Abstract
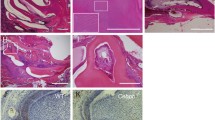
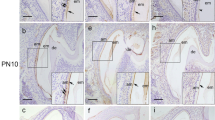
Reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) is a single membrane-anchored MMP-regulator and regulates matrix metalloproteinases (MMP) 2, 9 and 14. In turn, MMPs are endopeptidases that play a pivotal role in remodeling ECM. In this work, we decided to evaluate expression pattern of RECK in growing rat incisor during, specifically focusing out amelogenesis process. Based on different kinds of ameloblasts, our results showed that RECK expression was conducted by secretory and post-secretory ameloblasts. At the secretory phase, RECK was localized in the infra-nuclear region of the ameloblast, outer epithelium, near blood vessels, and in the stellate reticulum. From the transition to the maturation phases, RECK was strongly expressed by non-epithelial immuno-competent cells (macrophages and/or dendritic-like cells) in the papillary layer. From the transition to the maturation stage, RECK expression was increased. RECK mRNA was amplified by RT-PCR from whole enamel organ. Here, we verified the presence of RECK mRNA during all stages of amelogenesis. These events were governed by ameloblasts and by non-epithelial cells residents in the enamel organ. Concluding, we found differential expression of MMPs-2, -9 and RECK in the different phases of amelogenesis, suggesting that the tissue remodeling is rigorously controlled during dental mineralization.



Similar content being viewed by others
References
Accorsi-Mendonca T, Zambuzzi WF, da Silva Paiva KB, Pereira Lauris JR, Cestari TM, Taga R, Granjeiro JM (2005) Expression of metalloproteinase 2 in the cell response to porous demineralized bovine bone matrix. J Mol Histol 36:311–316
Annabi B, Lachambre M, Bousquet-Gagnon N, Page M, Gingras D, Beliveau R (2001) Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem J 353:547–553
Bartlett JD, Simmer JP (1999) Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425–441
Caron C, Xue J, Sun X, Simmer JP, Bartlett JD (2001) Gelatinase A (MMP-2) in developing tooth tissues and amelogenin hydrolysis. J Dent Res 80:1660–1664
Echizenya M, Kondo S, Takahashi R, Oh J, Kawashima S, Kitayama H, Takahashi C, Noda M (2005) The membrane-anchored MMP-regulator RECK is a target of myogenic regulatory factors. Oncogene 24:5850–5857
Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG (2004) Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 15:678–687
Goldberg M, Septier D, Bourd K, Hall R, George A, Goldberg H, Menashi S (2003) Immunohistochemical localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the forming rat incisor. Connect Tissue Res 44:143–153
Hannas AR, Pereira JC, Granjeiro JM, Tjaderhane L (2007) The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65:1–13
Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D (2001) Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci USA 98:13693–13698
Kallenbach E (1971) Electron microscopy of the differentiating rat incisor ameloblast. J Ultrastruct Res 35:508–531
Kawashima S, Imamura Y, Chandana EP, Noda T, Takahashi R, Adachi E, Takahashi C, Noda M (2008) Localization of the membrane-anchored MMP-regulator RECK at the neuromuscular junctions. J Neurochem 104:376–385
Kim HN, Chung HS (2008) Caveolin-1 inhibits membrane-type 1 matrix metalloproteinase activity. BMB Rep 41:858–862
Kondo S, Shukunami C, Morioka Y, Matsumoto N, Takahashi R, Oh J, Atsumi T, Umezawa A, Kudo A, Kitayama H, Hiraki Y, Noda M (2007) Dual effects of the membrane-anchored MMP regulator RECK on chondrogenic differentiation of ATDC5 cells. J Cell Sci 120:849–857
Kumamoto H, Ooya K (2006) Immunohistochemical detection of MT1-MMP, RECK, and EMMPRIN in ameloblastic tumors. J Oral Pathol Med 35:345–351
Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP (2008) Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 389:695–700
Miki T, Takegami Y, Okawa K, Muraguchi T, Noda M, Takahashi C (2007) The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J Biol Chem 282:12341–12352
Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EP, Omura A, Miki T, Takahashi R, Matsumoto N, Ludwig A, Noda M, Takahashi C (2007) RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat Neurosci 10:838–845
Nanci A, Slavkin HC, Smith CE (1987) Immunocytochemical and radioautographic evidence for secretion and intracellular degradation of enamel proteins by ameloblasts during the maturation stage of amelogenesis in rat incisors. Anat Rec 217:107–123
Nishikawa S, Sasaki F (1999) Internalization of amelogenin by dendritic cells of the papillary layer during transition and early maturation stages. Histochem Cell Biol 112:301–305
Noirey N, Staquet MJ, Gariazzo MJ, Serres M, Andre C, Schmitt D, Vincent C (2002) Relationship between expression of matrix metalloproteinases and migration of epidermal and in vitro generated langerhans cells. Eur J Cell Biol 81:383–389
Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR (2004) Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett 563:129–134
Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M (2001) The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107:789–800
Omura A, Matsuzaki T, Mio K, Ogura T, Yamamoto M, Fujita A, Okawa K, Kitayama H, Takahashi C, Sato C, Noda M (2009) RECK forms cowbell-shaped dimers and inhibits matrix metalloproteinase-catalyzed cleavage of fibronectin. J Biol Chem 284:3461–3469
Remacle A, Murphy G, Roghi C (2003) Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci 116:3905–3916
Sabharanjak S, Sharma P, Parton RG, Mayor S (2002) GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2:411–423
Sakuraba I, Hatakeyama J, Hatakeyama Y, Takahashi I, Mayanagi H, Sasano Y (2006) The MMP activity in developing rat molar roots and incisors demonstrated by in situ zymography. J Mol Histol 37:87–93
Sasahara RM, Brochado SM, Takahashi C, Oh J, Maria-Engler SS, Granjeiro JM, Noda M, Sogayar MC (2002) Transcriptional control of the RECK metastasis/angiogenesis suppressor gene. Cancer Detect Prev 26:435–443
Shapiro SD (1998) Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol 10:602–608
Sina A, Lord-Dufour S, Annabi B (2009) Cell-based evidence for aminopeptidase N/CD13 inhibitor actinonin targeting of MT1-MMP-mediated proMMP-2 activation. Cancer Lett 279:171–176
Smith CE, Nanci A (1989) A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat Rec 225:257–266
Staquet MJ, Piccardi N, Piccirilli A, Vincent C, Schmitt D, Msika P (2004) Novel protein kinase C and matrix metalloproteinase inhibitors of vegetable origin as potential modulators of Langerhans cell migration following hapten-induced sensitization. Int Arch Allergy Immunol 133:348–356
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Takagi S, Simizu S, Osada H (2009) RECK negatively regulates matrix metalloproteinase-9 transcription. Cancer Res 69:1502–1508
Takano Y, Kawahara I, Hoshino M, Takeuchi K, Maeda T, Ohshima H, Hanaizumi Y, Kawano Y (1996) Dendritic cells: a novel cellular component of the rat incisor enamel organ appearing in the late stages of enamel maturation. Adv Dent Res 10:94–104
Tallant C, Marrero A, Gomis-Ruth FX (2009) Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta
Uekita T, Itoh Y, Yana I, Ohno H, Seiki M (2001) Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol 155:1345–1356
Webster NL, Crowe SM (2006) Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol 80:1052–1066
Wu X, Gan B, Yoo Y, Guan JL (2005) FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell 9:185–196
Acknowledgments
The authors are grateful to the technical assistance of Daniele Ceolin, Gustavo N Zanelatto and Tânia M. Cestari. Special thanks to Dr. Professor Victor Arana-Chavez (Sao Paulo Dental School-USP) for helpful assistance in dissection of enamel organ. This work was supported by grants from the FAPESP (08/53003-9, 07/04148-1, 07/00216-2, 99/10655-5; 01/10707-7 and 07/04148-1), CAPES—(BEX 2415/04-6), and CNPq (475721/2003-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paiva, K.B.S., Zambuzzi, W.F., Accorsi-Mendonça, T. et al. Rat forming incisor requires a rigorous ECM remodeling modulated by MMP/RECK balance. J Mol Hist 40, 201–207 (2009). https://doi.org/10.1007/s10735-009-9231-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-009-9231-4