Abstract
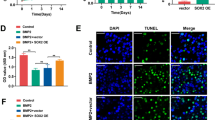
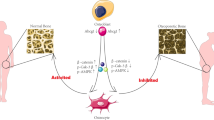
The imbalance between osteogenic and adipogenic differentiation of Bone marrow-derived mesenchymal stem cells (BMSCs) is involved in the occurrence and development of osteoporosis (OP). Previous studies have indicated the potential of phosphatase and actin regulator 1 (Phactr1) in regulating osteogenic and adipogenic differentiation of BMSCs. The present study aims to investigate the function and mechanism of Phactr1 in regulating osteogenic and adipogenic differentiation of BMSCs. Herein, the expression of Phactr1 in bone and adipose tissue of OP rats was determined by immunohistochemical. BMSCs were subjected to osteogenic and adipogenic differentiation, and transfected with Phactr1 overexpression lentivirus, small interference RNA (siRNA) and KD025 (selective ROCK2 inhibitor). The relationship between Phactr1 and ROCK2 was detected by Co-IP experiment. The expression of Phactr1, Runx2, C/EBPα, RhoA and ROCK2 was detected by Western blot. Calcium nodule and lipid droplets were determined by alizarin red and Oil red O staining. Interestingly, Phactr1 increased in both bone and adipose tissue of OP rats. During osteogenic differentiation, Phactr1 decreased and active RhoA, ROCK2 increased, while overexpression Phactr1 inhibits the increase of Runx2. Phactr1 increased and active RhoA decreased, ROCK2 did not changed during adipogenic differentiation. While, Knockdown Phactr1 inhibits the increase of C/EBPα. Phactr1 and ROCK2 were combined in osteogenic differentiation, but not in adipogenic differentiation. By using KD025, the decrease of Phactr1 and increase of Runx2 were inhibited respectively in osteogenic differentiation. Meanwhile, when ROCK2 was inhibited, Phactr1, C/EBPα were significantly increased in adipogenic differentiation. These findings indicated that Phactr1 negatively regulates bone mass by inhibiting osteogenesis and promoting adipogenesis of BMSCs by activating RhoA/ROCK2.






Similar content being viewed by others
References
Arnsdorf EJ, Tummala P, Kwon RYCR et al (2009) Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 122:546–553. https://doi.org/10.1242/jcs.036293
Chen B, Lin T, Yang X et al (2016) Intermittent parathyroid hormone (1–34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway. Exp Ther Med 11:2399–2406. https://doi.org/10.3892/etm.2016.3177
Chen L, Wu C, Wei D et al (2021) Biomimetic mineralized microenvironment stiffness regulated BMSCs osteogenic differentiation through cytoskeleton mediated mechanical signaling transduction. Mater Sci Eng C Mater Biol Appl 119:111613. https://doi.org/10.1016/j.msec.2020.111613
Cui LH, Shin MH, Chung EK et al (2005) Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int 16:1975–1981. https://doi.org/10.1007/s00198-005-1977-2
Fils-Aimé N, Dai M, Guo J et al (2013) MicroRNA-584 and the protein phosphatase and actin regulator 1 (PHACTR1), a new signaling route through which transforming growth factor-β Mediates the migration and actin dynamics of breast cancer cells. J Biol Chem 288:11807–11823. https://doi.org/10.1074/jbc.M112.430934
Fu R, Liu Q, Song G et al (2013) Spreading area and shape regulate apoptosis and differentiation of osteoblasts. Biomed Mater 8:055005. https://doi.org/10.1088/1748-6041/8/5/055005
James AW (2013) Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013:684736. https://doi.org/10.1155/2013/684736
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16:S3–S7. https://doi.org/10.1007/s00198-004-1702-6
Justesen J, Stenderup K, Ebbesen EN et al (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171. https://doi.org/10.1023/a:1011513223894
Ke X, Do DC, Li C et al (2019) RAS homolog family member A/Rho-associated protein kinase 1 signaling modulates lineage commitment of mesenchymal stem cells in asthmatic patients through lymphoid enhancer-binding factor 1. J Allergy Clin Immunol 143:1560-1574.e6. https://doi.org/10.1016/j.jaci.2018.08.023
Khan AU, Qu R, Fan T et al (2020) A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res Ther 11:283. https://doi.org/10.1186/s13287-020-01789-2
Li L, Tam L, Liu L et al (2011) Wnt-signaling mediates the anti-adipogenic action of lysophosphatidic acid through cross talking with the Rho/Rho associated kinase (ROCK) pathway. Biochem Cell Biol 89:515–521. https://doi.org/10.1139/o11-048
Lu C, Dong X, Yu WP et al (2020) Inorganic phosphate-osteogenic induction medium promotes osteogenic differentiation of valvular interstitial cells via the BMP-2/Smad1/5/9 and RhoA/ROCK-1 signaling pathways. Am J Transl Res 12:3329–3345
Martin AR, Sornay-Rendu E, Chandler JM et al (2002) The impact of osteoporosis on quality-of-life: the OFELY cohort. Bone 31:32–36. https://doi.org/10.1016/s8756-3282(02)00787-1
Nakano S, Inoue K, Xu C et al (2019) G-protein Gα13 functions as a cytoskeletal and mitochondrial regulator to restrain osteoclast function. Sci Rep 9:4236. https://doi.org/10.1038/s41598-019-40974-z
Oliveira MC, Vullings J, van de Loo F (2020) Osteoporosis and osteoarthritis are two sides of the same coin paid for obesity. Nutrition 70:110486. https://doi.org/10.1016/j.nut.2019.04.001
Pollard TD, Cooper JA (2009) Actin, a central player in cell shape and movement. Science 326:1208–1212. https://doi.org/10.1126/science.1175862
Qiu W, Andersen TE, Bollerslev J et al (2007) Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res 22:1720–1731. https://doi.org/10.1359/jbmr.070721
Riggs BL, Melton LJ 3rd (1995) The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17:505S-511S. https://doi.org/10.1016/8756-3282(95)00258-4
Seo CH, Jeong H, Feng Y et al (2014) Micropit surfaces designed for accelerating osteogenic differentiation of murine mesenchymal stem cells via enhancing focal adhesion and actin polymerization. Biomaterials 35:2245–2252. https://doi.org/10.1016/j.biomaterials.2013.11.089
Titorencu I, Jinga V, Constantinescu E et al (2007) Proliferation, differentiation and characterization of osteoblasts from human BM mesenchymal cells. Cytotherapy 9:682–696. https://doi.org/10.1080/14653240701561329
Tong Z, Liu Y, Xia R et al (2020) F-actin regulates osteoblastic differentiation of mesenchymal stem cells on TiO2 nanotubes through MKL1 and YAP/TAZ. Nanoscale Res Lett 15:183. https://doi.org/10.1186/s11671-020-03415-9
Wang S, Chen C, Su K et al (2016) Angiotensin II induces reorganization of the actin cytoskeleton and myosin light-chain phosphorylation in podocytes through rho/ROCK-signaling pathway. Ren Fail 38:268–275. https://doi.org/10.3109/0886022X.2015.1117896
Yang Y, Wang X, Wang Y et al (2019) Influence of cell spreading area on the osteogenic commitment and phenotype maintenance of mesenchymal stem cells. Sci Rep 9:6891. https://doi.org/10.1038/s41598-019-43362-9
Zhang Q, Zhou J, Wang Q et al (2020) Association between bone mineral density and lipid profile in chinese women. Clin Interv Aging 15:1649–1664. https://doi.org/10.2147/CIA.S266722
Zhu H, Guo ZK, Jiang XX et al (2010) A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 5(3):550–560. https://doi.org/10.1038/nprot.2009.238
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable efforts and comments on this paper.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (Grant No. 31570976), and Guangzhou Science, Technology and Innovation Commission (Grant No. 201604020148).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design: BC; performed the experiments: WL, ZC, XM, SZ and ZW; data analysis and interpretation and wrote the manuscript: WL, WC, DF; drafting the article or critically revising it for important intellectual content: BC; final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Ethical approval
The present study was approved by the Animal Care Committee of Sun Yat-Sen University [No. (2019)196] and was performed in accordance with the guidelines for the use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, W., Chen, Z., Mo, X. et al. Phactr1 negatively regulates bone mass by inhibiting osteogenesis and promoting adipogenesis of BMSCs via RhoA/ROCK2. J Mol Histol 53, 119–131 (2022). https://doi.org/10.1007/s10735-021-10031-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-021-10031-z