Abstract
Extracellular matrix (ECM) production and nucleus pulposus (NP) cell migration increase under periodic mechanical stress (PMS), but the underpinning regulatory mechanism remains unclear. This work aimed to examine the regulatory effects of cytoskeleton-lipid raft-integrin α1 signaling in NP cells exposed to PMS. Briefly, In NP cells, cytoskeleton rearrangement, lipid raft aggregation and integrin α1 expression in the stress and control groups were assessed by immunofluorescent staining and immunoblot. In addition, cell migration and ECM gene expression were detected by a scratch test and quantitative reverse transcription polymerase chain reaction (qRT‑PCR), respectively. As a result, PMS up-regulated ECM gene expression and enhanced NP cell migration (both P < 0.05), accompanied by increased integrin α1, lipid raft, caveolin-3, F-actin and β-tubulin amounts. Pretreatment with the lipid raft inhibitor methyl-β-cyclodextrin (MβCD) or small interfering RNA (siRNA) targeting caveolin-3 resulted in decreased ECM mRNA synthesis and cell migration induced by PMS (both P < 0.05); meanwhile, integrin α1 expression was also reduced. F-actin and β-tubulin inhibition by cytochalasin D and colchicine, respectively, not only reduced ECM mRNA synthesis and cell migration (both P < 0.05), but also disrupted lipid raft and caveolin-3 amount increases induced by PMS in NP cells. In conclusion, PMS promotes ECM mRNA up-regulation and cell migration through the cytoskeleton-lipid raft-integrin α1 signaling pathway, inhibiting cytoskeleton and lipid rafts could block the cellular effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disc degeneration disease(DDD)is a common ailment in the spine surgery department. Although the pathogenesis of DDD remains unclear, mechanical stress plays an important role in this process. In vivo, the physiological stress in the intervertebral disc varies with the different posture of the body (Wilke, 1999), which will affect the development, regeneration and degeneration of disc (Kroeber, 2005; Guehring, 2006). In vitro, it is known that mechanical stress can regulate NP cell bioactivities (Kuo, 2014; Feng, 2018; Kim, 2009). On the one hand, appropriate periodic mechanical stress (PMS) can promote NP cell proliferation and extracellular matrix (ECM) synthesis (Gao, 2018; Gao, 2016a; Gao, 2016b). On the other hand, the abnormal stress environment is one of the most important factors leading to NP cell degeneration (Kuo, 2014). However, the process in which mechanical stress signals are converted into chemical ones in NP cells needs further investigation.
Integrins constitute an important protein family on the surface of the cell membrane, which mediate cell adhesion, migration and other biological functions (Wu, 2019). Our previous study (Gao, 2016a) demonstrated that integrin α1 regulates ECM mRNA synthesis and NP cell migration under PMS. Integrin α1 can regulate the downstream phospholipase Cγ1 to convert mechanical signals into biochemical ones, but it remains unclear which protein regulates the pathway upstream of integrin α1.
Lipid rafts were firstly reported by researchers in 1997 (Simons, 1997) and systematically reviewed in 2002 (van, 2002). They constitute a heterogeneous structure located in the cell membrane and composed of cholesterol and sphingomyelin (Zajchowski, 2002). Most proteins in lipid rafts are associated with signal transduction (Katagiri, 2001; Harder 2004). The special structure of lipid rafts is helpful for cell signal transmission. For example, various proteins gather in lipid rafts, which will facilitate their interactions. It has been confirmed that lipid rafts play an important role in the stimulation feedback of different cells (such as lymphocytes (Verstraeten, 2010) and endothelial cells (Zeng, 2013) to mechanical stress, which is related to the structure and localization of lipid rafts on plasma membrane. But there is still a lack of relevant researches on the mechanism of lipid rafts participating in regulation of nucleus pulposus cells under the stress environment.
The cytoskeleton is a protein-fiber network structure in eukaryotes (Wang, 2020); it not only has critical functions in maintaining cellular morphology, external stress bearing, and maintaining the cell’s internal structure, but also contributes to regulating the metabolic function of cells. Besides, the cytoskeleton has been documented to interact with lipid rafts to affect cell signaling and function (Head, 2014).
In this study, we investigated the changes of integrin α1, lipid rafts and cytoskeleton in NP cells exposed to PMS as well as the associated regulatory mechanisms.
Materials and methods
Nucleus pulposus (NP) cell culture
NP cells from male Sprague-Dawley rats (4-week-old) were isolated and cultured as described in a previous study (Gao, 2016a). Briefly, the nucleus pulposus from rat thoracic and lumbar spines were collected and digested in 1.5% type II collagenase (Gibco; Thermo Fisher Scientific, Inc., USA) to get primary cells.Experiments involving animals had approval from the Ethics Committee of Nanjing Medical University. NP cells were passaged at approximately 80% confluence. Passage 2 NP cells at a density of 105/ml were seeded on slides (25 mm × 25 mm), and assessed in subsequent experiments.
Periodical mechanical stress equipment
A perfusion culture system with periodic stress field was designed by our team and produced by Taixing Experimental Instrument Factory (China). In short, this system consisted of a reciprocating intensifier pump and an air-tight cell culture device. It provides PMS in the range of 0-0.3 MPa at 0–1 Hz. PMS at 0-200 kPa (0.1 Hz) was selected for the experiments according to our previous studies (Gao, 2016b, Liang, 2017).
Experimental grouping
The experiments were carried out in two steps. First, the cells were assigned to the control and stress groups. The stress group underwent PMS, while control cells were not subjected to stress. The time of stress treatment depended on the different experiments. Briefly, 24 h stress treatment was performed for cell migration assay, and 8 h stress treatment was used for quantitative reverse transcription polymerase chain reaction (qRT‑PCR)and immunoblot. Then, cell migration assay, qRT‑PCR, lipid raft aggregation, cytoskeleton rearrangement and integrin α1 expression were detected in different groups. Secondly, the cells were pretreated with small interfering RNA (siRNA) targeting caveolin-3, methyl-β-cyclodextrin (MβCD), colchicine and cytochalasin D, respectively, before exposure to PMS. Then, the abovementioned assays were repeated.
Disruption of lipid rafts and the cytoskeleton
MβCD (C4555, Sigma-Aldrich, USA) is known as an inhibitor that disrupts the accumulation of lipid rafts (Deng, 2008). In order to investigate the biological effects of disrupted lipid rafts under stress environment, cells were pretreated with 20 mM MβCD for 60 min before the subsequent assays. Cytochalasin D (C2618, Sigma-Aldrich) and colchicine (C3915, Sigma-Aldrich) are F-actin and β-tubulin inhibitors, respectively. In order to assess the biological effects of F-actin or β-tubulin disruption under stress environment, cells were pretreated with 3µM cytochalasin D or 20µM colchicine for 90 min before the subsequent assays.
Cell transfection
The siRNA sequences were manufactured by Shanghai GenePharma (China): caveolin-3 siRNA 1, forward 5’- GUGAGCUACACCACUUUCATT − 3’ and reverse 5’- UGAAAGUGGUGUAGCUCACTT − 3’; caveolin-3 siRNA 2, forward 5’- GCUACCUGAUUGAGAUCCATT − 3’ and reverse 5’- UGGAUCUCAAUCAGGUAGCTT − 3’; caveolin-3 siRNA 3, forward 5’- GCAACAUUAAGGUGGUGCUTT − 3’ and reverse 5’- AGCACCACCUUAAUGUUGCTT − 3’; negative control (NC) siRNA, forward 5’- UUCUCCGAACGUGUCACGUTT-3’ and reverse 5’- ACGUGACACGUUCGGAGAATT-3’. For transfection, siRNA (75 pM) was mixed with Lipofectamine 2000 (Invitrogen, USA) in 50 µl Opti‑MEM medium (Gibco; USA) as directed by both manufacturers. Then, slides were incubated for 6 h before the subsequent experiments.
Immunoblot
Immunoblot was carried out as described in a previous report (Gao, 2016b). In brief, following treatment with RIPA lysis buffer (P0013, Beyotime, China), total protein was quantitated with a BCA protein assay kit (P0012, Beyotime).
After separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the protein bands underwent transfer onto polyvinylidene fluoride (PVDF) membranes. This was followed by overnight incubation (4℃) with primary antibodies raised against integrin α1 (1:1,000; sc-271,034, Santa Cruz, USA), caveolin-1 (1:1,000; sc-894, Santa Cruz), caveolin-2 (1:1,000; ab2911; Abcam, UK), caveolin-3 (1:1,000; ab2912; Abcam), and GAPDH (1:5,000; AP0063; Bioworld Technology, USA). Following washing with PBS, goat anti-mouse (GAM007; MultiSciences Technology, USA) or anti-rabbit (GAR0072; MultiSciences Technology) IgG-HRP was added for 1 h at ambient. Finally, Immobilon™ Western Chemiluminescent HRP substrate reagent (EMD Millipore, USA) was employed for development. Immunoreactive bands were captured and assessed on a Bio-Rad Gel Doc Imaging System (Bio-Rad Laboratories, USA).
Quantitative reverse transcription polymerase chain reaction (qRT‑PCR)
Total RNA from rat NP cells was extracted with TRIzol regent (Invitrogen) and reverse transcribed with a PrimeScript RT Master Mix kit (RR036, Takara, Japan). Then, qRT‑PCR was carried out with a SYBR Premix Ex Taq II kit (RR820, Takara) on a Step One Plus Real-Time PCR system. Primers were: aggrecan, forward 5’-CCCTACCCTTGCTTCTCCA-3’ and reverse 5’-CTTGAGAGGCACTCATCAATGT-3’; Col2A, forward 5’-GACCCCCAGGTTCTAATGG-3’ and reverse 5’GCACCTTTGGGACCATCTT-3’; β‑actin, forward 5’-GCAGAAGGAGATTACTGCCCT-3’ and reverse 5’-GCTGATCCACATCTGCTGGAA-3’.
Cellular immunofluorescent staining
Cell samples underwent treatment with 5 µg/ml of cholera toxin B-fluorescein isothiocyanate (CTB-FITC; c34775; Thermo Fisher Scientific, USA) at room temperature for 30 min. Then, cells were permeabilized using 0.1% Triton X-100 after fixation with 4% paraformaldehyde. This was followed by successive incubations with primary (4 °C, overnight) and secondary (4 °C, 2 h in the dark) antibodies. The primary antibodies were raised against β-Tubulin (1:500; ab009, MultiSciences Technology), F-actin (1:500; ab205; Abcam) and caveolin-3 (1:1,000; ab2912; Abcam). The secondary antibodies were as follows: Donkey anti-rabbit IgG-Alexa Fluor® 647 (A31573; Thermo Fisher Scientific) and Goat anti-mouse IgG-Alexa Fluor® 555 (A21424; Thermo Fisher Scientific). Finally, DAPI (D9542; Sigma-Aldrich) was used for counterstaining at ambient for 20 min before observation under an A1 laser confocal microscope (Nikon, Japan).
Cell migration assay
The migratory capacity of NP cells was evaluated by a scratch test as previously described (Gao, 2016a). Briefly, the scratch test was performed when cells on slides were about 80% confluent. Following treatment, a 200 µl pipette tip was used to produce scratches. Then, the cells were cultured in an incubator with serum-free medium at 37˚C, 5% CO2 for 24 h. After observation under a microscope (Olympus, Japan), further analysis was performed with ImageJ 1.43 (imagej.nih.gov).
Statistical analysis
Data are mean ± standard deviation (SD), and GraphPad Prism (GraphPad Software, USA) was utilized for analysis. Unpaired t-test was performed to compare group pairs. Multiple group comparisons were performed by one-way ANOVA followed by Dunnett’s multiple comparisons. P < 0.05 indicated statistical significance.
Results
PMS promotes ECM mRNA up-regulation, cell migration and integrin α1 upregulation
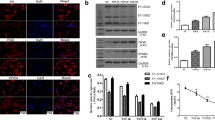
As reported previously by our team (Gao, 2016a), in comparison with control cells, PMS (0.2 MPa; 0.1 Hz; 24 h) markedly enhanced cell migration (P < 0.05, Fig. 1a). PMS (0.2 MPa; 0.1 Hz; 8 h) upregulated ECM-associated Col2A1 and aggrecan (P < 0.05, Fig. 1b), and upregulated integrin α1 (Fig. 1c).
Promotion effects of PMS on ECM production, cell migration and integrin α1 expression. (a) NP cell migration was evaluated by a scratch assay. The difference between scratch width at 0 and 24 h divided by scratch width at 0 h was equal to the percent of wound healing (Scale bar=100 μm). Data were expressed as mean ± standard deviation (SD) (n=5). *P<0.05, compared with the control group. (b) RT-qPCR analysis for mRNA expression of Col2A1 and aggrecan after 8 h stress treatment. Data were expressed as mean ± SD. *P<0.05, compared with the control group. (c) Integrin α1 protein expression was detected by Western blot after 8 h stress treatment. (d) The results of Western blot for caveolin-1, caveolin-2 and caveolin-3 in control and 8 h stress group
PMS promotes lipid raft aggregation and caveolin-3 upregulation in NP cells
CTB-FITC staining reflects lipid raft aggregation. In comparison with control cells, lipid raft aggregation was obvious at different time-points (2 h, 4 and 8 h) of stress treatment, especially in the 8 h stress treatment group (Fig. 2). Then, 8 h stress treatment was selected for subsequent assays. It was found that 8 h stress treatment upregulated caveolin-3, but not caveolin-1 or caveolin-2 by cellular western blot (Fig. 1d).
Lipid rafts and caveolin-3 are required for PMS-induced ECM mRNA up-regulation, cell migration and integrin α1 upregulation
In order to further explore the regulatory mechanism of lipid rafts and caveolin-3 under stress environment, the siRNA technology and MβCD were employed to inhibit caveolin-3 and lipid rafts, respectively. First, siRNA sequences (siRNA 2) with the best inhibitory effects on caveolin-3 (Fig. 3a) were selected. After pretreatment of NP cells with siRNA 2 and MβCD, respectively, inhibition of lipid rafts and caveolin-3 significantly reduced cell migration (P < 0.05, Fig. 3b) and ECM mRNA production (P < 0.05, Fig. 3c) in comparison with control cells under PMS. We further assessed the associations of lipid rafts, caveolin-3 and integrin α1. Both cellular immunofluorescent staining (Fig. 3d) and Western blot (Fig. 3e) demonstrated that inhibition of lipid rafts and caveolin-3, respectively, suppressed PMS-induced integrin α1 upregulation.
Role of lipid rafts and caveolin-3 in PMS-induced ECM production, cell migration and integrin α1 upregulation. (a) Western blot for caveolin-3 protein expression after blocking via siRNA. (b) NP cell migration was evaluated by a scratch assay after 24 h stress treatment. Representative images were showed in the control group, stress group, stress + MβCD group and stress + siRNA group. Data were expressed as mean ± SD (n = 5). *P<0.05, compared with the control group. #P<0.05, compared with the stress group. @P<0.05, compared with the stress group. (c) After 8 h stress treatment, RT-qPCR analysis for mRNA expression of Col2A1 and aggrecan in the control group, stress group, stress + MβCD group and stress + siRNA group. *P<0.05, compared with the control group. #P<0.05, compared with the stress group. @P<0.05, compared with the stress group. (d) After 8 h stress treatment, cellular immunofluorescent staining for lipid rafts, caveolin-3 and integrin α1 expression in the control group, stress group, stress + MβCD group and stress + siRNA group (Scale bar=10 μm). (e) After 8 h stress treatment, Western blot for integrin α1 protein expression in the control group, stress group, stress + MβCD group and stress + siRNA group
PMS increases F-actin and β-tubulin amounts
Cellular immunofluorescent staining showed that F-actin and β-tubulin amounts were obviously increased after 2 h, 4 and 8 h of stress treatment in comparison with control cells (Fig. 4a and b).
Cellular immunofluorescent staining for F-actin and β-tubulin expression. (a) Cellular immunofluorescent staining for observing F-actin expression at 0 h, 2 h, 4 and 8 h time-point after stress treatment (Scale bar=10 μm). (b) Cellular immunofluorescent staining for observing β-tubulin expression at 0 h, 2 h, 4 and 8 h time-point after stress treatment (Scale bar=10 μm)
F-actin and β-tubulin are required for PMS induced increase of lipid rafts and caveolin-3 upregulation in NP cells
To further investigate the regulatory relationships among cytoskeleton, lipid rafts and caveolin-3, the amounts of lipid rafts and caveolin-3 were examined after pretreatment of NP cells with F-actin (cytochalasin D) and β-tubulin (colchicine) inhibitors, respectively. Figure 5a shows that in comparison with control cells, PMS increased the amounts of lipid rafts, caveolin-3 and β-Tubulin, which were reduced after treatment with colchicine. Similarly, in comparison with control cells, stress treatment elevated the amounts of lipid rafts, caveolin-3 and F-actin, which were also decreased by treatment with cytochalasin D (Fig. 5b). After pretreatment of NP cells with colchicine and cytochalasin D, respectively, inhibition of β-tubulin and F-actin could significantly reduce cell migration (P < 0.05, Fig. 5d) and ECM mRNA level (P < 0.05, Fig. 5e) in comparison with control cells under PMS.
Role of F-actin and β-tubulin in PMS‑induced increase of lipid rafts and caveolin-3 upregulation in NP cells. (a) After 8 h stress treatment, cellular immunofluorescent staining for lipid rafts, β-Tubulin and caveolin-3 expression in the control group, stress group and stress + Colchicine group (Scale bar=10 μm). (b) After 8 h stress treatment, cellular immunofluorescent staining for lipid rafts, F-actin and caveolin-3 expression in the control group, stress group and stress + Cytochalasin D group (Scale bar=10 μm). (c) After 8 h stress treatment, Western blot for caveolin-3 protein expression in the control group, stress group, stress + Colchicine group and stress + Cytochalasin D group. (d) NP cell migration was evaluated by a scratch assay after 24 h stress treatment. Representative images were showed in the control group, stress group, stress + Colchicine group and stress + Cytochalasin D group. Data were expressed as mean ± SD (n = 5). *P<0.05, compared with the control group. #P<0.05, compared with the stress group. @P<0.05, compared with the stress group. (e) After 8 h stress treatment, RT-qPCR analysis for mRNA expression of Col2A1 and aggrecan in the control group, stress group, stress + Colchicine group and stress + Cytochalasin D group. Data were expressed as mean ± SD. *P<0.05, compared with the control group. #P<0.05, compared with the stress group. @P<0.05, compared with the stress group
Discussion
This study assessed the transduction of mechanical signals to biochemical ones in NP cells. The results showed that the cytoskeleton, lipid rafts (especially the lipid raft component caveolin-3) and integrin α1 participate in mechanical-biochemical signal transmission in NP cells.
Integrin subunits α1, α5 and α6 have been detected in intervertebral disc tissues (Nettles, 2004). Some integrins are important in regulating the attachment of NP cells to laminins (Bridgen, 2013) as well as NP cell survival in hypoxia (Risbud, 2005). Our previous report demonstrated that integrin α1 is critical in regulating migration and ECM mRNA production in NP cells under stress environment (Gao, 2016a). In this study, corroborating our previous findings, PMS upregulated integrin α1 in NP cells in a lipid raft dependent manner. Our results showed that integrin α1 was significantly downregulated after MβCD-mediated destruction of lipid rafts (Dykstra, 2003) in NP cells. Relevant evidences confirm that lipid rafts and integrin interact to regulate cell migration, regeneration and proliferation (Bi, 2018, Antelmi, 2013; Lee, 2016). However, in the process of mechanical-biochemical signal transmission, we firstly elucidated the relationship between lipid rafts and integrin α1.
In this study, CTB-FITC was used to label lipid rafts. CTB directly binds to GM1 gangliosides in lipid rafts, promoting lipid raft clustering in cells (Deng, 2008, Bi, 2001). The above results confirmed that compared with the control group, mechanical stress induced lipid raft aggregation significantly. Caveolae is the most important component of lipid rafts (Huo, 2003), while caveolin represents a marker protein of caveolae, with three variants including caveolin-1, caveolin-2 and caveolin-3 (Williams, 2004). Several studies have focused on caveolin-1 in the intervertebral disc. For instance, it was reported that caveolin-1 is upregulated with disc degeneration (Bach, 2016) but not in association with age (Heathfield, 2008). Furthermore, caveolin‑1 participates in NP cell senescence after oxidative injury (Ding, 2017) and may constitute an ideal target for the treatment of IVD degeneration (Smolders, 2013). However, no report has assessed the role of caveolin-3 in NP cells. As shown above, caveolin-3 amounts were significantly elevated in comparison with those of caveolin-1and 2 under mechanical stress. After blocking the caveolin-3 protein, extracellular matrix amounts and NP cell migration were decreased significantly compared with the stress group, again showing that caveolin-3 has a critical function in signal regulation under stress stimulation.
Previous studies demonstrated that the cytoskeleton is critical in modulating metabolic function in NP cells. The cytoskeleton consists of three parts, including microtubules (MTs), microfilaments (MFs), and intermediate filaments (IFs) (Pegoraro, 2017). Microtubules are mainly composed of tubulin, while microfilaments are mostly made of actin. It is known that leptin can upregulate β-actin, F-actin and vimentin in NP cells, inducing cytoskeleton remodeling through the RhoA/ROCK pathway (Li, 2013; Li 2014). Li et al. demonstrated that cyclic tensile strain can stimulate F-actin reorganization in NP cells and upregulate type II collagen (Li, 2011). Similarly, our results showed that PMS promoted cytoskeleton (β-Tubulin and F-actin) rearrangement in NP cells. Based on these findings, we further assessed whether cytoskeleton rearrangement was related to lipid raft aggregation and caveolin-3 upregulation. Following treatment with cytochalasin D and colchicine to inhibit F-actin and β-tubulin, respectively, it was found that lipid raft aggregation and caveolin-3 upregulation in NP cells induced by stress were also significantly weakened. Although it is known that lipid raft aggregation depends on cytoskeleton activation (Badizadegan, 2004, Head, 2006), this is the first time to draw similar conclusions under mechanical stress stimulation.
In vivo, the development, degeneration and regeneration of the intervertebral disc are inseparable from the stress environment. Therefore, this study of the nucleus pulposus cell regulation mechanism under the stress environment is closer to reality and has more profound significance. We demonstrated that the Src-GIT1-ERK1/2 pathway (Gao, 2018) and integrin α1-PLCγ1 (Gao, 2016a) were involved in the regulation chain. Combined with this study, from cytoskeleton, cell membrane structure (lipid rafts) and transmembrane protein integrin α1 to transporters in cytoplasm, we have basically clarified the transmission of signal pathways under stress environment. However, the limitation of this study is that all data is based on monolayer cell culture system, the further research will be performed in the three-dimensional cell culture system in the future.
Overall, this work demonstrated that PMS could promote cytoskeleton rearrangement and induce lipid raft aggregation in NP cells, resulting in integrin α1 upregulation and increased ECM mRNA production and cell migration (Fig. 6). These results reveal the regulatory mechanism underlying the biological functions of NP cells under stress environment. These findings provide a theoretical basis for further DDD research.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable requests.
Abbreviations
- DDD:
-
Disc degeneration disease
- PMS:
-
periodic mechanical stress
- ECM:
-
extracellular matrix
- NP:
-
Nucleus pulposus
- MβCD:
-
methyl-β-cyclodextrin
- CTB-FITC:
-
cholera toxin B-fluorescein isothiocyanate
References
Antelmi E, Cardone RA, Greco MR, Rubino R, Di Sole F, Martino NA, Casavola V, Carcangiu M, Moro L, Reshkin SJ (2013) ß1 integrin binding phosphorylates ezrin at T567 to activate a lipid raft signalsome driving invadopodia activity and invasion. PLoS ONE 8:e75113
Bach FC, Zhang Y, Miranda-Bedate A, Verdonschot LC, Bergknut N, Creemers LB, Ito K, Sakai D, Chan D, Meij BP, Tryfonidou MA (2016) Increased caveolin-1 in intervertebral disc degeneration facilitates repair, vol 18. Arthritis research & therapy, p 59
Badizadegan K, Wheeler HE, Fujinaga Y, Lencer WI (2004) Trafficking of cholera toxin-ganglioside GM1 complex into golgi and induction of toxicity depend on actin cytoskeleton. Am J Physiol Cell Physiol 287:C1453–1462
Bi J, Wang R, Zeng X (2018) Lipid rafts regulate the lamellipodia formation of melanoma A375 cells via actin cytoskeleton-mediated recruitment of β1 and β3 integrin. Oncol Lett 16:6540–6546
Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, Altman A (2001) Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol 2:556–563
Bridgen DT, Gilchrist CL, Richardson WJ, Isaacs RE, Brown CR, Yang KL, Chen J, Setton LA (2013) Integrin-mediated interactions with extracellular matrix proteins for nucleus pulposus cells of the human intervertebral disc. J Orthop research: official publication Orthop Res Soc 31:1661–1667
Deng GM, Tsokos GC (2008) Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. J immunology: official J Am Association Immunologists 181:4019–4026
Ding L, Zeng Q, Wu J, Li D, Wang H, Lu W, Jiang Z, Xu G (2017) Caveolin–1 regulates oxidative stress–induced senescence in nucleus pulposus cells primarily via the p53/p21 signaling pathway in vitro. Mol Med Rep 16:9521–9527
Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK (2003) Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol 21:457–481
Feng C, Yang M, Zhang Y, Lan M, Huang B, Liu H, Zhou Y (2018) Cyclic mechanical tension reinforces DNA damage and activates the p53-p21-Rb pathway to induce premature senescence of nucleus pulposus cells. Int J Mol Med 41:3316–3326
Gao G, He J, Nong L, Xie H, Huang Y, Xu N, Zhou D (2016a) Periodic mechanical stress induces the extracellular matrix expression and migration of rat nucleus pulposus cells by upregulating the expression of intergrin α1 and phosphorylation of downstream phospholipase Cγ1. Mol Med Rep 14:2457–2464
Gao G, Li H, Huang Y, Yin J, Jiang Y, Xu N, Zhou D, Nong L, Ren K (2018) Periodic mechanical stress induces Extracellular Matrix expression and Migration of Rat Nucleus Pulposus cells through Src-GIT1-ERK1/2 signaling pathway. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 50:1510–1521
Gao G, Shen N, Jiang X, Sun H, Xu N, Zhou D, Nong L, Ren K (2016b) Periodic mechanical stress activates EGFR-dependent Rac1 mitogenic signals in rat nucleus pulpous cells via ERK1/2. 469:723–730Biochemical and biophysical research communications
Guehring T, Unglaub F, Lorenz H, Omlor G, Wilke HJ, Kroeber MW (2006) Intradiscal pressure measurements in normal discs, compressed discs and compressed discs treated with axial posterior disc distraction: an experimental study on the rabbit lumbar spine model. European spine journal: official publication of the european spine Society, the european spinal deformity Society, and the european section of the cervical spine Research Society. 15:597–604
Harder T (2004) Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr Opin Immunol 16:353–359
Head BP, Patel HH, Insel PA (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838:532–545
Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA (2006) Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281:26391–26399
Heathfield SK, Le Maitre CL, Hoyland JA (2008) Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration, vol 10. Arthritis research & therapy, p R87
Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K (2003) Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem 278:11561–11569
Katagiri YU, Kiyokawa N, Fujimoto J (2001) A role for lipid rafts in immune cell signaling. Microbiol Immunol 45:1–8
Kim DH, Kim SH, Heo SJ, Shin JW, Lee SW, Park SA, Shin JW (2009) Enhanced differentiation of mesenchymal stem cells into NP-like cells via 3D co-culturing with mechanical stimulation. J Biosci Bioeng 108:63–67
Kroeber M, Unglaub F, Guehring T, Nerlich A, Hadi T, Lotz J, Carstens C (2005) Effects of controlled dynamic disc distraction on degenerated intervertebral discs: an in vivo study on the rabbit lumbar spine model. Spine 30:181–187
Kuo YJ, Wu LC, Sun JS, Chen MH, Sun MG, Tsuang YH (2014) Mechanical stress-induced apoptosis of nucleus pulposus cells: an in vitro and in vivo rat model. J Orthop science: official J Japanese Orthop Association 19:313–322
Lee SS, Lee SJ, Lee SH, Ryu JM, Lim HS, Kim JS, Song EJ, Jung YH, Lee HJ, Kim CH, Han HJ (2016) Netrin-1-Induced Stem Cell Bioactivity contributes to the regeneration of injured tissues via the lipid raft-dependent integrin α6β4 signaling pathway. Sci Rep 6:37526
Li S, Jia X, Duance VC, Blain EJ (2011) The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study, vol 21. European cells & materials, pp 508–522
Li Z, Liang J, Wu WK, Yu X, Yu J, Weng X, Shen J (2014) Leptin activates RhoA/ROCK pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int J Mol Sci 15:1176–1188
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G, Liu J (2013) The role of leptin on the organization and expression of cytoskeleton elements in nucleus pulposus cells. J Orthop research: official publication Orthop Res Soc 31:847–857
Liang W, Li Z, Wang Z, Zhou J, Song H, Xu S, Cui W, Wang Q, Chen Z, Liu F, Fan W (2017) Periodic mechanical stress INDUCES chondrocyte proliferation and Matrix Synthesis via CaMKII-Mediated Pyk2 signaling. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 42:383–396
Nettles DL, Richardson WJ, Setton LA (2004) Integrin expression in cells of the intervertebral disc. J Anat 204:515–520
Pegoraro AF, Janmey P, Weitz DA (2017) Mechanical Properties of the Cytoskeleton and cells. Cold Spring Harbor perspectives in biology, p 9
Risbud MV, Guttapalli A, Albert TJ, Shapiro IM (2005) Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine 30:2503–2509
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Smolders LA, Meij BP, Onis D, Riemers FM, Bergknut N, Wubbolts R, Grinwis GC, Houweling M, Groot Koerkamp MJ, van Leenen D, Holstege FC, Hazewinkel HA, Creemers LB, Penning LC, Tryfonidou MA (2013) Gene expression profiling of early intervertebral disc degeneration reveals a down-regulation of canonical wnt signaling and caveolin-1 expression: implications for development of regenerative strategies, vol 15. Arthritis research & therapy, p R23
van Meer G (2002) Cell biology. The different hues of lipid rafts. Science 296:855–857
Verstraeten SV, Mackenzie GG, Oteiza PI (2010) The plasma membrane plays a central role in cells response to mechanical stress. Biochim Biophys Acta 1798:1739–1749
Wang TT, Wei QC, Zhang ZT, Lin MT, Chen JJ, Zhou Y, Guo NN, Zhong XC, Xu WH, Liu ZX, Han M, Gao JQ (2020) AIE/FRET-based versatile PEG-Pep-TPE/DOX nanoparticles for cancer therapy and real-time drug release monitoring. Biomaterials Sci 8:118–124
Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE (1999) New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 24:755–762
Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5:214
Wu X, Wang K, Hua W, Li S, Liu X, Song Y, Zhang Y, Shao Z, Li S, Yang C (2019) Fibronectin induced ITGβ1/FAK-dependent apoptotic pathways determines the fate of degenerative NP cells. J Orthop research: official publication Orthop Res Soc 37:439–448
Zajchowski LD, Robbins SM (2002) Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem 269:737–752
Zeng Y, Waters M, Andrews A, Honarmandi P, Ebong EE, Rizzo V, Tarbell JM (2013) Fluid shear stress induces the clustering of heparan sulfate via mobility of glypican-1 in lipid rafts. Am J Physiol Heart Circ Physiol 305:H811–820
Funding
This study supported by the General Project of Jiangsu Provincial Department of Health(H2019025), Six Talent Peaks Project, Jiangsu Provincial Finance Department (WSW-186), Special Funds for Key Plan (Social Development) of Jiangsu Province (BE2020650), Changzhou Science and Technology Bureau Project (CZ20200037), Jintan Science and Technology Plan Project (KJ201926) and the Wuxi Health Commission Foundation (J201811).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Effects of periodic mechanical stress on cytoskeleton dependent lipid raft-induced integrin ɑ1 activation in rat nucleus pulposus cells”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gongming Gao and Kewei Ren contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10735_2023_10112_MOESM1_ESM.tif
Supplementary Material 1: The other two Western blot images for caveolin-3 protein expression after blocking via siRNA (Fig. 3a)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, G., Ren, K., Chen, L. et al. Effects of periodic mechanical stress on cytoskeleton dependent lipid raft-induced integrin ɑ1 activation in rat nucleus pulposus cells. J Mol Histol 54, 67–75 (2023). https://doi.org/10.1007/s10735-023-10112-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10112-1