Abstract
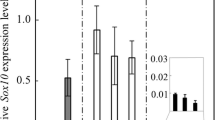
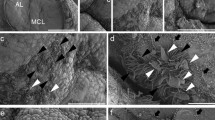
Although δ-crystallin (δ-crys), also known as lens protein, is transiently expressed in Rathke’s pouch (RP) of the chick embryo, detailed temporal and spatial expression patterns have been obscure. In this study, to understand the relationship between the δ-crys mRNA-expressing region and RP formation, we examined the embryonic expression pattern of δ-crys mRNA in the primordium of the adenohypophysis. δ-crys mRNA expression was initially found at stage 15 anterior to the foregut and posterior to the invaginated oral ectoderm. After RP formation, the δ-crys mRNA was expressed in the post-ventral region of RP and the anterior region of RP. δ-crys mRNA expression was then restricted to the cephalic lobe of the pituitary gland. From stage 20, the δ-crys and alpha-glycoprotein subunit (αGSU) mRNA-expressing regions were almost completely overlapping. The αGSU mRNA-expressing region is thought to be the primordium of the pars tuberalis, and these regions were overlapped with the Lhx3 mRNA-expressing region. The intensity of δ-crys mRNA expression gradually decreased with development and completely disappeared by stage 34. These results suggest that the embryonic chick pituitary gland consists of two different regions labeled with δ-crys and Lhx3.



Similar content being viewed by others
References
Aizawa S, Hoshino S, Sakata I, Adachi A, Yashima S, Hattori A, Sakai T (2007) Diurnal change of thyroid-stimulating hormone mRNA expression in the rat pars tuberalis. J Neuroendocrinol 19(11):839–846. doi:10.1111/j.1365-2826.2007.01603.x
Baravanov V (1977) Expression of δ-crystallin in the adenohypophysis of chick embryos. Dokl Acad Sci USSR 234:195–198
Dellmann HD, Stoeckel ME, Hindelang-Gertner C, Porte A, Stutinsky F (1974) A comparative ultrastructural study of the pars tuberalis of various mammals, the chicken and the newt. Cell Tissue Res 148(3):313–329
Ede DA, Kelly WA (1964) Developmental abnormalities in the head region of the talpid mutant of the fowl. J Embryol Exp Morphol 12:161–182
Ellsworth BS, Butts DL, Camper SA (2008) Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Develop Biol 313(1):118–129. doi:10.1016/j.ydbio.2007.10.006
Ericson J, Norlin S, Jessell TM, Edlund T (1998) Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125(6):1005–1015
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. Developmental dynamics : an official publication of the American Association of Anatomists. 195 (4):231–272. doi:10.1002/aja.1001950404
Japon MA, Rubinstein M, Low MJ (1994) In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem Off J Histochem Soc 42(8):1117–1125
Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H (1998) Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125(13):2521–2532
Kameda Y, Miura M, Ohno S (2000) Expression of the common alpha-subunit mRNA of glycoprotein hormones during the chick pituitary organogenesis, with special reference to the pars tuberalis. Cell Tissue Res 299(1):71–80
Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, Carmignac D, Lovell-Badge R, Robinson IC, Dattani MT (2006) Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Investig 116(9):2442–2455. doi:10.1172/JCI28658
Kioussi C, O’Connell S, St-Onge L, Treier M, Gleiberman AS, Gruss P, Rosenfeld MG (1999) Pax6 is essential for establishing ventral-dorsal cell boundaries in pituitary gland development. Proc Nat Acad Sci USA 96(25):14378–14382
Kondoh H, Uchikawa M, Yoda H, Takeda H, Furutani-Seiki M, Karlstrom RO (2000) Zebrafish mutations in Gli-mediated hedgehog signaling lead to lens transdifferentiation from the adenohypophysis anlage. Mech Dev 96(2):165–174
Kondoh H, Uchikawa M, Kamachi Y (2004) Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int J Develop Biol 48(8–9):819–827. doi:10.1387/ijdb.041868hk
Maseki Y, Nakamura K, Iwasawa A, Zheng J, Inoue K, Sakai T (2004) Development of gonadotropes in the chicken embryonic pituitary gland. Zoolog Sci 21(4):435–444
Mullis PE (2001) Transcription factors in pituitary development. Mol Cell Endocrinol 185(1–2):1–16
Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T (2008) Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452(7185):317–322. doi:10.1038/nature06738
Rathke H (1838) Über die Entstehung der Glandula pituitaria. Arch Anat Physiol Wiss Med 1838:482–485
Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R (2004) SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet 36(3):247–255. doi:10.1038/ng1309
Sakai T, Sakamoto S, Ijima K, Matsubara K, Kato Y, Inoue K (1999) Characterization of TSH-positive cells in foetal rat pars tuberalis that fail to express Pit-1 factor and thyroid hormone beta2 receptors. J Neuroendocrinol 11(3):187–193
Sakamoto S, Nakamura K, Inoue K, Sakai T (2000) Melatonin stimulates thyroid-stimulating hormone accumulation in the thyrotropes of the rat pars tuberalis. Histochem Cell Biol 114(3):213–218
Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T (2002) Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 23(3):531–536
Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ (2003) Transcriptional control during mammalian anterior pituitary development. Gene 319:1–19
Schwind J (1928) The development of the hypophysis cerebri of the albino rat. Am J Anat 41:295–319
Seessel A (1877) Zur Entwicklungsgeschichte des Vorderdarms. Arch Anat Physiol Wiss Med 1877:449–467
Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW (1990) Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 4(5):695–711
Stoeckel ME, Hindelang C, Klein MJ, Poissonnier M, Felix JM (1993) Early expression of the glycoprotein hormone alpha-subunit in the pars tuberalis of the rat pituitary gland during ontogenesis. Neuroendocrinology 58(6):616–624
Takagi H, Nagashima K, Inoue M, Sakata I, Sakai T (2008) Detailed analysis of formation of chicken pituitary primordium in early embryonic development. Cell Tissue Res 333(3):417–426. doi:10.1007/s00441-008-0647-z
Treier M, O’Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG (2001) Hedgehog signaling is required for pituitary gland development. Development 128(3):377–386
Ueda Y, Okada TS (1986) Transient expression of a ‘lens-specific’ gene, delta-crystallin, in the embryonic chicken adenohypophysis. Cell Diff 19(3):179–185
Wittkowski W, Bockmann J, Kreutz MR, Bockers TM (1999) Cell and molecular biology of the pars tuberalis of the pituitary. Int Rev Cytol 185:157–194
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, M., Shiina, T., Aizawa, S. et al. Detailed analysis of the δ-crystallin mRNA-expressing region in early development of the chick pituitary gland. J Mol Hist 43, 273–280 (2012). https://doi.org/10.1007/s10735-012-9407-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9407-1