granulosa cell proliferation.PLoS One, 6(7), e21415
Zhang XM et al (2013) Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 523(1):82–87
Zheng Y et al (2019) Loss-of-function mutations with circadian rhythm regulator Per1/Per2 lead to premature ovarian insufficiency†. Biol Reprod 100(4):1066–1072
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest/Competing interestsAll the authors listed have significantly contributed to the manuscript and there is no disclosure of any conflict of interest.
Author information
Authors and Affiliations
Contributions
A.Y. conceived and coordinated the study, and analyzed experiments. G.B designed experimental groups and performed the analysis of food intake and body weight changes, morphological evaluation of ovaries. G.B. and E.Y. performed follicle counting. G.B., T.O., and E.Y. performed immunofluorescence staining and image analysis. G.B. and T.O. performed a western blot. A.Y. and G.B. wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Approval of Yeditepe University Animal Ethical Committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bora, G., Önel, T., Yıldırım, E. et al. Circadian regulation of mTORC1 signaling via Per2 dependent mechanism disrupts folliculogenesis and oocyte maturation in female mice. J Mol Histol 54, 217–229 (2023). https://doi.org/10.1007/s10735-023-10126-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10126-9