Abstract
Proteolysis of extracellular matrix is an important requirement for embryonic development and is instrumental in processes such as morphogenesis, angiogenesis, and cell migration. Efficient remodeling requires controlled spatio-temporal expression of both the proteases and their inhibitors. Protein C inhibitor (PCI) effectively blocks a range of serine proteases, and recently has been suggested to play a role in cell differentiation and angiogenesis. In this study, we mapped the expression pattern of PCI throughout mouse development using in situ hybridization and immunohistochemistry. We detected a wide-spread, yet distinct expression pattern with prominent PCI levels in skin including vibrissae, and in fore- and hindgut. Further sites of PCI expression were choroid plexus of brain ventricles, heart, skeletal muscles, urogenital tract, and cartilages. A strong and stage-dependent PCI expression was observed in the developing lung. In the pseudoglandular stage, PCI expression was present in distal branching tubules whereas proximal tubules did not express PCI. Later in development, in the saccular stage, PCI expression was restricted to distal bronchioli whereas sacculi did not express PCI. PCI expression declined in postnatal stages and was not detected in adult lungs. In general, embryonic PCI expression indicates multifunctional roles of PCI during mouse development. The expression pattern of PCI during lung development suggests its possible involvement in lung morphogenesis and angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein C inhibitor (PCI, also known as SERPINA5; reviews in Geiger 2007, Suzuki 2008) is a glycoprotein that belongs to the serine protease inhibitor (serpin) family, which includes both serine protease inhibitors and non-inhibitory members (Rau et al. 2007; Silverman et al. 2001). These serine protease inhibitors interact with the active sites of their target serine proteases as suicide substrates by the formation of covalent enzyme-inhibitor complexes, which results in enzymatically inactive proteases. Many inhibitors of the coagulation and fibrinolytic cascades belong to the serpin family, including antithrombin, heparin cofactor 2, plasminogen activator inhibitor PAI-1 and 2, alpha-2 plasmin inhibitor, and PCI (Rau et al. 2007). PCI is a multifunctional serpin that can act both as a protease inhibitor of the coagulation (activated protein C, factor Xa, factor XIa, protein Z-dependent protease inhibitor and plasma kallikrein), the fibrinolytic (urokinase-type plasminogen activator (uPA) and tissue-type PA (tPA)), and the fertilization system (acrosin and prostate specific antigen) (Christensson and Lilja 1994; Ecke et al. 1992; Espana et al. 1989; Geiger et al. 1989; Marlar and Griffin 1980; Meijers et al. 1988; Suzuki et al. 1983; Zheng et al. 1994). It also acts as a non-inhibitory serpin by binding retinoic acid (Jerabek et al. 2001) and by attenuating breast cancer cell growth, metastasis and angiogenesis (Asanuma et al. 2007; Suzuki and Hayashi 2007). A role of PCI in tissue repair and regeneration has been suggested by its inhibitory action on the activator of the hepatocyte growth factor (HGF; Hayashi et al. 2007). In humans, PCI mRNA is expressed in many tissues, including liver, kidney, pancreas, skin, and the male and female reproductive organs (Geiger 2007; Krebs et al. 1999) while PCI protein was additionally found in different body fluids, including plasma, urine, synovial fluid, sweat, tears, milk, seminal plasma, Graaf follicle fluid, and cerebrospinal fluid (Laurell et al. 1992). In rodents, PCI is predominantly expressed in the male and female reproductive organs (Uhrin et al. 2000; Wagenaar et al. 2000; Wakita et al. 1998). In humans, the functional role in vivo was tested in venous thrombosis, where elevated levels of PCI constituted a mild risk factor for thrombosis (Meijers et al. 2002) indicating that of the possible actions of PCI: anticoagulant by inhibition of coagulation enzymes, anti-antifibrinolytic by inhibition of the thrombin activatable fibrinolysis inhibitor (TAFI) activation by thrombin-thrombomodulin, anti-anticoagulant by inhibition of activated protein C and protein C activation by thrombomodulin, and antifibrinolytic by the inhibition of urokinase, the last two options predominate in vivo. In rodents, the functional role of PCI has been studied in vivo in genetically modified mice (Hayashi et al. 2004; Nishii et al. 2006; Uhrin et al. 2000; Wagenaar et al. 2000). Male homozygous PCI deficient mice are sterile due to severely impaired spermatogenesis probably caused by a disruption of the Sertoli cell barrier (Uhrin et al. 2000). In transgenic mice overexpressing PCI in multiple organs, including the lung, the role of PCI in protection against monocrotaline-induced pulmonary hypertension was described (Nishii et al. 2006).
Because little is known about embryonic expression of PCI, we studied the spatial and temporal expression of PCI during the development of the mouse.
We observed a tissue specific expression of PCI in organs derived from all three germ layers. Some sites of PCI expression suggest its involvement in the proteolysis of extracellular matrix in connection with morphogenetic processes and/or angiogenesis. A prominent and distinct spatio-temporal expression pattern was found in the developing lung which suggests a role for PCI in controlling ductal outgrowth and development of the capillary plexus of sacculi and alveoli. Other sites of PCI expression may suggest a role for PCI in developmentally important cellular processes such as growth factor signaling.
Materials and methods
Animals
Mouse rearing and experiments were performed independently in the labs in The Netherlands (in situ hybridization) and in Austria (immunohistochemistry). The research protocols were approved by the Institutional Animal Care and Use Committee of the Universities of Utrecht and Vienna. The FVB/NAU strain was used for in situ hybridization and the mixed 129S/v:Swiss (50%:50%) strain was used for immunohistochemistry. Timed-pregnant mice were kept in a 12 h dark-light cycle and fed a standard chow diet ad libitum. Breeding pairs were allowed access overnight. Pregnant mice were killed by decapitation and embryos were delivered by hysterectomy after a gestation of 8.5, 9.5, 10.5, 11.5, 12.5, 14.5 and 18.5 days for in situ hybridization and after 9.5, 11.5, 12.5, 14.5 and 16.5 days for immunohistochemistry. Neonatal (at the day of birth; spontaneous birth occurs 20 days after conception), days 1 and 2 and adult mice were sacrificed under Nembutal anesthesia by transecting the abdominal blood vessels and lungs were removed. Whole embryo’s (embryonic days 8.5–16.5) and fetal (embryonic day 18.5), neonatal days 1 and 2, and adult lungs were fixed with buffered formaldehyde (4% paraformaldehyde in phosphate buffered saline, pH 7.4) for 24 h at 4°C, and embedded in paraffin after dehydration in a graded alcohol series and xylene. From gestational days 8.5–16.5 whole embryos were studied. Hereafter, tissue was removed due to difficulties with sectioning of bony structures. Since highest PCI expression was observed in the developing embryonic lung, only lung tissue was studied in ED 18.5, neonatal days 1 and 2, and adult mice.
In situ hybridization
Paraffin sections (7 μm) from embryonic and neonatal tissue were cut and mounted onto 3-aminopropyltriethanol silane coated slides (Henderson 1989). The in situ hybridization procedure was based on the method described by Wilkinson (Wilkinson 1992; Wilkinson and Nieto 1993). Sections were deparaffined, digested with proteinase K (Roche, Almere, The Netherlands), postfixed in 4% paraformaldehyde, treated with 0.2 M HCl to block endogenous alkaline phosphatase activity, acetylated with acetic anhydride dissolved in triethanolamine and hybridized with digoxygenine-labeled probe (5–50 ng per section) in hybridization buffer overnight at 55°C. The hybridization buffer contained 9/10 (volume) hybridization mixture: 50% formamide, 2× SSC, 1× Denhardt’s solution and 10% dextran sulphate and 1/10 (volume) probe solution: 0.1–1 ng/μl cRNA probe and 1 μg/μl yeast RNA in distilled water. After hybridization, high stringency washing was performed in 50% formamide/2× SSC at 55°C. Sections were treated with RNase A, rinsed in 2× SSC, blocked with lamb serum (Invitrogen, Paisley, England) and incubated with anti-digoxygenine-alkaline phosphatase conjugated Fab fragments (Roche, Almere, The Netherlands) for 2 h. Sections were washed extensively with Tris-buffered saline (TBS; 100 mM Tris, 150 mM NaCl, pH 7.5) and incubated with NBT/BCIP solution (Roche, Almere, The Netherlands), dissolved in 6% polyvinyl alcohol, overnight in the dark. The staining reaction was terminated with TE-buffer (10 mM Tris/HCl, 1 mM EDTA, pH 8.0). Subsequently, sections were counterstained with nuclear fast red, dehydrated with an ethanol series, and mounted in Euparal. As negative controls, hybridizations using sense probes or anti-sense probes on RNase pretreated sections were performed, which did not give any staining at all (Fig. 1b, d). We studied a minimum of 3 animals per time-point.
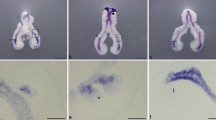
Controls for detection of PCI expression in lung tissue at embryonic days (ED) 14.5 (a–d) and 16.5 (e–h), respectively. In situ hybridization with antisense (a, c) and sense (b, d) probes; immunohistochemistry with (e, g) and without primary antibody (f, h). (c, d, g, h) high power magnifications of boxed areas in (a, b, e, f). Specific labeling can be found in distal branching tubules of the lung (arrows), whereas proximal tubules are devoid of staining (asterisks). The corresponding structures in controls are unstained (arrows, asterisks). In controls of immunohistochemistry (f, h) the lumina of blood vessels are slightly positive which is caused by the sensitive amplification system able to detect biotin in residual mouse plasma. In (a) PCI expression can also be observed within the cartilaginous ribs whereas they are devoid of label in controls (b). Lu = lung, Ri = ribs, VCI = vena cava inferior
Digoxygenine-labeled anti-sense cRNA probes to mouse PCI were made by in vitro transcription using pBluescript as a vector, containing the full length cDNA of mouse PCI obtained from I.M.A.G.E. Consortium, CloneID, 680998 (Lennon et al. 1996).
Immunohistochemistry
Immune-staining was performed on paraffin sections using antigen retrieval and signal enhancement as described previously (Uhrin et al. 2007). In short, de-paraffinated sections were incubated with 1% H2O2 in methyl alcohol for 30 min to block endogenous peroxidase activity and hydrated through a descending series of ethanol. Subsequently, antigen unmasking was achieved by steaming with 0.01 M citric acid buffer (pH 6.2) for 15 min. Slides were washed in TBS and digested with 0.5 μg/ml proteinase K (Roche Diagnostics, Vienna, Austria) in TBS supplemented with 2 mM CaCl2 for 2.5 min at 37°C. After 4 washes for 10 min in TBS, sections were incubated with 10% BSA (bovine serum albumin, fraction V, Roche Diagnostics, Vienna, Austria) in TBS for 30 min to reduce unspecific binding of antibodies and with a mouse-specific polyclonal rabbit anti-PCI antibody in TBS-BSA overnight at 4°C. The antibody was raised against recombinant mouse PCI as described previously (Uhrin et al. 2000). Hereafter, sections were incubated with a biotinylated goat anti- rabbit antibody (Dako, Vienna, Austria) and peroxidase-conjugated avidin (Vector, Burlingame, USA) in TBS-BSA for 30 min at room temperature. After each incubation, sections were washed 4 times for 10 min in TBST (0.05 % Tween20 in TBS). After peroxidase activity was visualized with metal-enhanced DAB (Roche Diagnostics, Vienna, Austria) sections were counter-stained with methyl green, dehydrated in an ascending series of ethanol and mounted with Eukitt.
For controls, the primary antibody was omitted which resulted in no specific staining (Fig. 1f, h).
Results
Specificity of in situ hybridization and immunohistochemistry detecting PCI expression in mouse embryos
In situ hybridization using anti-sense probes resulted in a distinct spatio-temporal expression pattern described below whereas use of sense probes yielded no signal (Fig. 1a–d). Likewise, immunohistochemistry where the first antibody was omitted erased any specific signal (Fig. 1e–h).
Embryonic PCI expression on EDs 8.5–12.5
PCI mRNA expression as detected by in situ hybridization was below the detection level on embryonic days (EDs) 8.5–10.5. PCI protein expression could be detected in the developing embryonic myocardium of the heart tube from ED 9.5 onwards (Fig. 2a).
PCI expression at embryonic days (ED) 9.5 (a), 11.5 and 12.5, respectively (b–j). In situ hybridization (b, c, e, g, i) and immunohistochemistry (a, d, f, h, j) on formaldehyde fixed paraffin sections. a Early expression of PCI in myocytes of the heart at ED 9.5; b PCI positive cells in choroid plexus (arrowheads) in the lateral ventricle of the brain; c skin of upper and lower jaws; d PCI in myocardium at ED 11.5; note absence of staining in non-myocardial cells including the cushion material of the atrioventricular canal and the developing valves in the outflow tract; e, presence of PCI in oesophagus and lung and absence in trachea; f, g pseudoglandular stage of lung development; note presence of PCI in distal tubules whereas proximal tubules are devoid of staining (asterisks); h, i PCI in cardiac region of stomach where the PCI-positive oesophagus enters (arrow) and in gonad; j PCI expression in hindgut, urethra and skin of anal bay. AB = anal bay, At = atrium, CM = cushion material, Go = gonad, Hg = hindgut, LJ = lower jaw, Lu = lung, Oe = oesophagus, OFT = outflow tract, St = stomach, To = tongue, Tr = trachea, UJ = upper jaw, Ur = urethra, Ve = ventricle
On ED 11.5 and 12.5 PCI mRNA and protein expression in the cranial region was detected in the epithelial cells of the choroid plexus, which protrudes into the lumen of the lateral and fourth ventricle (Fig. 2b) and in the developing skin of the upper and lower jaws (Fig. 2c). In the thoracic region PCI was expressed in the myocardium of the heart (Fig. 2d) with decreasing staining intensity through development, in the epithelial lining of the oesophagus (Fig. 2e), and in the notochord (not shown, see Fig. 4h for later stage). In the respiratory tract, PCI-positive cells were present in the epithelial cells of the embryonic lung, whereas staining was absent in the epithelial lining of the trachea (Figs. 2e–g, 3g in later stage). In the late embryonic, early pseudoglandular stage of lung development PCI was already differentially expressed in the bronchial epithelial cells showing only expression in the most distally localized branching bronchioli (Fig. 2f, g). PCI expression was present in the oesophagus extending into the cardia region of the stomach (Fig. 2h, i). In the abdominal region, PCI label was found in the developing reproductive organs, including the gonads and in the urogenital sinus, in the (hind)gut and the skin of the anal bay (Fig. 2h, j). Weak PCI expression in the developing vertebrae could only be detected at the protein level (not shown, see Fig. 4h for later stage).
PCI expression in the head and thoracic region at embryonic days (ED) 14.5 and 16.5, respectively. In situ hybridization (a, c, d) and immunohistochemistry (b, e–h) on formaldehyde fixed paraffin sections. High power magnifications of the boxed areas in (c, e) are presented in (d, f). a Positive ependymal cells of choroid plexus; b PCI expression in skin of snout, nasal cavity and developing vibrissae; c thoracic and upper abdominal part at ED 14.5; note PCI expression in lung, thoracal muscles (arrow), diaphragm (double arrow), and intercostal muscles (arrowhead); d high power magnification of boxed area in (c); pseudoglandular stage of lung development with positive distal bronchioli, whereas proximal tubules are negative (asterisks); e at ED 16.5 onset of ossification in ribs with label in both cartilaginous and osseous compartments; pseudoglandular stage lung; f high power magnification of boxed area in (e) with positivity in distal branching bronchioli and no staining in proximal tubules (asterisks); g PCI positive label in oesophagus but not in trachea; h extremely latero-sagittal section through rib cage at ED 14.5 shows PCI-positive intercostal and rib-cage muscles and in skin. He = heart, Li = liver, LJ = lower jaw, Lu = lung, NC = nasal cavity, Oe = oesophagus, Ri = rib, To = Tongue, Tr = trachea, UJ = upper jaw, Ve = ventricle
PCI expression in the abdominal region at embryonic days (ED) 14.5 and 16.5, respectively. In situ hybridization (b–f) and immunohistochemistry (a, g, h) on formaldehyde fixed paraffin sections. a Little or no staining in liver, gallbladder and bile ducts, intestine, pancreas; b, c strong PCI expression in oesophagus and sporadic expression in gut epithelia; strong expression in skin of hindlimb; d strong label in urethra and skin of anal bay; e scattered label in tubules of kidney, in particular in branching ureter/collecting tubules; f PCI expression in testis, epididymis, and kidney; g in paws at ED 16.5, strong expression is found in skin, interdigital webs (arrows) and in the zone of beginning ossification in diaphyses (arrowheads); h in the vertebral column, PCI expression can be found around areas of developing intervertebral discs (arrows) and in nucleus pulposus (arrowhead) consistent with positive staining of notochord in previous stages. AB = anal bay; CBD = common bile duct; CD = cystic duct; CHD = common hepatic duct; Du = duodenum; Ep = epididymis; Gb = gallbladder; Gu = gut, Hl = hindlimb; Ki = kidney, Li = liver, Oe = oesophagus, Pa = pancreas, St = stomach; Te = testis; Ur = urethra
Fetal PCI expression on EDs 14.5–16.5
On ED 14.5–16.5 in the cranial region PCI was expressed in the developing brain in the choroid plexus (Fig. 3a), in the epithelial lining of the nasal and oral cavities and in the skin of the nose including developing vibrissae (Fig. 3b).
In the thoracic region highest PCI expression was observed in the developing lung, which has proceeded towards the late pseudoglandular stage of development. PCI was expressed in the cuboidal bronchial epithelium of the most distally localized branching bronchioli. Expression was absent in the proximal columnar epithelial cells of the bronchioli and bronchi (Fig. 3c–f). PCI was present in the epithelial lining of the oesophagus and was absent from trachea (Fig. 3g). In addition, PCI expression could be detected in the muscles of the thorax, intercostal muscles and in the diaphragm (Fig. 3c, e, h).
In the abdominal region, PCI expression was largely absent from liver and pancreas (Fig. 4a). PCI positive labeling was found in oesophagus, in the proximal part of stomach epithelia, and in a scattered pattern in epithelial cells lining the gut (Fig. 4b, c). In urogenital organs, strong expression was found in urethra (Fig. 4d), and weaker expression was present in tubular epithelial cells of the kidney (Fig. 4e, f). PCI was present in the tubular structures of the developing reproductive organs, including the testis and epididymis (Fig. 4f). A high level of expression was observed in the skin of the developing urogenital region (Fig. 4d) whereas in other parts of the skin the level of PCI expression varied (e.g. Figs. 4b, g, and 3b, h). In addition, PCI was expressed in cartilages and in interdigital webs of the paws (Fig. 4g) and around intervertebral disks of the vertebral column including the receding notochord (Fig. 4h).
Postnatal PCI expression in the developing lung
In the early saccular stage of lung development on ED 18.5 PCI was expressed at high levels in the terminal bronchial epithelium, whereas expression was low or even absent in the sacculi (Fig. 5a, b). On neonatal days 1 and 2, when lung development is still in the saccular stage, PCI was expressed in epithelial cells of terminal bronchioli and at very low levels in alveolar type 2 cells whereas alveolar type 1 cells were negative (Fig. 5c–f). No PCI mRNA and protein expression could be detected in adult lungs (Fig. 5g, h).
PCI expression in the lung at embryonic day (ED) 18.5 (a, b), postnatal days 1 (c, d) and 2 (e, f), and in adults (g, h). In situ hybridization (a, b, e, f) and immunohistochemistry (c, d, g, h) on formaldehyde fixed paraffin sections. a early saccular stage of lung development (ED 18.5); strong label in columnar epithelia of terminal bronchioli and some label in type 2 alveolar epithelial cells can be observed; type 1 epithelial cells of sacculi are devoid of signal; b high power magnification of boxed area in (a); c–f PCI expression at postnatal days 1 (c, d) and 2 (e, f), lung in saccular phase; note PCI-expression in terminal bronchii in Clara cells until the bronchoalveolar duct junction (arrows); faint staining of type 2 alveolar cells, type 1 alveolar cells are negative; d, f high power magnifications of boxed areas in (c, e); g no PCI expression can be found in normal adult lung; h high power magnification of boxed area in (g). S = sacculus, TB = terminal bronchiolus
Discussion
The serine protease inhibitor PCI (SERPINA5) has initially been described in humans to be involved in the regulation of hemostasis and fibrinolysis (Ecke et al. 1992; Espana et al. 1989; Geiger et al. 1989; Marlar and Griffin 1980; Meijers et al. 1988; Suzuki et al. 1983). Recently it became clear that PCI fulfills many other functions under normal and pathological conditions. Some functions where PCI is involved, such as extracellular matrix remodeling, are pivotal in embryonic developmental processes. Thus, we mapped the expression pattern of PCI mRNA and protein throughout mouse development.
Using immunohistochemistry, we first detected signal on ED 9.5 whereas embryos at this stage were devoid of label after in situ hybridization. It should be noted, that initial experiments to detect PCI by immunohistochemistry resulted in low signal intensity. Satisfactory signal intensity was only achieved when antigen retrieval methodology and signal amplification inherent to the biotin-avidin system was applied. This approach likely resulted in a superior level of sensitivity of immunohistochemistry over in situ hybridization which may explain the discrepancy in detection of the temporal onset of PCI expression using the two methods.
The embryonic and fetal expression pattern of PCI suggests involvement in different developmental processes. However, it has previously been shown that PCI-deficient (PCI−/−) mice were viable although PCI−/− males were infertile (Uhrin et al. 2000). This suggests that the role of PCI during development is redundant and other factors can compensate for the lack of functional PCI levels. Consequently, the exact role of PCI at the different expression sites remains to be elucidated.
Some sites of PCI expression are consistent with the well characterized function of PCI in regulating extracellular matrix proteolysis, which is of eminent importance during morphogenesis. For instance, expression of PCI in the developing hair anlagen of the snout falls into this category. Another well established fact is the presence of PCI in many body liquids such as in cerebrospinal fluid in humans (Laurell et al. 1992). Thus it appears comprehensible that PCI is expressed in the ependymal cells of choroid plexus where the cerebrospinal fluid is secreted into the ventricles.
Expression of PCI in the skin during mouse development coincides with the previously described presence of PCI antigen in the normal human epidermis and its constitutive expression by keratinocytes in culture (Krebs et al. 1999) where PCI could provide protease inhibitory activity. Further possible functions of PCI in the developing skin might involve protection from active proteases present in the amniotic fluid, such as uPA and tPA (Verkleij-Hagoort et al. 2007), or regulation of morphogen or growth factor supply in the epidermis, as PCI binds retinoids (Jerabek et al. 2001) and hepatocyte growth factor (HGF) both present in developing skin and amniotic fluid (Laurell et al. 1992; Srivastava et al. 1999).
Other sites of PCI expression are more difficult to reconcile with known functions of PCI. Presence of PCI in the interdigital webs of the paws and in the receding notochord indicates involvement in cell death and apoptosis. To our knowledge, no reports exist on direct involvement of PCI in apoptosis so far. However, in endothelial cells activated protein C (APC), which is inhibited by PCI, blocks p53-induced apoptosis (Cheng et al. 2003). It is thus tempting to speculate that PCI may act proapoptotic by binding of repressive factors of apoptotic pathways or, alternatively, PCI might be involved in triggering devascularization which in turn might induce apoptosis by an indirect mechanism. Furthermore, PCI protein could accumulate on apoptotic cells, since it binds to phosphatidylserine (Malleier et al. 2007), which is exposed on the surface of apoptotic cells.
Concerning PCI expression in developing skeletal and cardiac muscles, in a recent proteome analysis of differentiating C2C12 muscle cells an up-regulation of serpins was found and it was speculated that serpins may be involved in myogenic differentiation and/or in myoblast migration (Chan et al. 2007). Additionally, PCI might play a role via interaction with HGF which has also been shown to be involved in myoblast migration and muscle formation (Dietrich et al. 1999).
The expression of PCI in developing gonads is of particular interest as we reported recently the expression of PCI in post-natal and adult mouse testis (Uhrin et al. 2007) and could previously show that PCI−/− mice display severely impaired spermatogenesis (Uhrin et al. 2000). Our data on the up-regulation of PCI on ED 12.5 are consistent with a recent paper demonstrating sex-dimorphic gonadal upregulation of PCI where PCI expression is found in developing testes but not in ovaries (Odet et al. 2004). Leydig cells were shown to be the source of PCI expression and the authors speculate that PCI in Leydig cells might be involved in the control of tissue proteolysis as Leydig cells produce both PCI and its target protease uPA (Habert et al. 2001; Jerabek et al. 2001). It was proposed that PCI might be involved in retinoic signaling because both Leydig cells and PCI are targets of retinoids (Odet et al. 2004). Further speculations about possible roles of PCI include regulation of angiogenesis as developing testes display a more extensive angiogenetic activity than ovaries.
PCI protein was also detected in cartilages where PCI was present in areas of chondrocyte hypertrophy before matrix degradation and onset of ossification. In vertebrae, PCI was present in areas of future intervertebral discs. Interestingly, in humans, serpins A1 and A3 have recently been shown to be expressed in fetal cartilages, are up-regulated during differentiation of cartilages (Pogue et al. 2004), and involvement of these serpins in matrix homeostasis was hypothesized (Boeuf et al. 2008; Pogue et al. 2004).
The absence of mouse PCI in the embryonic liver coincides with our previous findings suggesting that PCI in adult mouse, contrary to the situation in humans, is not a plasma protein (Uhrin et al. 2000).
Concerning expression of PCI in the developing lung we found that in the pseudoglandular stage PCI is expressed in the most distally branching tubules. In the saccular phase, PCI is expressed in the bronchiolar epithelia and is absent from saccular epithelia. PCI expression ceases in the perinatal period and is undetectable in adult lungs. Several putative functions for PCI in the developing mouse lung can be postulated: (1) PCI is directly involved in lung morphogenesis participating in branching of the bronchiolar tree by controlling extracellular proteolysis; (2) PCI is secreted into amniotic fluid and the lung may represent a source of PCI production and secretion into the amniotic fluid; (3) PCI could act through interaction with HGF which is expressed in lung mesenchyme (Sonnenberg et al. 1993); (4) notably, PCI expression could not be detected in the epithelia responsible for gas exchange in sacculi and alveoli at later stages of development. PCI has recently been shown to exert inhibitory effects on angiogenesis in a tumor model in immuno-deficient mice, in Matrigel implant assays and in rat corneal angiogenesis assays (Asanuma et al. 2007). It was speculated that this effect might be brought about by competition with the potent angiogenesis-inducing factor VEGF for heparin binding sites (Asanuma et al. 2007). Thus, down-regulation of PCI in alveoli could be necessary for alveolar capillarisation, a hallmark of saccular phase of lung development and crucial for proper post-natal lung functionality. Conversely, expression of PCI in cuboidal epithelia of bronchi and bronchioli may help to prevent this process.
In conclusion, we found that the expression patterns of PCI show a tissue specific distribution during mouse embryonic development. The data presented in this study point to PCI as an important factor for mouse development. On the other hand we reported previously, that, with the exception of male infertility, all PCI−/− mice develop phenotypically normal (Uhrin et al. 2000). This indicates that the functions of PCI can be compensated by other factors and it will be interesting to learn which factors are able to take over the functions of PCI in PCI−/− mice.
References
Asanuma K, Yoshikawa T, Hayashi T, Akita N, Nakagawa N, Hamada Y, Nishioka J, Kamada H, Gabazza EC, Ido M, Uchida A, Suzuki K (2007) Protein C inhibitor inhibits breast cancer cell growth, metastasis and angiogenesis independently of its protease inhibitory activity. Int J Cancer 121:955–965
Boeuf S, Steck E, Pelttari K, Hennig T, Buneb A, Benz K, Witte D, Sultmann H, Poustka A, Richter W (2008) Subtractive gene expression profiling of articular cartilage and mesenchymal stem cells: serpins as cartilage-relevant differentiation markers. Osteoarthritis Cartilage 16:48–60
Chan XC, McDermott JC, Siu KW (2007) Identification of secreted proteins during skeletal muscle development. J Proteome Res 6:698–710
Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV (2003) Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med 9:338–342
Christensson A, Lilja H (1994) Complex formation between protein C inhibitor and prostate-specific antigen in vitro and in human semen. Eur J Biochem 220:45–53
Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C (1999) The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126:1621–1629
Ecke S, Geiger M, Resch I, Jerabek I, Sting L, Maier M, Binder BR (1992) Inhibition of tissue kallikrein by protein C inhibitor. Evidence for identity of protein C inhibitor with the kallikrein binding protein. J Biol Chem 267:7048–7052
Espana F, Berrettini M, Griffin JH (1989) Purification and characterization of plasma protein C inhibitor. Thromb Res 55:369–384
Geiger M (2007) Protein C inhibitor, a serpin with functions in- and outside vascular biology. Thromb Haemost 97:343–347
Geiger M, Huber K, Wojta J, Stingl L, Espana F, Griffin JH, Binder BR (1989) Complex formation between urokinase and plasma protein C inhibitor in vitro and in vivo. Blood 74:722–728
Habert R, Lejeune H, Saez JM (2001) Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol 179:47–74
Hayashi T, Nishioka J, Kamada H, Asanuma K, Kondo H, Gabazza EC, Ido M, Suzuki K (2004) Characterization of a novel human protein C inhibitor (PCI) gene transgenic mouse useful for studying the role of PCI in physiological and pathological conditions. J Thromb Haemost 2:949–961
Hayashi T, Nishioka J, Nakagawa N, Kamada H, Gabazza EC, Kobayashi T, Hattori A, Suzuki K (2007) Protein C inhibitor directly and potently inhibits activated hepatocyte growth factor activator. J Thromb Haemost 5:1477–1485
Henderson C (1989) Aminoalkylsilane: an inexpensive, simple preparation for slide adhesion. Journal of Histotechnology 12:123–124
Jerabek I, Zechmeister-Machhart M, Binder BR, Geiger M (2001) Binding of retinoic acid by the inhibitory serpin protein C inhibitor. Eur J Biochem 268:5989–5996
Krebs M, Uhrin P, Vales A, Prendes-Garcia MJ, Wojta J, Geiger M, Binder BR (1999) Protein C inhibitor is expressed in keratinocytes of human skin. J Invest Dermatol 113:32–37
Laurell M, Christensson A, Abrahamsson PA, Stenflo J, Lilja H (1992) Protein C inhibitor in human body fluids. Seminal plasma is rich in inhibitor antigen deriving from cells throughout the male reproductive system. J Clin Invest 89:1094–1101
Lennon G, Auffray C, Polymeropoulos M, Soares MB (1996) The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics 33:151–152
Malleier JM, Oskolkova O, Bochkov V, Jerabek I, Sokolikova B, Perkmann T, Breuss J, Binder BR, Geiger M (2007) Regulation of protein C inhibitor (PCI) activity by specific oxidized and negatively charged phospholipids. Blood 109:4769–4776
Marlar RA, Griffin JH (1980) Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J Clin Invest 66:1186–1189
Meijers JC, Kanters DH, Vlooswijk RA, van Erp HE, Hessing M, Bouma BN (1988) Inactivation of human plasma kallikrein and factor XIa by protein C inhibitor. Biochemistry 27:4231–4237
Meijers JC, Marquart JA, Bertina RM, Bouma BN, Rosendaal FR (2002) Protein C inhibitor (plasminogen activator inhibitor-3) and the risk of venous thrombosis. Br J Haematol 118:604–609
Nishii Y, Gabazza EC, Fujimoto H, Nakahara H, Takagi T, Bruno N, D’Alessandro-Gabazza CN, Maruyama J, Maruyama K, Hayashi T, Adachi Y, Suzuki K, Taguchi O (2006) Protective role of protein C inhibitor in monocrotaline-induced pulmonary hypertension. J Thromb Haemost 4:2331–2339
Odet F, Guyot R, Leduque P, Le Magueresse-Battistoni B (2004) Evidence for similar expression of protein C inhibitor and the urokinase-type plasminogen activator system during mouse testis development. Endocrinology 145:1481–1489
Pogue R, Sebald E, King L, Kronstadt E, Krakow D, Cohn DH (2004) A transcriptional profile of human fetal cartilage. Matrix Biol 23:299–307
Rau JC, Beaulieu LM, Huntington JA, Church FC (2007) Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost 5(Suppl 1):102–115
Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O’Donnell E, Salvesen GS, Travis J, Whisstock JC (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276:33293–33296
Sonnenberg E, Meyer D, Weidner KM, Birchmeier C (1993) Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 123:223–235
Srivastava MD, Lippes J, Srivastava BI (1999) Hepatocyte growth factor in human milk and reproductive tract fluids. Am J Reprod Immunol 42:347–354
Suzuki K (2008) The multi-functional serpin, protein C inhibitor: beyond thrombosis and hemostasis. J Thromb Haemost 6:2017–2026
Suzuki K, Hayashi T (2007) Protein C and its inhibitor in malignancy. Semin Thromb Hemost 33:667–672
Suzuki K, Nishioka J, Hashimoto S (1983) Protein C inhibitor. Purification from human plasma and characterization. J Biol Chem 258:163–168
Uhrin P, Dewerchin M, Hilpert M, Chrenek P, Schöfer C, Zechmeister-Machart M, Krönke G, Vales A, Carmeliet P, Binder BR, Geiger M (2000) Disruption of the protein C inhibitor gene results in impaired spermatogenesis and male infertility. J Clin Invest 106:1531–1539
Uhrin P, Schofer C, Zaujec J, Ryban L, Hilpert M, Weipoltshammer K, Jerabek I, Pirtzkall I, Furtmuller M, Dewerchin M, Binder BR, Geiger M (2007) Male fertility and protein C inhibitor/plasminogen activator inhibitor-3 (PCI): localization of PCI in mouse testis and failure of single plasminogen activator knockout to restore spermatogenesis in PCI-deficient mice. Fertil Steril 88:1049–1057
Verkleij-Hagoort AC, Ursem NT, Hop WC, Geurts-Moespot A, Steegers EA, Sweep FC, Steegers-Theunissen RP (2007) Complex congenital malformations and the impact of the plasminogen activator system and beta-hCG in amniotic fluid. Eur J Obstet Gynecol Reprod Biol 135:47–52
Wagenaar GTM, van Vuuren AJH, Girma M, Tiekstra MJ, Kwast L, Koster JG, Rijneveld AW, Elisen MGLM, van der Poll T, Meijers JCM (2000) Characterization of transgenic mice that secrete functional human protein C inhibitor into the circulation. Thromb Haemost 83:93–101
Wakita T, Hayashi T, Yuasa H, Nishioka J, Kawamura J, Suzuki K (1998) Molecular cloning, tissue distribution and androgen regulation of rat protein C inhibitor. FEBS Lett 429:263–268
Wilkinson DG, Nieto MA (1993) Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol 225:361–373
Zheng X, Geiger M, Ecke S, Bielek E, Donner P, Eberspacher U, Schleuning WD, Binder BR (1994) Inhibition of acrosin by protein C inhibitor and localization of protein C inhibitor to spermatozoa. Am J Physiol 267:C466–C472
Acknowledgments
This work was supported by grants from the Austrian Science Fund FWF P16230-B04 to P. Uhrin and P17337-B09, P20248-B09 to M. Geiger.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wagenaar, G.T.M., Uhrin, P., Weipoltshammer, K. et al. Expression patterns of protein C inhibitor in mouse development. J Mol Hist 41, 27–37 (2010). https://doi.org/10.1007/s10735-010-9259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-010-9259-5